Sustainability in Internal Medicine: A Year-Long Ward-Wide Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrolment and Study Timeframe
2.2. Assessment of Patient Complexity and Intensity of Care
2.3. Assistance Parameters
2.4. Individual and Global Ward Data Aggregation
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. General Clinical Features and Outcomes
3.2. Mortality
3.3. Length of Hospitalisation
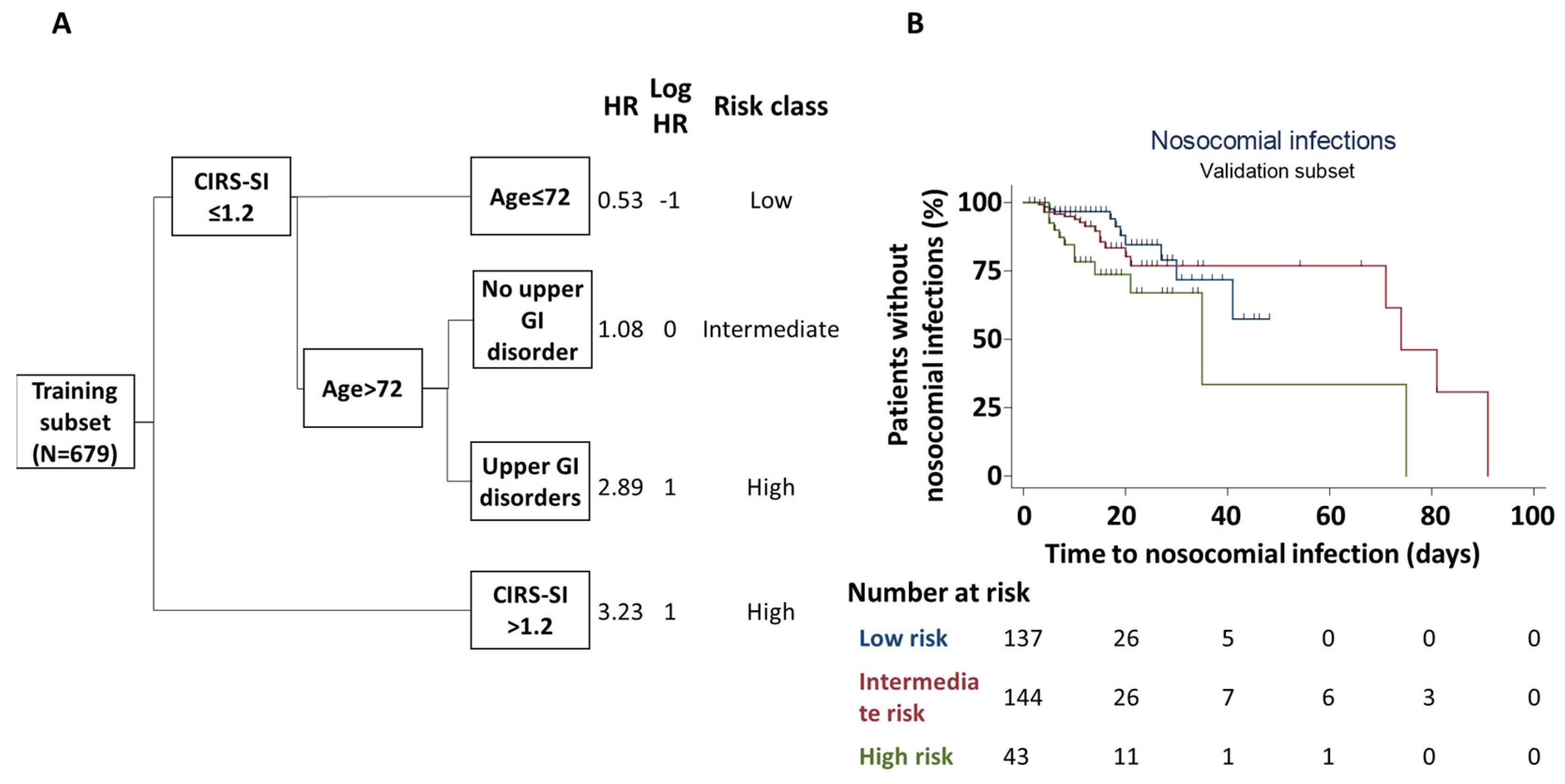
3.4. Nosocomial Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, H.J.; Morra, D.; Caesar, M.; Carter, M.W.; Abrams, H. Understanding hospital and emergency department congestion: An examination of inpatient admission trends and bed resources. CJEM 2010, 12, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Haklai, Z.; Glick, S.; Benbassat, J. Determinants of hospital utilization: The content of medical inpatient care in Israel. Isr. Med. Assoc. J. 2000, 2, 339–342. [Google Scholar] [PubMed]
- Dieleman, J.L.; Squires, E.; Bui, A.L.; Campbell, M.; Chapin, A.; Hamavid, H.; Horst, C.; Li, Z.; Matyasz, T.; Reynolds, A.; et al. Factors Associated With Increases in US Health Care Spending, 1996–2013. JAMA 2017, 318, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Buurman, B.M.; Frenkel, W.J.; Abu-Hanna, A.; Parlevliet, J.L.; de Rooij, S.E. Acute and chronic diseases as part of multimorbidity in acutely hospitalized older patients. Eur. J. Intern. Med. 2016, 27, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Maniam, B.; Leavell, H. The silver tsunami: Evaluating the impact of population aging in the US. J. Bus. Behav. Sci. 2017, 29, 153–169. [Google Scholar]
- Arlotti, M.; Aguilar-Hendrickson, M. The vicious layering of multilevel governance in Southern Europe: The case of elderly care in Italy and Spain. Soc. Policy Adm. 2018, 52, 646–661. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Pforsich, H.; Lin, V.W. Physician workforce in the United States of America: Forecasting nationwide shortages. Hum. Resour. Health 2020, 18, 8. [Google Scholar] [CrossRef]
- “Sustainable”. In Merriam-Webster.com Dictionary; 2023; Available online: https://www.merriam-webster.com/dictionary/sustainable (accessed on 13 October 2023).
- Brundtland, G.H. Report of the World Commission on Environment and Development: “Our Common Future.”; UN: New York City, NY, USA, 1987. [Google Scholar]
- Prante, F.J.; Bramucci, A.; Truger, A. Decades of Tight Fiscal Policy Have Left the Health Care System in Italy Ill-Prepared to Fight the COVID-19 Outbreak. Inter. Econ. 2020, 55, 147–152. [Google Scholar] [CrossRef]
- Verma, A.A.; Guo, Y.; Kwan, J.L.; Lapointe-Shaw, L.; Rawal, S.; Tang, T.; Weinerman, A.; Cram, P.; Dhalla, I.A.; Hwang, S.W.; et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The General Medicine Inpatient Initiative (GEMINI) retrospective cohort study. CMAJ Open 2017, 5, E842–E849. [Google Scholar] [CrossRef]
- Martin, S.; Longo, F.; Lomas, J.; Claxton, K. Causal impact of social care, public health and healthcare expenditure on mortality in England: Cross-sectional evidence for 2013/2014. BMJ Open 2021, 11, e046417. [Google Scholar] [CrossRef]
- The Economist Educational Foundation. Sustainable Healthcare: Doctor Shortages. 2023. Available online: https://talk.economistfoundation.org/festivals/festival-2022/sustainable-healthcare-doctor-shortages/ (accessed on 19 October 2023).
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Salvi, F.; Miller, M.D.; Grilli, A.; Giorgi, R.; Towers, A.L.; Morichi, V.; Spazzafumo, L.; Mancinelli, L.; Espinosa, E.; Rappelli, A.; et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J. Am. Geriatr. Soc. 2008, 56, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Reis Miranda, D.; Moreno, R.; Iapichino, G. Nine equivalents of nursing manpower use score (NEMS). Intensive Care Med. 1997, 23, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Canti, V.; Moiola, L.; Magnoni, M.; Rovere-Querini, P.; Coletto, L.A.; Dagna, L.; Manfredi, A.A.; Bozzolo, E.P. Performance of SLE responder index and lupus low disease activity state in real life: A prospective cohort study. Int. J. Rheum. Dis. 2019, 22, 1752–1761. [Google Scholar] [CrossRef]
- Kaye, K.S.; Marchaim, D.; Chen, T.Y.; Baures, T.; Anderson, D.J.; Choi, Y.; Sloane, R.; Schmader, K.E. Effect of nosocomial bloodstream infections on mortality, length of stay, and hospital costs in older adults. J. Am. Geriatr. Soc. 2014, 62, 306–311. [Google Scholar] [CrossRef]
- Blecker, S.; Shine, D.; Park, N.; Goldfeld, K.; Scott Braithwaite, R.; Radford, M.J.; Gourevitch, M.N. Association of weekend continuity of care with hospital length of stay. Int. J. Qual. Health Care 2014, 26, 530–537. [Google Scholar] [CrossRef]
- Schoenfeld, D.J. The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika 1981, 68, 316–319. [Google Scholar] [CrossRef]
- Launay, C.P.; Annweiler, C.; de Decker, L.; Kabeshova, A.; Fantino, B.; Beauchet, O. Risk of in-hospital mortality following emergency department admission: Results from the geriatric EDEN cohort study. J. Nutr. Health Aging 2014, 18, 83–86. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Spitznagel, E.L., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004, 291, 2441–2447. [Google Scholar] [CrossRef]
- Munier-Marion, E.; Benet, T.; Regis, C.; Lina, B.; Morfin, F.; Vanhems, P. Hospitalization in double-occupancy rooms and the risk of hospital-acquired influenza: A prospective cohort study. Clin. Microbiol. Infect. 2016, 22, 461.e7–461.e9. [Google Scholar] [CrossRef]
- van Vught, L.A.; Klein Klouwenberg, P.M.; Spitoni, C.; Scicluna, B.P.; Wiewel, M.A.; Horn, J.; Schultz, M.J.; Nurnberg, P.; Bonten, M.J.; Cremer, O.L.; et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA 2016, 315, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Dimick, J.B.; Pronovost, P.J.; Heitmiller, R.F.; Lipsett, P.A. Intensive care unit physician staffing is associated with decreased length of stay, hospital cost, and complications after esophageal resection. Crit. Care Med. 2001, 29, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.; Price, R.; Barr, K.; McAuley, S.; Allen, J.; Clinton, A.; Phillips, G.; Marwick, C.; McMurdo, M.; Witham, M. S13 Incidence and Risk Factors for the Development of Hospital Acquired Pneumonia in Older Hospitalised Patients; BMJ Publishing Group Ltd.: London, UK, 2014. [Google Scholar]
- Dorfman, R.; Khayat, Z.; Sieminowski, T.; Golden, B.; Lyons, R. Application of personalized medicine to chronic disease: A feasibility assessment. Clin. Transl. Med. 2013, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.J.; Shapiro, M.; Hays, R.D.; Afifi, A.; Vazirani, S.; Ward, C.R.; Ettner, S.L. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J. Nurs. Adm. 2006, 36, 79–85. [Google Scholar] [CrossRef]
- Chen, Y.R.; Yang, Y.; Wang, S.C.; Chou, W.Y.; Chiu, P.F.; Lin, C.Y.; Tsai, W.C.; Chang, J.M.; Chen, T.W.; Ferng, S.H.; et al. Multidisciplinary care improves clinical outcome and reduces medical costs for pre-end-stage renal disease in Taiwan. Nephrol. 2014, 19, 699–707. [Google Scholar] [CrossRef]
- Antonioli, P.; Bolognesi, N.; Valpiani, G.; Morotti, C.; Bernardini, D.; Bravi, F.; Di Ruscio, E.; Stefanati, A.; Gabutti, G. A 2-year point-prevalence surveillance of healthcare-associated infections and antimicrobial use in Ferrara University Hospital, Italy. BMC Infect. Dis. 2020, 20, 75. [Google Scholar] [CrossRef]
- Fabbian, F.; De Giorgi, A.; Boari, B.; Misurati, E.; Gallerani, M.; Cappadona, R.; Cultrera, R.; Manfredini, R.; Rodriguez Borrego, M.A.; Lopez-Soto, P.J. Infections and internal medicine patients: Could a comorbidity score predict in-hospital mortality? Medicine 2018, 97, e12818. [Google Scholar] [CrossRef]
- Lizioli, A.; Privitera, G.; Alliata, E.; Antonietta Banfi, E.M.; Boselli, L.; Panceri, M.L.; Perna, M.C.; Porretta, A.D.; Santini, M.G.; Carreri, V. Prevalence of nosocomial infections in Italy: Result from the Lombardy survey in 2000. J. Hosp. Infect. 2003, 54, 141–148. [Google Scholar] [CrossRef]
- Raoofi, S.; Pashazadeh Kan, F.; Rafiei, S.; Hosseinipalangi, Z.; Noorani Mejareh, Z.; Khani, S.; Abdollahi, B.; Seyghalani Talab, F.; Sanaei, M.; Zarabi, F.; et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef]
- De Angelis, G.; Murthy, A.; Beyersmann, J.; Harbarth, S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin. Microbiol. Infect. 2010, 16, 1729–1735. [Google Scholar] [CrossRef]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Robertson, C.; Pan, J.; Kennedy, S.; Haahr, L.; Manoukian, S.; Mason, H.; Kavanagh, K.; Graves, N.; Dancer, S.J.; et al. Impact of healthcare-associated infection on length of stay. J. Hosp. Infect. 2021, 114, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tess, B.H.; Glenister, H.M.; Rodrigues, L.C.; Wagner, M.B. Incidence of hospital-acquired infection and length of hospital stay. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.D.; Dai, C.; Srivastava, S.; Smith, C.A.; Gill, S.S. Risk factors, costs and complications of delayed hospital discharge from internal medicine wards at a Canadian academic medical centre: Retrospective cohort study. BMC Health Serv. Res. 2019, 19, 935. [Google Scholar] [CrossRef]
- Lankford, M.G.; Zembower, T.R.; Trick, W.E.; Hacek, D.M.; Noskin, G.A.; Peterson, L.R. Influence of role models and hospital design on hand hygiene of healthcare workers. Emerg. Infect. Dis. 2003, 9, 217–223. [Google Scholar] [CrossRef]
- Mauro, M.; Giancotti, M. Italian responses to the COVID-19 emergency: Overthrowing 30 years of health reforms? Health Policy 2021, 125, 548–552. [Google Scholar] [CrossRef]
- Garattini, L.; Bozzetto, M.; Remuzzi, G.; Freemantle, N.; Nobili, A. Primary care in a National Health Service: Time for radical reform. Fam. Pract. 2022, 39, 994–995. [Google Scholar] [CrossRef]
- Loughnane, C.; Murphy, A.; Mulcahy, M.; McInerney, C.; Walshe, V. Have bailouts shifted the burden of paying for healthcare from the state onto individuals? Ir. J. Med. Sci. 2019, 188, 5–12. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Trabucchi, M.; De Leo, D. Nursing homes or besieged castles: COVID-19 in northern Italy. Lancet Psychiatry 2020, 7, 387–388. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Ternero-Vega, J.E.; Diaz-Jimenez, P.; Conde-Guzman, C.; Nieto-Martin, M.D.; Moreno-Gavino, L.; Delgado-Cuesta, J.; Rincon-Gomez, M.; Gimenez-Miranda, L.; Navarro-Amuedo, M.D.; et al. Death risk stratification in elderly patients with covid-19. A comparative cohort study in nursing homes outbreaks. Arch. Gerontol. Geriatr. 2020, 91, 104240. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, E.; Rivasi, G.; Bulgaresi, M.; Barucci, R.; Lorini, C.; Balzi, D.; Faraone, A.; Fortini, G.; Vaccaro, G.; Del Lungo, I.; et al. Caring for nursing home residents with COVID-19: A “hospital-at-nursing home” intermediate care intervention. Aging Clin. Exp. Res. 2021, 33, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Eden, S.; Shintani, A.; Morandi, A.; Schnelle, J.; Dittus, R.S.; Storrow, A.B.; Ely, E.W. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad. Emerg. Med. 2011, 18, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Rayman, G.; Akpan, A.; Cowie, M.; Evans, R.; Patel, M.; Posporelis, S.; Walsh, K. Managing patients with comorbidities: Future models of care. Future Healthc. J. 2022, 9, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Carroll, C.B.; Close, J.; Gordon, A.L.; O’Brien, J.; Quinn, T.J.; Rochester, L.; Sayer, A.A.; Shenkin, S.D.; van der Velde, N.; et al. Research with older people in a world with COVID-19: Identification of current and future priorities, challenges and opportunities. Age Ageing 2020, 49, 901–906. [Google Scholar] [CrossRef]
- Ahmed, H.; Carmody, J.B. On the Looming Physician Shortage and Strategic Expansion of Graduate Medical Education. Cureus 2020, 12, e9216. [Google Scholar] [CrossRef] [PubMed]
- Zink, B.J. Learning from our history. J. Emerg. Med. 2008, 35, 1–3. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Li, S.; Kuo, Y.F. Association of the Work Schedules of Hospitalists With Patient Outcomes of Hospitalization. JAMA Intern. Med. 2020, 180, 215–222. [Google Scholar] [CrossRef]
- Querido, S.J.; de Rond, M.E.J.; Wigersma, L.; Ten Cate, O. Some residents drop out of specialty training. How important is prior clinical experience? A survey among residents in the Netherlands. GMS J. Med. Educ. 2023, 40, Doc5. [Google Scholar] [CrossRef]
- Coaccioli, S. Medicine of complexity: The modern internal medicine. Clin. Ter. 2010, 161, 9–11. [Google Scholar]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.J.; Andersen, K.G. COVID-19 testing: One size does not fit all. Science 2021, 371, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Lemiech-Mirowska, E.; Kiersnowska, Z.M.; Michalkiewicz, M.; Depta, A.; Marczak, M. Nosocomial infections as one of the most important problems of healthcare system. Ann. Agric. Environ. Med. 2021, 28, 361–366. [Google Scholar] [CrossRef] [PubMed]

| All Patients (N = 1073) | Women (n = 445) | Men (n = 628) | |
|---|---|---|---|
| Demographics and outcomes | |||
| Women: n (%) | 445 (41) | 445 (100) | 0 (0) |
| Age: median (IQR) | 74 (62–82) | 73 (61–81) | 75 (64–82) |
| In-hospital deaths: n (%) | 119 (11) | 48 (11) | 71 (11) |
| Nosocomial infections: n (%) | 136 (13) | 56 (13) | 80 (13) |
| Time to discharge (days): median (IQR) | 12 (8–20) | 12 (8–21) | 12 (8–19) |
| Morbidity | |||
| ADL/IADL dependence: n (%) | 436 (41) | 181 (41) | 255 (41) |
| Cardiovascular disorders: n (%) | 745 (69) | 300 (67) | 445 (71) |
| Cardiac disorders: n (%) | 566 (53) | 216 (49) | 350 (56) |
| Hypertension: n (%) | 583 (54) | 242 (54) | 341 (54) |
| Pulmonary disorders: n (%) | 517 (48) | 205 (46) | 312 (50) |
| Renal disorders: n (%) | 362 (34) | 134 (30) | 228 (36) |
| Upper gastrointestinal tract disorders: n (%) | 103 (10) | 41 (9) | 62 (10) |
| Lower gastrointestinal tract disorders: n (%) | 105 (10) | 51 (11) | 54 (9) |
| Liver disorders: n (%) | 170 (16) | 62 (14) | 108 (17) |
| Metabolic disorders: n (%) | 425 (40) | 187 (42) | 238 (38) |
| Immune-mediated disorders: n (%) | 180 (17) | 110 (25) | 70 (11) |
| Neoplastic disorders: n (%) | 350 (33) | 132 (30) | 218 (35) |
| End-stage neoplastic disorders: n (%) | 76 (7) | 33 (7) | 43 (7) |
| Neurological disorders: n (%) | 439 (41) | 179 (40) | 260 (41) |
| Psychiatric disorders: n (%) | 100 (9) | 51 (11) | 49 (8) |
| Immunocompromised subjects: n (%) | 226 (21) | 106 (24) | 120 (19) |
| CIRS total score: median (IQR) | 9 (6–13) | 9 (5–12) | 9 (6–13) |
| CIRS severity score: median (IQR) | 0.7 (0.4–0.9) | 0.6 (0.4–0.9) | 0.7 (0.5–1.0) |
| CIRS comorbidity score: median (IQR) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.5 (2.0–5.0) |
| Intensity of care | |||
| Continuous vital signs monitoring *: n (%) | 1045 (97) | 432 (97) | 613 (98) |
| Any oxygen support: n (%) | 589 (55) | 222 (50) | 367 (58) |
| Nasal cannulas or Venturi’s mask: n (%) | 488 (45) | 183 (41) | 305 (49) |
| Non-invasive ventilation: n (%) | 109 (10) | 42 (9) | 67 (11) |
| Intravenous treatments: n (%) | 999 (93) | 399 (90) | 600 (96) |
| Vasoactive circulation support with one drug at least once: n (%) | 17 (2) | 7 (2) | 10 (2) |
| Vasoactive circulation support with more than one drug at least once: n (%) | 0 (0) | 0 (0) | 0 (0) |
| Dialysis at least once: n (%) | 14 (1) | 3 (1) | 11 (2) |
| At least one in-ward non-standard procedure: n (%) | 177 (16) | 75 (17) | 102 (16) |
| At least one exit from the ward for other procedures: n (%) | 760 (71) | 315 (71) | 445 (71) |
| Patients requiring surgery: n (%) | 27 (3) | 14 (3) | 13 (2) |
| NEMS average score: median (IQR) | 18 (16–19) | 18 (16–19) | 18 (16–19) |
| Variable | Positive | Negative | p | ||
|---|---|---|---|---|---|
| n | Length of Stay (Days) | n | Length of Stay (Days) | ||
| ADL/IADL dependence | 230 | 15 (9–25) | 415 | 11 (8–19) | <0.001 |
| Neurological disorders | 245 | 14 (9–23) | 400 | 12 (8–20) | 0.029 |
| Any infection | 441 | 13 (9–22) | 204 | 11 (7–19) | 0.002 |
| Any nosocomial infection | 81 | 25 (18–35) | 564 | 11 (8–18) | <0.001 |
| Nosocomial infection, n = 1 | 72 | 24 (17–32) | <0.001 | ||
| Nosocomial infection, n > 1 | 9 | 62 (59–89) | <0.001 | ||
| Nosocomial infection, n = 2 | 5 | 59 (32–60) | 0.001 | ||
| Nosocomial infection, n = 3 | 2 | 68 (62–63) | 0.016 | ||
| Nosocomial infection, n = 4 | 2 | 113 (90–136) | 0.014 | ||
| Admitted from nursing homes or ICU | 62 | 26 (16–49) | 583 | 12 (8–19) | <0.001 |
| Requiring surgery | 19 | 22 (14–42) | 626 | 12 (8–21) | 0.001 |
| Any oxygen support | 334 | 14 (9–22) | 311 | 12 (7–19) | 0.003 |
| NIMV users | 55 | 16 (10–25) | 590 | 12 (8–21) | 0.016 |
| Variable | Coefficient | Standard Error | p |
|---|---|---|---|
| Individual variables | |||
| ADL/IADL dependency | 2.01 | 0.62 | 0.001 |
| Neurological disorders | 0.17 | 0.55 | 0.759 |
| Any infection | 1.89 | 0.51 | <0.001 |
| Nosocomial infection, n = 1 | 5.57 | 1.46 | <0.001 |
| Nosocomial infection, n > 1 | 25.11 | 11.65 | 0.031 |
| Admitted from nursing home or ICU | 10.30 | 1.91 | <0.001 |
| Requiring surgery | 5.22 | 2.69 | 0.053 |
| Any oxygen support | 1.85 | 0.66 | 0.005 |
| Number of on-standard in-ward procedures | 1.11 | 0.69 | 0.109 |
| Exits from the Unit | 2.67 | 0.19 | <0.001 |
| Average NEMS | −0.13 | 0.07 | 0.083 |
| Unit-related variables | |||
| Unit average length of hospitalisation | 0.09 | 0.11 | 0.405 |
| Unit average number of infected patients | 4.88 | 5.25 | 0.353 |
| Unit average NIMV users | −1.58 | 5.61 | 0.778 |
| Patient/physician ratio | 0.35 | 0.10 | <0.001 |
| Intercept | −3.58 | 4.37 | 0.411 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, G.A.; Damanti, S.; Caruso, P.F.; Mette, F.; Pagliula, G.; Cariddi, A.; Sartorelli, S.; Falbo, E.; Scotti, R.; Di Terlizzi, G.; et al. Sustainability in Internal Medicine: A Year-Long Ward-Wide Observational Study. J. Pers. Med. 2024, 14, 115. https://doi.org/10.3390/jpm14010115
Ramirez GA, Damanti S, Caruso PF, Mette F, Pagliula G, Cariddi A, Sartorelli S, Falbo E, Scotti R, Di Terlizzi G, et al. Sustainability in Internal Medicine: A Year-Long Ward-Wide Observational Study. Journal of Personalized Medicine. 2024; 14(1):115. https://doi.org/10.3390/jpm14010115
Chicago/Turabian StyleRamirez, Giuseppe A., Sarah Damanti, Pier Francesco Caruso, Francesca Mette, Gaia Pagliula, Adriana Cariddi, Silvia Sartorelli, Elisabetta Falbo, Raffaella Scotti, Gaetano Di Terlizzi, and et al. 2024. "Sustainability in Internal Medicine: A Year-Long Ward-Wide Observational Study" Journal of Personalized Medicine 14, no. 1: 115. https://doi.org/10.3390/jpm14010115
APA StyleRamirez, G. A., Damanti, S., Caruso, P. F., Mette, F., Pagliula, G., Cariddi, A., Sartorelli, S., Falbo, E., Scotti, R., Di Terlizzi, G., Dagna, L., Praderio, L., Sabbadini, M. G., Bozzolo, E. P., & Tresoldi, M. (2024). Sustainability in Internal Medicine: A Year-Long Ward-Wide Observational Study. Journal of Personalized Medicine, 14(1), 115. https://doi.org/10.3390/jpm14010115







