Pharmacogenetic Variants Associated with Fluoxetine Pharmacokinetics from a Bioequivalence Study in Healthy Subjects
Abstract
:1. Introduction
2. Methods
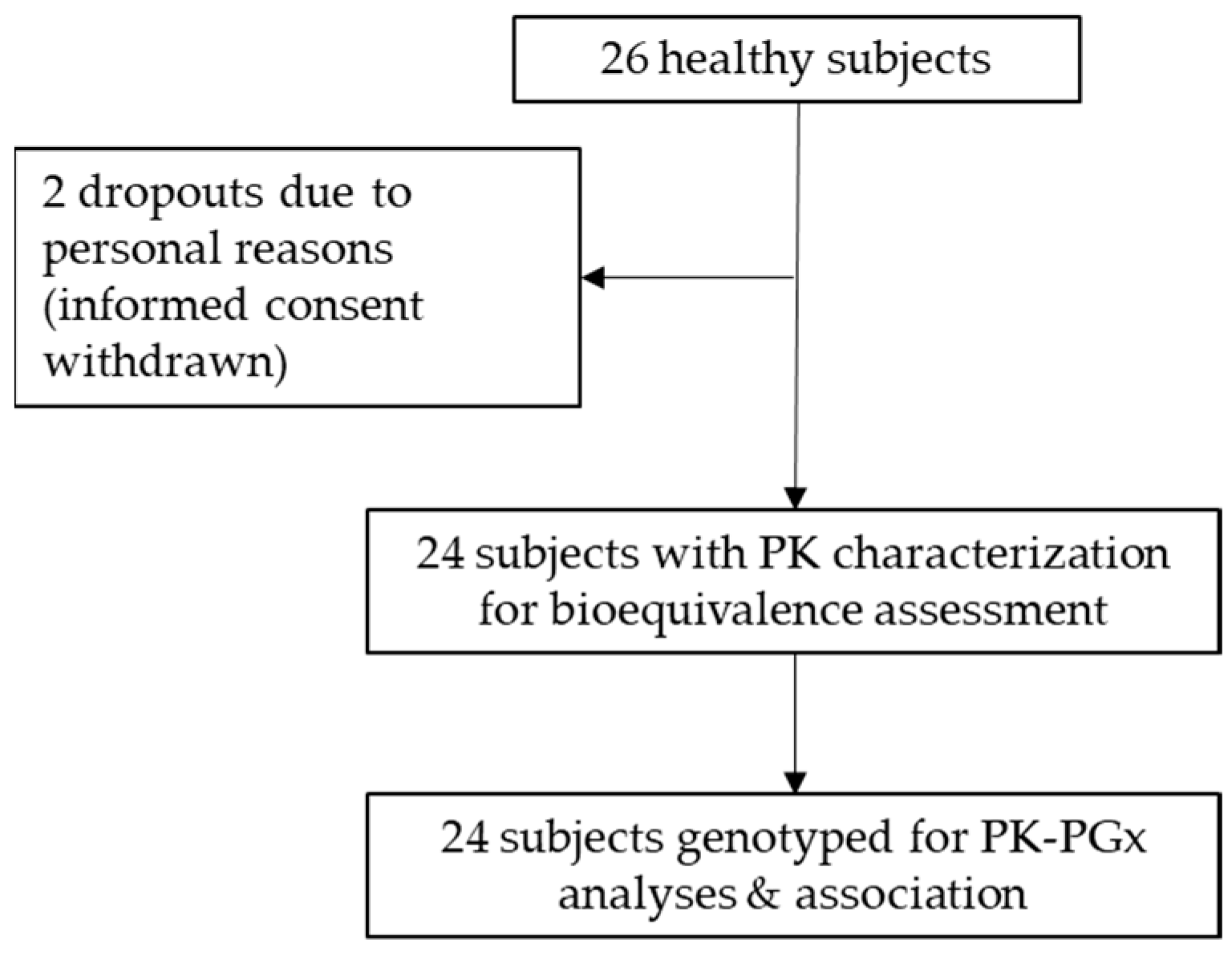
2.1. Bioequivalence Clinical Trial
2.2. Plasma Fluoxetine Determination
2.3. Genetic and Pharmacogenomic Analyses
2.4. Pharmacokinetics and Statistical Analysis
2.5. Genotyping and Genotype-Phenotype Association
3. Results
3.1. Bioequivalence Clinical Trial
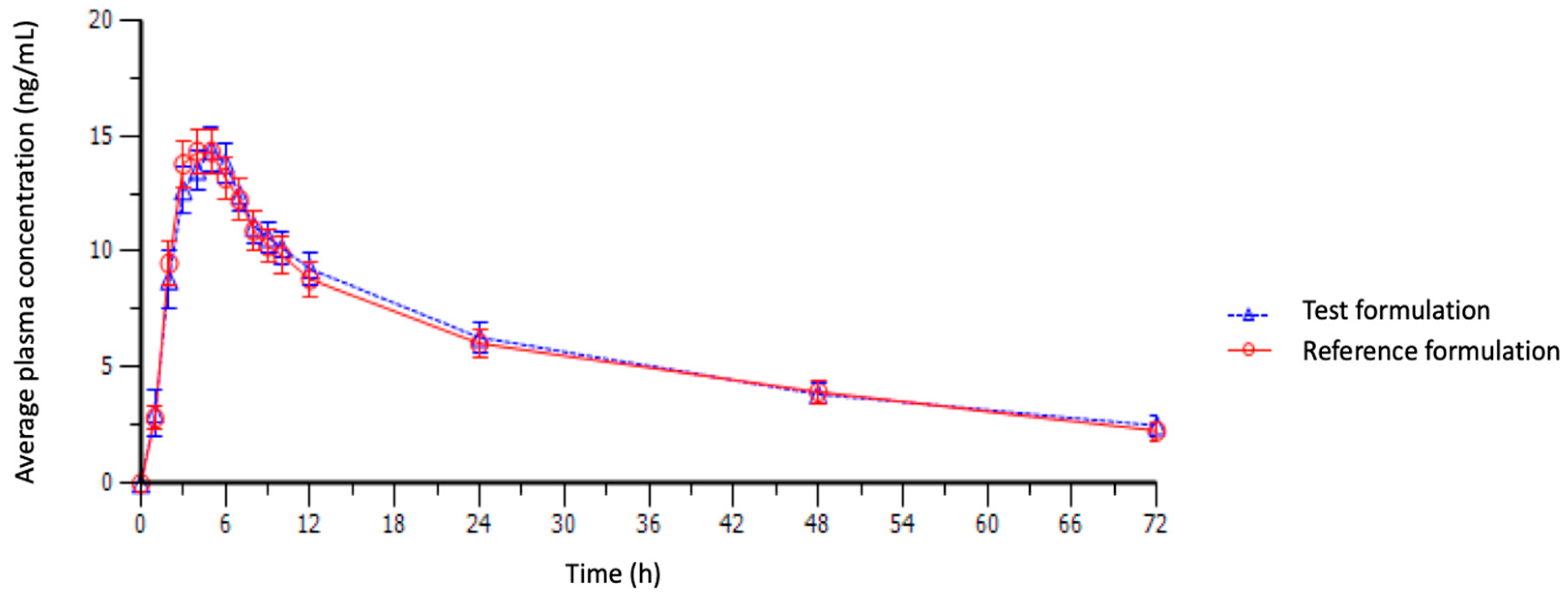
3.2. Plasma Fluoxetine Determination
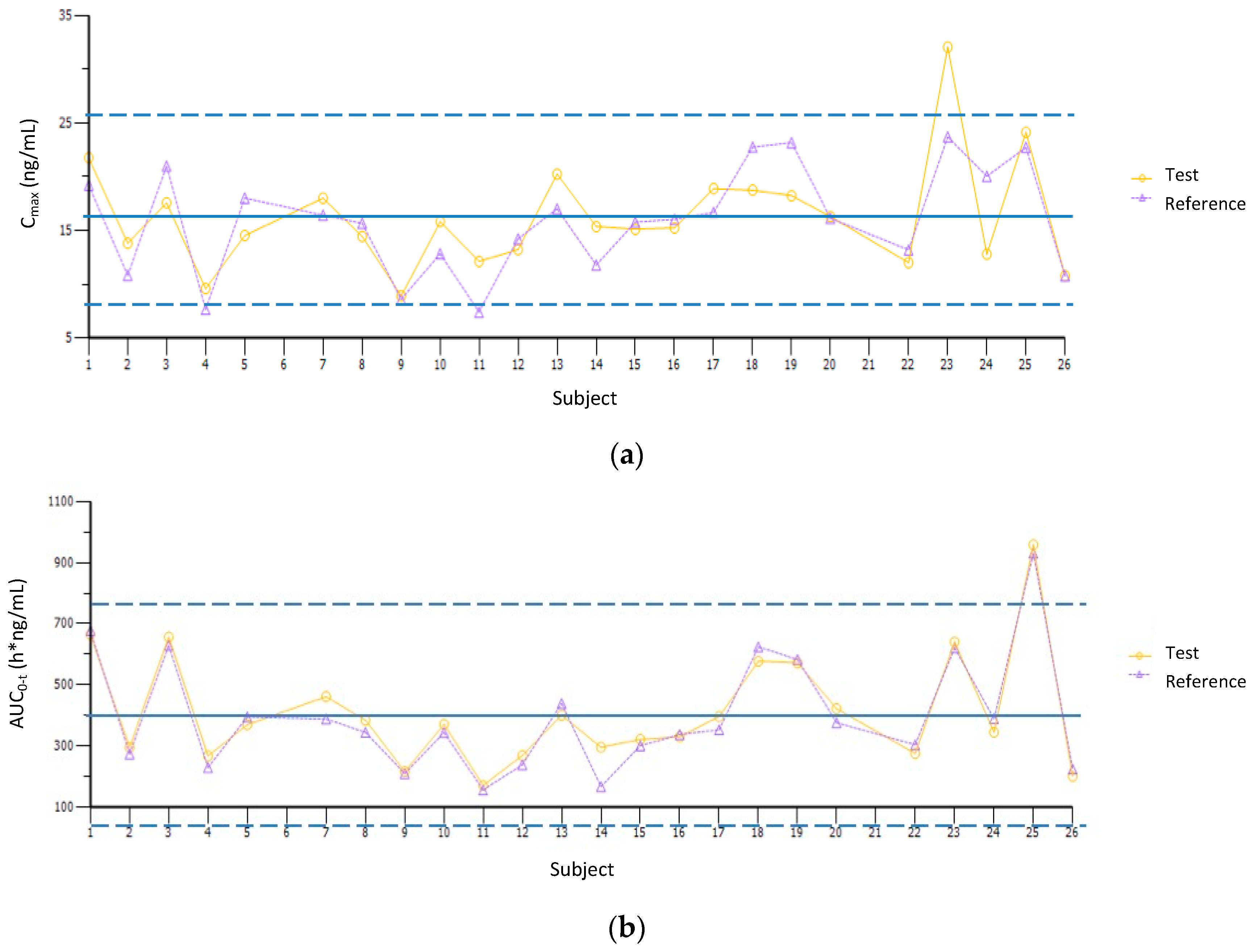
3.3. Pharmacokinetic Parameters and Bioequivalence Testing
3.4. Pharmacogenomic Analyses
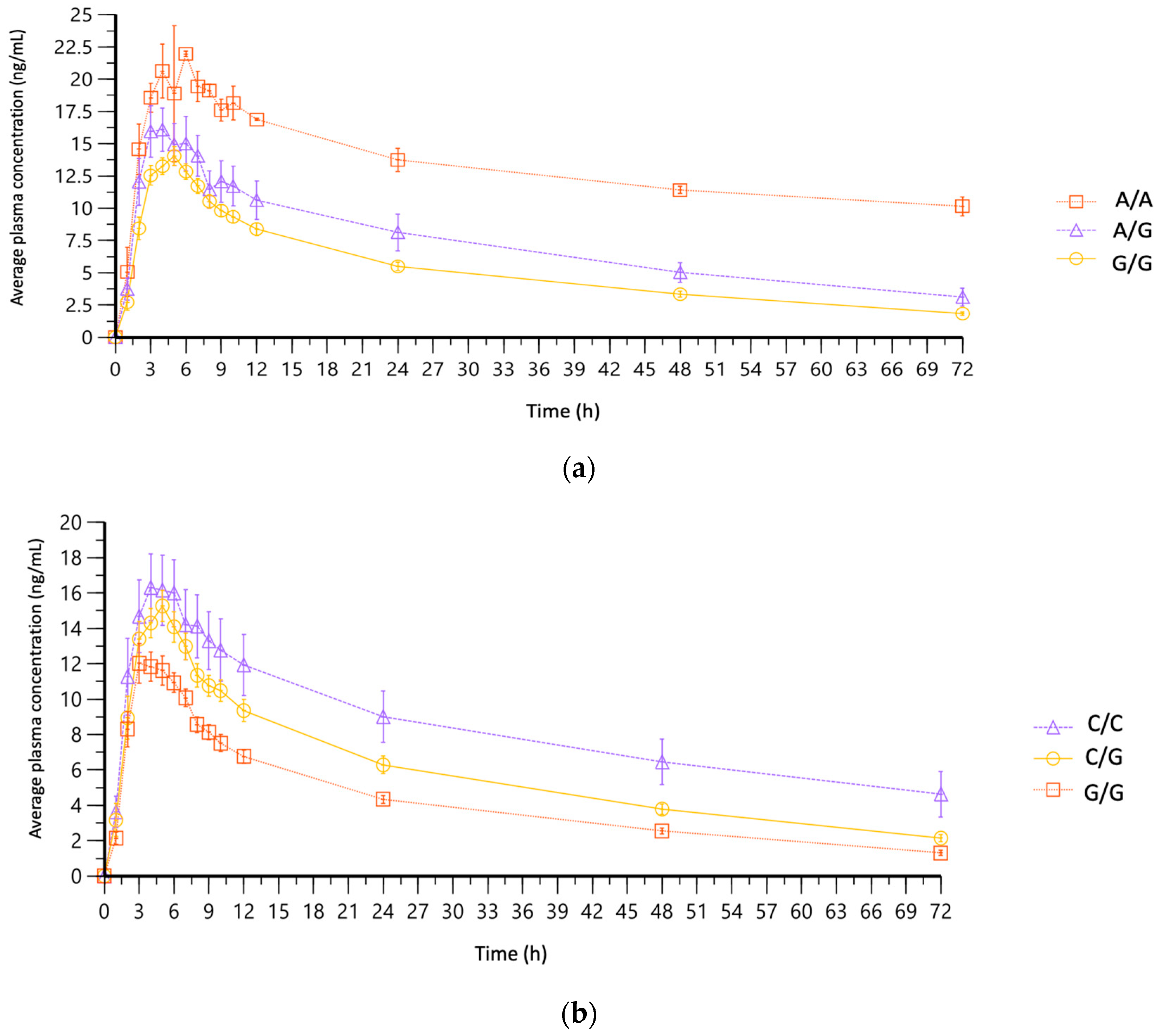
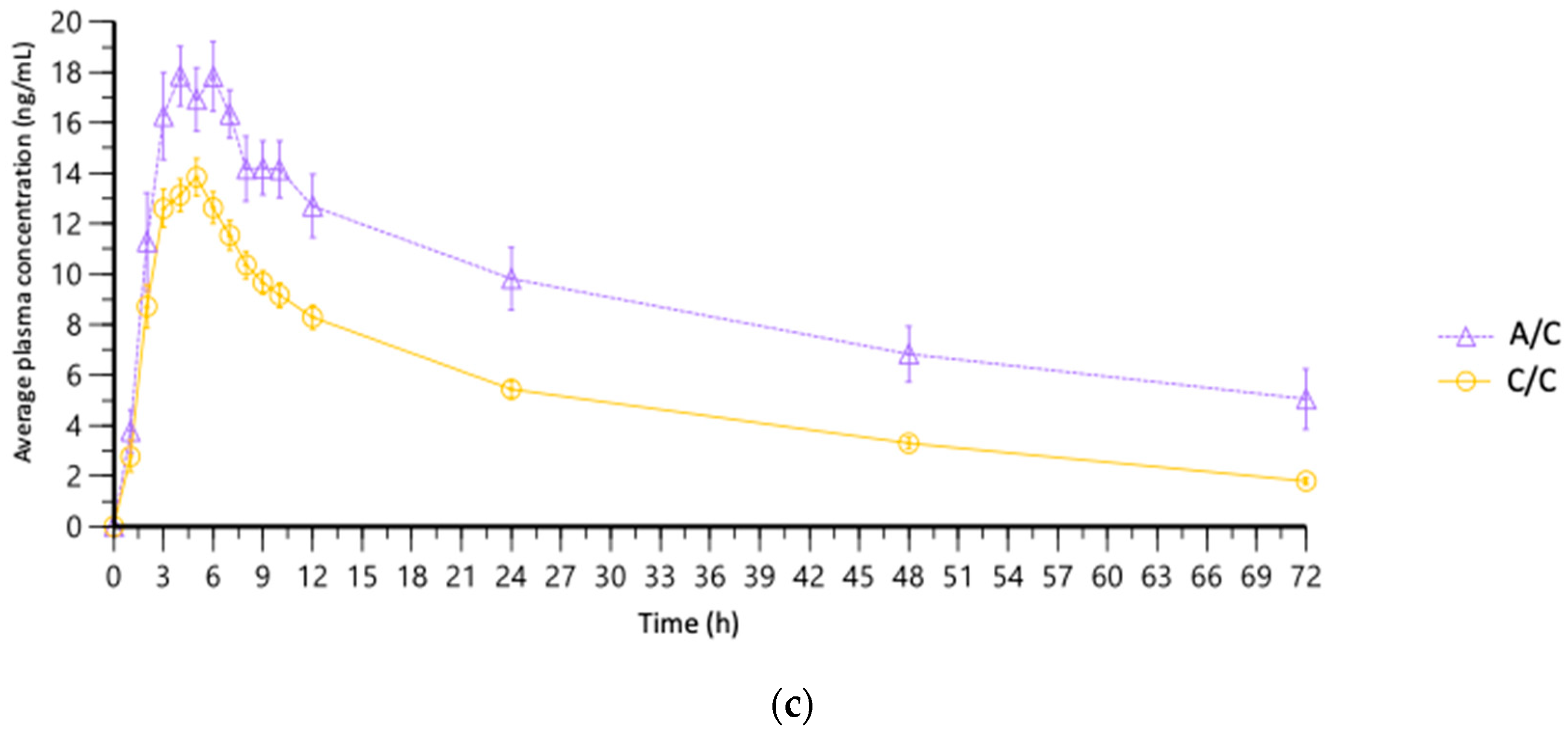
3.5. Association of PK Parameters with Pharmacogenomic Variants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 5 May 2023).
- Elsevier, B.V. Scopus. Available online: https://www.scopus.com/home.uri (accessed on 5 May 2023).
- Maruf, A.A.; Greenslade, A.; Arnold, P.D.; Bousman, C. Antidepressant Pharmacogenetics in Children and Young Adults: A Systematic Review. J. Affect. Disord. 2019, 254, 98–108. [Google Scholar] [CrossRef]
- PharmGKB Fluoxetine. Available online: https://www.pharmgkb.org/chemical/PA449673 (accessed on 5 July 2023).
- Iosifescu, D.V. Pharmacogenomic Testing for Next-Step Antidepressant Selection: Still a Work in Progress. JAMA 2022, 328, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Cicali, E.J.; Elchynski, A.L.; Cook, K.J.; Houder, J.T.; Thomas, C.D.; Smith, D.M.; Elsey, A.; Johnson, J.A.; Cavallari, L.H.; Wiisanen, K. How to Integrate CYP2D6 Phenoconversion into Clinical Pharmacogenetics: A Tutorial. Clin. Pharmacol. Ther. 2021, 110, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2D6 and CYP2C19 Genotypes and Dosing of Tricyclic Antidepressants: 2016 Update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- COFEPRIS. Norma Oficial Mexicana NOM-177-SSA1-2013; Que Establece las Pruebas y Procedimientos Para Demostrar Que un Medicamento es Intercambiable. Requisitos a Que Deben Sujetarse los Terceros Autorizados Que Realicen las Pruebas de Intercambiabilidad. Requisitos Para Realizar los Estudios de Biocomparabilidad. Requisitos a Que Deben Sujetarse los Terceros Autorizados, Centros de Investigación o Instituciones Hospitalarias Que Realicen las Pruebas de Biocomparabilidad; Diario Oficial de la Federación (DOF). Published on 20 September 2013. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5314833&fecha=20/09/2013#gsc.tab=0 (accessed on 20 May 2023).
- Clinicaltrials.gov. Studies Found for: Fluoxetine, Prozac, Reconcile. Available online: https://www.clinicaltrials.gov/ct2/results/details?term=bioequivalence&intr=Prozac (accessed on 20 August 2023).
- COFEPRIS. Consultas de Ensayos Clínicos Registrados. Available online: http://siipris03.cofepris.gob.mx/Resoluciones/Consultas/ConWebRegEnsayosClinicos.asp (accessed on 20 August 2023).
- Moraes, M.O.; Lerner, F.E.; Corso, G.; Bezerra, F.A.F.; Moraes, M.E.A.; De Nucci, G. Fluoxetine Bioequivalence Study: Quantification of Fluoxetine and Norfluoxetine by Liquid Chromatography Coupled to Mass Spectrometry. J. Clin. Pharmacol. 1999, 39, 1053–1061. [Google Scholar] [CrossRef]
- Chow, S.-C.; Liu, J.-P. Design and Analysis of Bioavailability and Bioequivalence Studies, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; 760p. [Google Scholar]
- ICH (International Council for Harmonisation). Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). Published on 9 November 2016. Available online: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed on 20 May 2023).
- ICH (International Council for Harmonisation). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guidelines. Available online: https://www.ich.org/products/guidelines.html (accessed on 15 May 2023).
- WMA. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 5 July 2023).
- Illumina. Infinium® Global Screening Array-24. Available online: https://www.illumina.com/products/by-type/microarray-kits/infinium-global-screening.html (accessed on 2 May 2023).
- Certara. Phoenix WinNonlin. Available online: https://www.certara.com/software/phoenix-winnonlin/ (accessed on 5 May 2023).
- Lee, H.Y.; Lee, Y.J. The Data Analysis Tool for Average Bioequivalence (ABE) and Bioavailability (BA) (BEAR v2.7.7). Available online: http://pkpd.kmu.edu.tw/bear/ (accessed on 15 May 2023).
- IBM. SPSS Statistics. Available online: https://www.ibm.com/products/spss-statistics (accessed on 15 May 2023).
- Fan, J.; Liu, H. Statistical Analysis of Big Data on Pharmacogenomics. Adv. Drug Deliv. Rev. 2013, 65, 987–1000. [Google Scholar] [CrossRef]
- Cruz-Correa, O.F.; León-Cachón, R.B.R.; Barrera-Saldaña, H.A.; Soberón, X. Prediction of Atorvastatin Plasmatic Concentrations in Healthy Volunteers Using Integrated Pharmacogenetics Sequencing. Pharmacogenomics 2017, 18, 121–131. [Google Scholar] [CrossRef]
- Hodgson, K.; Mufti, S.J.; Uher, R.; McGuffin, P. Genome-Wide Approaches to Antidepressant Treatment: Working towards Understanding and Predicting Response. Genome Med. 2012, 4, 52. [Google Scholar] [CrossRef]
- PharmGKB. The Pharmacogenomics Knowledgebase. Available online: https://www.pharmgkb.org/ (accessed on 15 May 2023).
- Cariaso, M.; Lennon, G. SNPedia: A Wiki Supporting Personal Genome Annotation, Interpretation and Analysis. Nucleic Acids Res. 2012, 40, D1308–D1312. [Google Scholar] [CrossRef]
- Fairley, S.; Lowy-Gallego, E.; Perry, E.; Flicek, P. The International Genome Sample Resource (IGSR) Collection of Open Human Genomic Variation Resources. Nucleic Acids Res. 2020, 48, D941–D947. [Google Scholar] [CrossRef]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A Genome-Wide Mutational Constraint Map Quantified from Variation in 76,156 Human Genomes. bioRxiv 2022, 485034. [Google Scholar]
- Phan, J.; Yin, Y.; Zhang, H.; Qiang, W.; Shektman, E. Allele Frequency Aggregator (ALFA). Available online: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (accessed on 5 May 2023).
- Hertzberg, M.; Feldman, M.; Beckham, J.; Kudler, H.; Davidson, J. Lack of Efficacy for Fluoxetine in PTSD: A Placebo Controlled Trial in Combat Veterans. Ann. Clin. Psychiatry 2000, 12, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hongkaew, Y.; Gaedigk, A.; Wilffert, B.; Ngamsamut, N.; Kittitharaphan, W.; Limsila, P.; Sukasem, C. Relationship between CYP2D6 Genotype, Activity Score and Phenotype in a Pediatric Thai Population Treated with Risperidone. Sci. Rep. 2021, 11, 4158. [Google Scholar] [CrossRef]
- Lesser, I.M.; Myers, H.F.; Lin, K.-M.; Bingham Mira, C.; Joseph, N.T.; Olmos, N.T.; Schettino, J.; Poland, R.E. Ethnic Differences in Antidepressant Response: A Prospective Multi-Site Clinical Trial. Depress. Anxiety 2010, 27, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J.; Maguen, S.; Rabkin, J.G. Ethnic Differences in Response to Fluoxetine in a Controlled Trial with Depressed HIV-Positive Patients. Psychiatr. Serv. 1998, 49, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Micromedex. Fluoxetine Hydrochloride. Truven Health Analytics LLC. 2016. Available online: https://www.micromedexsolutions.com/ (accessed on 1 May 2023).
- PLM. PROZAC®20 (IPP). Diccionario de Especialidades Farmacéuticas. 2016. Available online: https://www.medicamentosplm.com/Home/productos/prozac_20_c%C3%A1psulas/50/101/9459/14 (accessed on 1 May 2023).
- Sagahón-Azúa, J.; Medellín-Garibay, S.E.; Chávez-Castillo, C.E.; González-Salinas, C.G.; Milán-Segovia, R.D.C.; Romano-Moreno, S. Factors Associated with Fluoxetine and Norfluoxetine Plasma Concentrations and Clinical Response in Mexican Patients with Mental Disorders. Pharmacol. Res. Perspect. 2021, 9, e00864. [Google Scholar] [CrossRef]
- Alfaro, C.L.; Lam, Y.W.F.; Simpson, J.; Ereshefsky, L. CYP2D6 Inhibition by Fluoxetine, Paroxetine, Sertraline, and Venlafaxine in a Crossover Study: Intraindividual Variability and Plasma Concentration Correlations. J. Clin. Pharmacol. 2000, 40, 58–66. [Google Scholar] [CrossRef]
- Sugihara, M.; Takeuchi, S.; Sugita, M.; Higaki, K.; Kataoka, M.; Yamashita, S. Analysis of Intra- and Intersubject Variability in Oral Drug Absorption in Human Bioequivalence Studies of 113 Generic Products. Mol. Pharm. 2015, 12, 4405–4413. [Google Scholar] [CrossRef]
- Reyes-Barron, C.; Tonarelli, S.; Delozier, A.; Briones, D.F.; Su, B.B.; Rubin, L.P.; Xu, C. Pharmacogenetics of Antidepressants, A Review of Significant Genetic Variants in Different Populations. Clin Depress. 2016, 2, 1000109. [Google Scholar] [CrossRef]
- Caudle, K.E.; Sangkuhl, K.; Whirl-Carrillo, M.; Swen, J.J.; Haidar, C.E.; Klein, T.E.; Gammal, R.S.; Relling, M.V.; Scott, S.A.; Hertz, D.L.; et al. Standardizing CYP 2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2020, 13, 116–124. [Google Scholar] [CrossRef]
- Deodhar, M.; Rihani, S.B.A.; Darakjian, L.; Turgeon, J.; Michaud, V. Assessing the Mechanism of Fluoxetine-Mediated CYP2D6 Inhibition. Pharmaceutics. 2021, 13, 148. [Google Scholar] [CrossRef]
- Hesse, L.M.; Venkatakrishnan, K.; Court, M.H.; von Moltke, L.L.; Duan, S.X.; Shader, R.I.; Greenblatt, D.J. CYP2B6 Mediates the in vitro Hydroxylation of Bupropion: Potential Drug Interactions with Other Antidepressants. Drug Metab. Dispos. 2000, 28, 1176–1183. [Google Scholar]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruaño, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef]
- Niitsu, T.; Fabbri, C.; Bentini, F.; Serretti, A. Pharmacogenetics in Major Depression: A Comprehensive Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 183–194. [Google Scholar] [CrossRef]
- Abbas, B.; Sabri, N.A.; El-Khouly, A.A. Association between Food/UGT2B7 Polymorphisms and Pharmacokinetics/Pharmacodynamics Properties of Indapamide in Healthy Humans. Biomedicines 2023, 11, 1501. [Google Scholar] [CrossRef]
| Test Product | Reference Product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax | AUC0–t | AUC0–∞ | tmax | t1/2 | Cmax | AUC0–t | AUC0–∞ | tmax | t1/2 | |
| Mean | 16.25 | 411.64 | 556.06 | 4.42 | 30.71 | 15.92 | 398.19 | 542.43 | 4.25 | 31.32 |
| Geometric mean | 15.59 | 377.86 | 473.26 | 4.25 | 28.90 | 15.12 | 360.06 | 457.47 | 4.07 | 29.02 |
| Standard deviation (S.D.) | 5.02 | 184.05 | 429.46 | 1.18 | 13.78 | 4.89 | 189.55 | 436.65 | 1.19 | 17.21 |
| Standard error of the mean (SEM) | 1.02 | 37.57 | 87.66 | 0.24 | 2.81 | 1.00 | 38.69 | 89.13 | 0.24 | 3.51 |
| Minimum | 8.92 | 171.86 | 200.73 | 2.00 | 15.42 | 7.42 | 157.33 | 171.14 | 2.00 | 19.24 |
| Median | 15.31 | 371.42 | 452.21 | 5.00 | 27.04 | 16.10 | 349.68 | 442.86 | 4.50 | 27.60 |
| Maximum | 32.05 | 957.96 | 2351.79 | 6.00 | 88.86 | 23.71 | 930.62 | 2383.39 | 6.00 | 106.88 |
| Coefficient of variation (%) | 30.90 | 44.70 | 77.20 | 26.60 | 44.90 | 30.70 | 47.60 | 80.50 | 28.00 | 55.00 |
| Parameter | Geometric Mean | T/R Ratio (%) | 90% Confidence Interval | Intrasubject Variability (%) | Intersubject Variability (%) | Power (%) | |
|---|---|---|---|---|---|---|---|
| Test (T) Product | Reference (R) Product | ||||||
| Cmax | 15.59 | 15.12 | 103.10 | 96.05–110.67 | 14.4% | 29.27% | 100.0% |
| AUC0–t | 377.85 | 360.05 | 104.94 | 99.87–110.28 | 10.0% | 44.97% | 100.0% |
| AUC0–∞ | 473.25 | 457.47 | 103.45 | 100.23–106.77 | 6.4% | 57.12% | 100.0% |
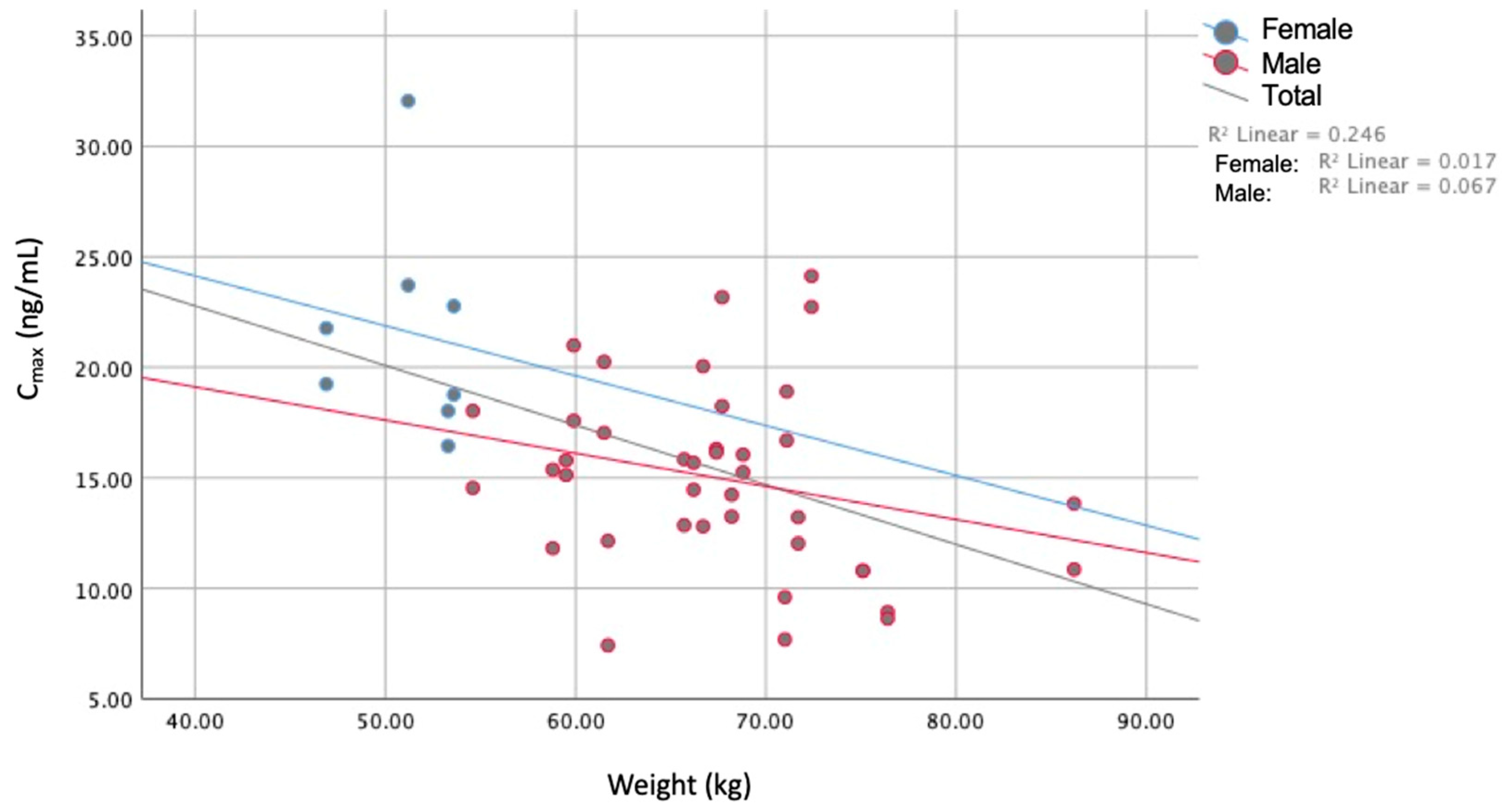
| BMI | Age | Weight | Height | Cmax | AUC0–t | AUC0–∞ | tmax | t1/2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI | Pearson Correlation | 1 | 0.481 * | 0.665 ** | 0.023 | −0.213 | −0.179 | −0.020 | 0.103 | 0.133 |
| p | 0.017 | 0.000 | 0.913 | 0.146 | 0.223 | 0.891 | 0.484 | 0.368 | ||
| Age | Pearson Correlation | 1 | 0.323 * | −0.014 | −0.344 * | −0.250 | −0.209 | 0.252 | 0.015 | |
| p | 0.123 | 0.950 | 0.017 | 0.087 | 0.153 | 0.084 | 0.921 | |||
| Weight | Pearson Correlation | 1 | 0.758 ** | −0.496 ** | −0.359 * | −0.113 | 0.003 | 0.129 | ||
| p | 0.000 | 0.000 | 0.012 | 0.446 | 0.981 | 0.380 | ||||
| Height | Pearson Correlation | 1 | −0.483 ** | −0.340 * | −0.141 | −0.078 | 0.056 | |||
| p | 0.001 | 0.018 | 0.339 | 0.598 | 0.705 | |||||
| Cmax | Pearson Correlation | 1 | 0.825 ** | 0.609 ** | −0.158 | 0.301 * | ||||
| p | 0.000 | 0.000 | 0.285 | 0.037 | ||||||
| AUC0–t | Pearson Correlation | 1 | 0.895 ** | 0.069 | 0.676 ** | |||||
| p | 0.000 | 0.640 | 0.000 | |||||||
| AUC0–∞ | Pearson Correlation | 1 | 0.086 | 0.902 ** | ||||||
| p | 0.559 | 0.000 | ||||||||
| tmax | Pearson Correlation | 1 | 0.158 | |||||||
| p | 0.282 | |||||||||
| t1/2 | Pearson Correlation | 1 | ||||||||
| p | ||||||||||
| Gene | SNV | Associated Haplotype | Genetic Variation | Annotated Variants | Clinical Annotations | Evidence Level (CPIC) | Classification | Related Drug | Function |
|---|---|---|---|---|---|---|---|---|---|
| CYP1A2 | rs2069514 | * 1C | 3860G>A | 17 | 2 | 3 | N/A | Antipsychotic | Efficacy |
| CYP1A2 | rs2069526 | * 1K | 739T>G | 7 | 1 | 3 | Intronic | Escitalopram | Toxicity |
| CYP2B6 | rs4802101 | 750T>C | 3 | 1 | 3 | 5′ end | Cyclophosphamide | Toxicity | |
| CYP2C19 | rs11188072 | 8 | 2 | 3 | N/A | Escitalopram | Dose | ||
| CYP2C8 | rs11572080 | * 3 | 58 | 14 | 3 | Missense | Rosiglitazone | ||
| CYP2C9 | rs28371686 | * 5 | 42619C>G | 23 | 5 | 1A | Missense | Phenytoin | Metabolism |
| CYP2D6 | rs1080985 | * 2A | 1496C>G | 3 | 2 | 3 | 3′ end | Thioridazine | Efficacy |
| CYP2D6 | rs1135840 | * 4, * 10 | 4180G>C | 828 | 82 | 3 | Missense | Fluoxetine | |
| CYP2D6 | rs16947 | * 21 | 2851C>T | 643 | 77 | 3 | Missense | Fluoxetine | |
| CYP2D6 | rs72549358 | * 28 | 2 | 1 | 4 | Intronic | Tamoxifen | ||
| CYP3A4 | rs2687116 | C>A | 2 | 0 | N/A | Intronic | -- | ||
| ABCB1 | rs1045642 | G>A | 580 | 100 | 3 | Missense | SSRI | Efficacy | |
| ABCB1 | rs1128503 | 1236T>C | 216 | 38 | 3 | Syno-nym | Methadone | Metabolism/PK | |
| ABCB1 | rs2032582 | 2677T>G/A | 315 | 49 | 3 | Missense | Fluoxetine | Efficacy | |
| SLC6A4 | rs1042173 | A>C | 2 | 2 | 3 | 3′ UTR | Ondansetron | Efficacy | |
| SLC6A4 | rs2066713 | G>A | 1 | 1 | 3 | Intronic | Ethanol | Toxicity | |
| SLC6A4 | rs25532 | 12 | 3 | N/A | |||||
| TPH1 | rs1799913 | G>T | 1 | 0 | N/A | Intronic | Lithium | Efficacy |
| Gene | rs | Allele | ALL | AFR | EAS | SAS | EUR | AMR | MXN |
|---|---|---|---|---|---|---|---|---|---|
| CYP1A2 | rs2069526 | G | 0.071 | 0.123 | 0.084 | 0.069 | 0.024 | 0.025 | 0.063 |
| CYP1A2 | rs2069514 | A | 0.209 | 0.313 | 0.281 | 0.080 | 0.020 | 0.362 | 0.413 |
| CYP2B6 | rs4802101 | T | 0.246 | 0.057 | 0.340 | 0.252 | 0.414 | 0.221 | 0.083 |
| CYP2C19 | rs11188072 | T | 0.156 | 0.246 | 0.015 | 0.136 | 0.224 | 0.120 | 0.083 |
| CYP2C8 | rs11572080 | T | 0.046 | 0.008 | 0.001 | 0.030 | 0.118 | 0.091 | 0.065 |
| CYP2C9 | rs28371686 | G | 0.005 | 0.017 | 0.000 | 0.000 | 0.000 | 0.001 | 0.021 |
| CYP2D6 | rs72549358 | T | 0.001 | 0.000 | 0.000 | 0.000 | 0.005 | 0.001 | 0.021 |
| CYP2D6 | rs1080985° | C | 0.162 | 0.051 | 0.103 | 0.240 | 0.241 | 0.239 | 0.292 |
| CYP2D6 | rs16947° | A | 0.368 | 0.568 | 0.135 | 0.357 | 0.338 | 0.332 | 0.326 |
| CYP2D6 | rs1135840° | C | 0.404 | 0.322 | 0.292 | 0.474 | 0.460 | 0.530 | 0.438 |
| CYP3A4 | rs2687116 | C | 0.220 | 0.721 | 0.005 | 0.045 | 0.030 | 0.102 | 0.083 |
| ABCB1 | rs1045642 | A | 0.395 | 0.150 | 0.398 | 0.575 | 0.518 | 0.428 | 0.438 |
| ABCB1 | rs1128503 | A | 0.416 | 0.136 | 0.627 | 0.587 | 0.416 | 0.403 | 0.458 |
| ABCB1 | rs2032582 | T | 0.049 | 0.001 | 0.134 | 0.050 | 0.018 | 0.059 | 0.479 |
| SLC6A4 | rs2066713 | A | 0.257 | 0.254 | 0.066 | 0.289 | 0.381 | 0.314 | 0.292 |
| SLC6A4 | rs1042173 | C | 0.485 | 0.185 | 0.822 | 0.552 | 0.437 | 0.542 | 0.375 |
| TPH1 | rs1799913 | T | 0.321 | 0.163 | 0.475 | 0.269 | 0.391 | 0.372 | 0.354 |
| Sample size | 5008 | 1322 | 1008 | 978 | 1006 | 694 | 24 | ||
| PK Parameter | Gene | Chromosome | SNV | Minor Allele | Coefficient (ADD) | p-Value |
|---|---|---|---|---|---|---|
| Cmax | ABCB1 | 7 | rs2032582 | C | −2.27 | 0.033 |
| ABCB1 | 7 | rs1128503 | G | −2.11 | 0.042 | |
| CYP2B6 | 19 | rs4802101 | A | 5.38 | 0.009 | |
| CYP2B6 | 19 | rs4803418 | G | −2.90 | 0.010 | |
| CYP2B6 | 19 | rs4803419 | A | −2.90 | 0.010 | |
| CYP2B6 | 19 | rs2279344 | G | 4.94 | 0.005 | |
| CYP2D6 | 22 | rs28371703 | A | 4.87 | 0.016 | |
| AUC0–t | CYP2B6 | 19 | rs4802101 | A | 219.30 | 0.022 |
| CYP2B6 | 19 | rs4803418 | G | −156.30 | 0.002 | |
| CYP2B6 | 19 | rs4803419 | A | −156.30 | 0.002 | |
| CYP2B6 | 19 | rs2279344 | G | 180.50 | 0.033 | |
| CYP2D6 | 22 | rs1135840 | C | 142.00 | 0.005 | |
| CYP2D6 | 22 | rs28371703 | A | 291.80 | 0.001 | |
| CYP2D6 | 22 | rs1065852 | A | 249.80 | 0.001 | |
| AUC0–∞ | CYP2B6 | 19 | rs4802101 | A | 568.30 | 0.020 |
| CYP2B6 | 19 | rs4803418 | G | −336.90 | 0.010 | |
| CYP2B6 | 19 | rs4803419 | A | −336.90 | 0.010 | |
| CYP2B6 | 19 | rs2279344 | G | 463.50 | 0.032 | |
| CYP2D6 | 22 | rs1135840 | C | 301.80 | 0.023 | |
| CYP2D6 | 22 | rs28371703 | A | 733.80 | 0.001 | |
| CYP2D6 | 22 | rs1065852 | A | 783.60 | 0.000 | |
| tmax | CYP2C9 | 10 | rs2256871 | G | −1.99 | 0.047 |
| t1/2 | SLC6A4 | 17 | rs1042173 | A | 9.89 | 0.049 |
| CYP2B6 | 19 | rs4802101 | A | 18.43 | 0.045 | |
| CYP2B6 | 19 | rs2279344 | G | 16.82 | 0.035 | |
| CYP2D6 | 22 | rs28371703 | A | 23.54 | 0.006 | |
| CYP2D6 | 22 | rs1065852 | A | 28.64 | 0.000 | |
| Cl | ABCB1 | 7 | rs2032582 | C | 14.20 | 0.018 |
| ABCB1 | 7 | rs1128503 | G | 11.77 | 0.048 | |
| CYP2B6 | 19 | rs4802101 | A | −24.28 | 0.047 | |
| CYP2B6 | 19 | rs4803418 | G | 14.00 | 0.035 | |
| CYP2B6 | 19 | rs4803419 | A | 14.00 | 0.035 | |
| CYP2B6 | 19 | rs2279344 | G | −22.78 | 0.031 | |
| CYP2D6 | 22 | rs28371703 | A | −26.79 | 0.022 | |
| CYP2D6 | 22 | rs1065852 | A | −21.36 | 0.043 |
| Parameter | Geometric Mean | T/R Ratio (%) | 90% Confidence Interval | Intrasubject Variability (%) | Intersubject Variability (%) | Power (%) | |
|---|---|---|---|---|---|---|---|
| Test (T) Product | Reference (R) Product | ||||||
| Cmax | 15.32 | 14.83 | 103.28 | 95.89–111.23 | 14.7% | 28.37% | 100.0% |
| AUC0–t | 363.16 | 346.11 | 104.93 | 99.61–110.53 | 10.3% | 40.75% | 100.0% |
| AUC0–∞ | 441.64 | 426.27 | 103.61 | 100.24–107.09 | 6.5% | 43.46% | 100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Tufinio, C.A.; Palma-Aguirre, J.A.; Gonzalez-Covarrubias, V. Pharmacogenetic Variants Associated with Fluoxetine Pharmacokinetics from a Bioequivalence Study in Healthy Subjects. J. Pers. Med. 2023, 13, 1352. https://doi.org/10.3390/jpm13091352
Díaz-Tufinio CA, Palma-Aguirre JA, Gonzalez-Covarrubias V. Pharmacogenetic Variants Associated with Fluoxetine Pharmacokinetics from a Bioequivalence Study in Healthy Subjects. Journal of Personalized Medicine. 2023; 13(9):1352. https://doi.org/10.3390/jpm13091352
Chicago/Turabian StyleDíaz-Tufinio, Carlos Alejandro, José Antonio Palma-Aguirre, and Vanessa Gonzalez-Covarrubias. 2023. "Pharmacogenetic Variants Associated with Fluoxetine Pharmacokinetics from a Bioequivalence Study in Healthy Subjects" Journal of Personalized Medicine 13, no. 9: 1352. https://doi.org/10.3390/jpm13091352
APA StyleDíaz-Tufinio, C. A., Palma-Aguirre, J. A., & Gonzalez-Covarrubias, V. (2023). Pharmacogenetic Variants Associated with Fluoxetine Pharmacokinetics from a Bioequivalence Study in Healthy Subjects. Journal of Personalized Medicine, 13(9), 1352. https://doi.org/10.3390/jpm13091352






