Cytokine Signatures for Lung Cancer Diagnosis in African American Populations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Information
2.2. Blood Collection and Plasma Preparation
2.3. The Measurement of the Cytokines in Plasma
2.4. Statistical Analysis
3. Results
3.1. High Sensitivity and Specificity in Cytokine Detection Using the FirePlex Immunoassay
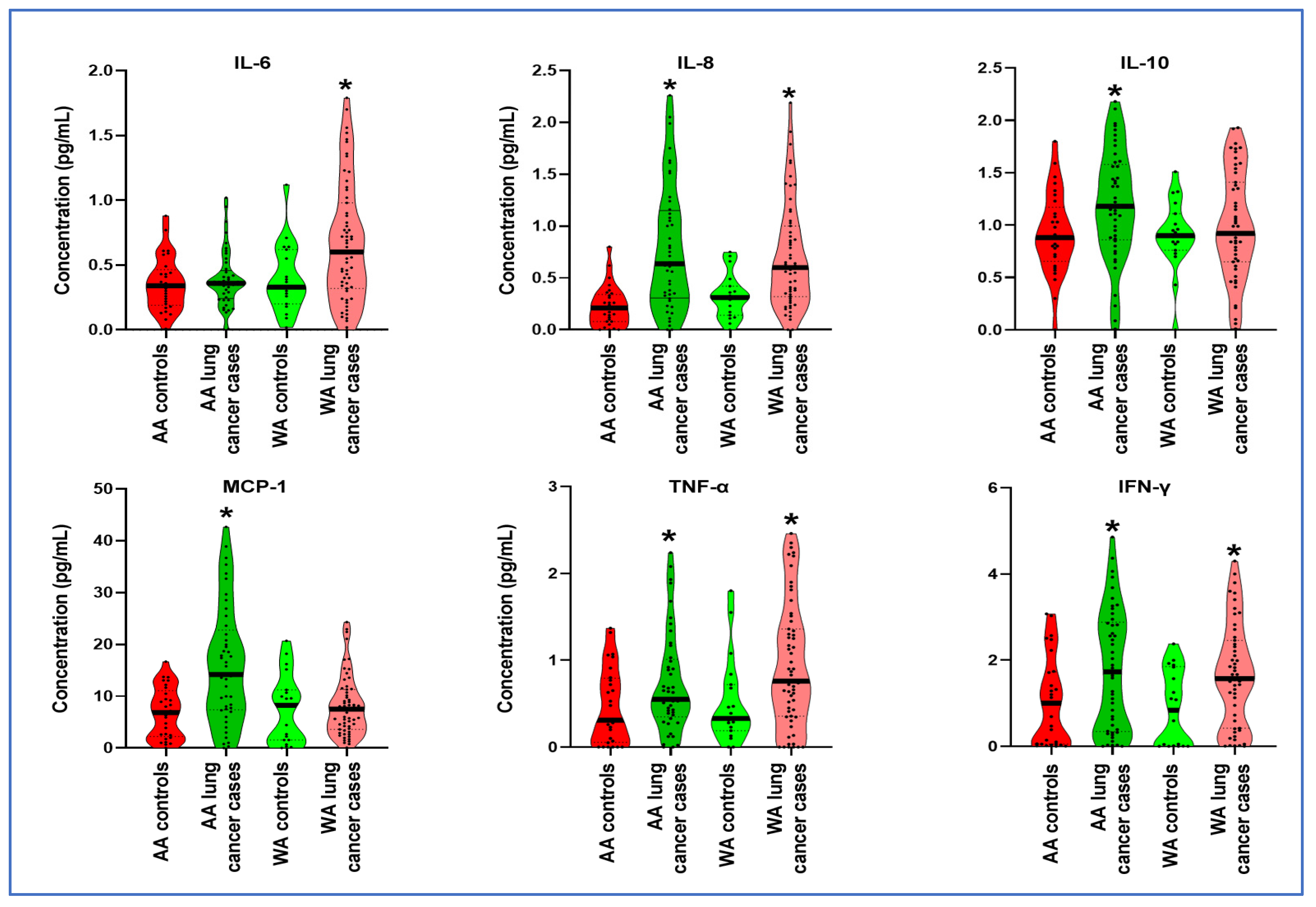
3.2. Distinct Cytokine Levels in Plasma across Various Ethnic Groups
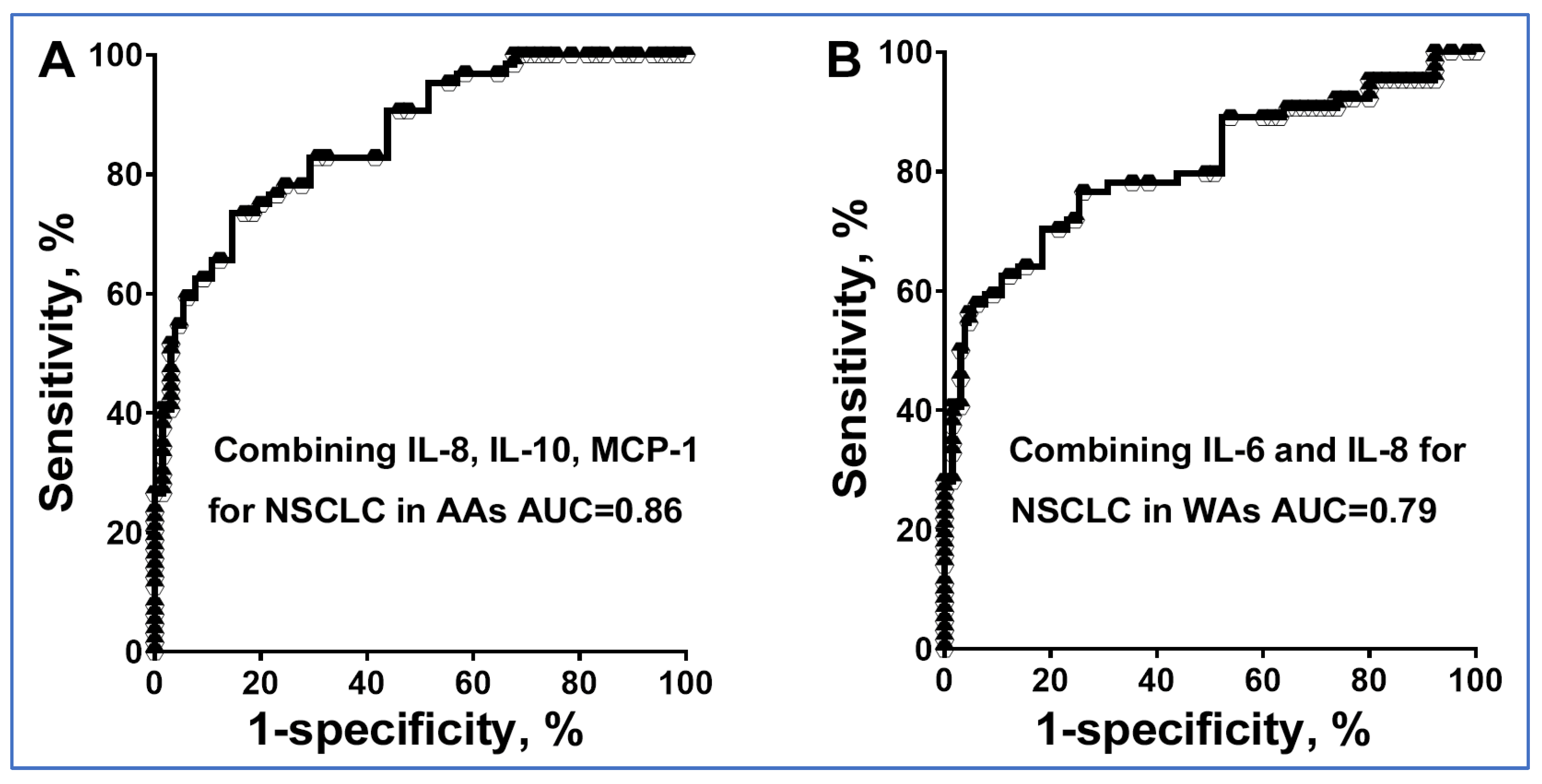
3.3. The Diagnostic Utility of Plasma Cytokine Biomarkers Varies with Ethnicity
3.4. Validating the Diagnostic Potential of Cytokine Biomarkers for Detecting Disparities in Lung Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cress, D.; Munoz-Antonia, T. Racial and Ethnic Differences in the Epidemiology and Genomics of Lung Cancer. Cancer Control 2016, 23, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M. Lung cancer health disparities. Carcinogenesis 2018, 39, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, K.A.; Wang, Z.; Aldrich, M.; Amos, C.I.; Blot, W.J.; Bowman, E.D.; Burdette, L.; Cai, Q.; Caporaso, N.; Chung, C.C.; et al. Genome-wide association study confirms lung cancer susceptibility loci on chromosomes 5p15 and 15q25 in an African-American population. Lung Cancer 2016, 98, 33–42. [Google Scholar] [CrossRef]
- Lerner, L.; Winn, R.; Hulbert, A. Lung cancer early detection and health disparities: The intersection of epigenetics and ethnicity. J. Thorac. Dis. 2018, 10, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hosgood, H.D.; Deng, L.; Ye, K.; Su, C.; Sharma, J.; Yang, Y.; Halmos, B.; Perez-Soler, R. Survival Disparities in Black Patients With EGFR-mutated Non-small-cell Lung Cancer. Clin. Lung Cancer 2020, 21, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Zingone, A.; Sinha, S.; Ante, M.; Nguyen, C.; Daujotyte, D.; Bowman, E.D.; Sinha, N.; Mitchell, K.A.; Chen, Q.; Yan, C.; et al. A comprehensive map of alternative polyadenylation in African American and European American lung cancer patients. Nat. Commun. 2021, 12, 5605. [Google Scholar] [CrossRef]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2021, 124, 315–332. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Ryan, B.M.; Pine, S.R.; Chaturvedi, A.K.; Caporaso, N.; Harris, C.C. A combined prognostic serum interleukin-8 and interleukin-6 classifier for stage 1 lung cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J. Thorac. Oncol. 2014, 9, 1494–1503. [Google Scholar] [CrossRef]
- Pine, S.R.; Mechanic, L.E.; Enewold, L.; Bowman, E.D.; Ryan, B.M.; Cote, M.L.; Wenzlaff, A.S.; Loffredo, C.A.; Olivo-Marston, S.; Chaturvedi, A.; et al. Differential Serum Cytokine Levels and Risk of Lung Cancer Between African and European Americans. Cancer Epidemiol. Biomark. Prev. 2016, 25, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Meaney, C.L.; Mitchell, K.A.; Zingone, A.; Brown, D.; Bowman, E.; Yu, Y.; Wenzlaff, A.S.; Neslund-Dudas, C.; Pine, S.R.; Cao, L.; et al. Circulating Inflammation Proteins Associated with Lung Cancer in African Americans. J. Thorac. Oncol. 2019, 14, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Leng, Q.; Zhan, M.; Jiang, F. A Plasma Long Noncoding RNA Signature for Early Detection of Lung Cancer. Transl. Oncol. 2018, 11, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Wang, Y.; Jiang, F. A Direct Plasma miRNA Assay for Early Detection and Histological Classification of Lung Cancer. Transl. Oncol. 2018, 11, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Lin, Y.; Jiang, F.; Lee, C.J.; Zhan, M.; Fang, H.; Wang, Y. A plasma miRNA signature for lung cancer early detection. Oncotarget 2017, 8, 111902–111911. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, Z.; Todd, N.W.; Zhang, H.; Liao, J.; Yu, L.; Guarnera, M.A.; Li, R.; Cai, L.; Zhan, M.; et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer 2011, 11, 374. [Google Scholar] [CrossRef]
- Shen, J.; Todd, N.W.; Zhang, H.; Yu, L.; Lingxiao, X.; Mei, Y.; Guarnera, M.; Liao, J.; Chou, A.; Lu, C.L.; et al. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab. Investig. 2011, 91, 579–587. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Liu, Y.N.; Chang, T.H.; Tsai, M.F.; Wu, S.G.; Tsai, T.H.; Chen, H.Y.; Yu, S.L.; Yang, J.C.; Shih, J.Y. IL-8 confers resistance to EGFR inhibitors by inducing stem cell properties in lung cancer. Oncotarget 2015, 6, 10415–10431. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Gong, K.; Guo, G.; Beckley, N.; Zhang, Y.; Yang, X.; Sharma, M.; Habib, A.A. Tumor necrosis factor in lung cancer: Complex roles in biology and resistance to treatment. Neoplasia 2021, 23, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, H.; Abe, Y.; Naruke, M.; Tokunaga, T.; Oshika, Y.; Kawakami, T.; Osada, H.; Nagata, J.; Kamochi, J.; Tsuchida, T.; et al. Significant correlation between interleukin 10 expression and vascularization through angiopoietin/TIE2 networks in non-small cell lung cancer. Clin. Cancer Res. 2001, 7, 1287–1292. [Google Scholar] [PubMed]
- Teng, M.W.; Darcy, P.K.; Smyth, M.J. Stable IL-10: A new therapeutic that promotes tumor immunity. Cancer Cell 2011, 20, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lan, J.; Tang, J.; Luo, N. MCP-1 targeting: Shutting off an engine for tumor development. Oncol. Lett. 2022, 23, 26. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Bai, F.; Ding, L.; Huang, Y.; Lu, C.; Chen, S.; Li, C.; Yue, X.; Liang, X.; et al. IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-kappaB. Cell Prolif. 2020, 53, e12776. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Z.; Sun, S.; Yu, Y.; Lv, D.; Cao, C.; Deng, Z. TGF-beta1, IL-6, and TNF-alpha in bronchoalveolar lavage fluid: Useful markers for lung cancer? Sci. Rep. 2014, 4, 5595. [Google Scholar] [CrossRef]
- Holdsworth, S.R.; Gan, P.Y. Cytokines: Names and Numbers You Should Care About. Clin. J. Am. Soc. Nephrol. 2015, 10, 2243–2254. [Google Scholar] [CrossRef]
| An exploratory Set | A Validation Set | ||||
|---|---|---|---|---|---|
| Cancer cases (n = 104) | Controls (n = 48) | Cancer cases (n = 58) | Controls (n = 58) | ||
| Age | 66.62 (SD 11.58) | 64.23 (SD 10.14) | Age | 65.68 (SD 10.93) | 65.43 (SD 10.27) |
| Sex | Sex | ||||
| Female | 14 | 5 | Female | 12 | 12 |
| Male | 90 | 43 | Male | 46 | 46 |
| Race | Race | ||||
| African Americans | 45 | 29 | African Americans | 28 | 28 |
| White Americans | 59 | 19 | White Americans | 30 | 30 |
| Smoking pack-years | 32.7 | 30.3 | Smoking pack-years | 36.4 | 33.9 |
| Stage | Stage | ||||
| Stage I | 28 | Stage I | 18 | ||
| Stage II | 16 | Stage II | 16 | ||
| Stage III | 26 | Stage III | 14 | ||
| Stage IV | 34 | Stage IV | 10 | ||
| Histological type | Histological type | ||||
| Adenocarcinoma | 52 | Adenocarcinoma | 32 | ||
| Squamous cell carcinoma | 52 | Squamous cell carcinoma | 26 | ||
| AA Controls | AA Cancer Patients | U Statistic | p | WA Controls | WA Cancer Patients | U Statistic | p | |
|---|---|---|---|---|---|---|---|---|
| IL-6 | 0.355 | 0.388 | 589.0 | 0.485 | 0.397 | 0.672 | 357.0 | 0.018 * |
| IL-8 | 0.228 | 0.784 | 245.5 | <0.0001 * | 0.323 | 0.709 | 268.5 | 0.008 * |
| IL-10 | 0.917 | 1.195 | 421.5 | 0.011 * | 0.906 | 0.999 | 440.0 | 0.561 |
| MCP-1 | 6.848 | 16.080 | 317.0 | 0.001 * | 7.533 | 8.087 | 510.0 | 0.560 |
| IFN-γ | 1.026 | 1.781 | 396.0 | 0.007 * | 0.8748 | 1.659 | 355.0 | 0.002 * |
| TNF-α | 0.470 | 0.739 | 0453.0 | 0.045 * | 0.518 | 0.941 | 285.0 | 0.013 * |
| Cytokines | AUC (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|---|
| IL-6 in WAs | 0.7035 (0.5846 to 0.8223) | 71.64 (59.31% to 81.99%) | 63.16 (38.36% to 83.71%) |
| IL-8 in WAs | 0.7440 (0.6297 to 0.8583) | 71.19 (57.92% to 82.24%) | 68.42 (43.45% to 87.42%) |
| IL-8 in AAs | 0.8076 (54.80% to 83.24%) | 75.86 (56.46% to 89.70%) | |
| MCP-1 in AAs | 0.7571 (0.6495 to 0.8647) | 68.89 (53.35% to 81.83%) | 62.07 (42.26% to 79.31%) |
| Combined IL-6 and IL-8 in WAs | 80.76 (0.7310 to 0.8842) | 76.27 (63.41% to 86.38%) | 73.68 (48.80% to 90.85%) |
| Combined IL-8, IL-10, and MCP-1 in AAs | 86.15 (0.8003 to 0.9228) | 75.56 (60.46% to 87.12%) | 79.31 (60.28% to 92.01%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Q.; Dhilipkannah, P.; Jiang, F. Cytokine Signatures for Lung Cancer Diagnosis in African American Populations. J. Pers. Med. 2024, 14, 117. https://doi.org/10.3390/jpm14010117
Leng Q, Dhilipkannah P, Jiang F. Cytokine Signatures for Lung Cancer Diagnosis in African American Populations. Journal of Personalized Medicine. 2024; 14(1):117. https://doi.org/10.3390/jpm14010117
Chicago/Turabian StyleLeng, Qixin, Pushpa Dhilipkannah, and Feng Jiang. 2024. "Cytokine Signatures for Lung Cancer Diagnosis in African American Populations" Journal of Personalized Medicine 14, no. 1: 117. https://doi.org/10.3390/jpm14010117
APA StyleLeng, Q., Dhilipkannah, P., & Jiang, F. (2024). Cytokine Signatures for Lung Cancer Diagnosis in African American Populations. Journal of Personalized Medicine, 14(1), 117. https://doi.org/10.3390/jpm14010117






