Efficacy of Combined Photobiomodulation Therapy with Supplements versus Supplements alone in Restoring Thyroid Gland Homeostasis in Hashimoto Thyroiditis: A Clinical Feasibility Parallel Trial with 6-Months Follow-Up
Abstract
1. Introduction
1.1. Hashimoto Thyroiditis Immunopathogenesis
1.2. Current Treatment Modalities of Hashimoto Thyroiditis
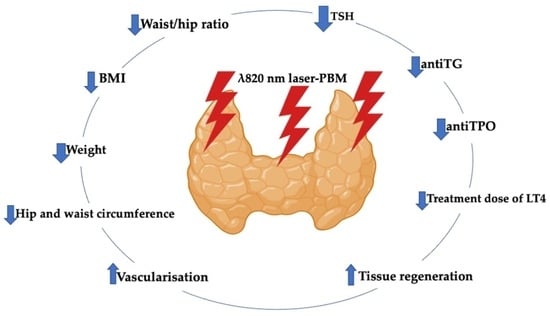
1.3. Photobiomodulation-Induced Antioxidant Effect in Restoring Homeostasis of Thyroid Gland
1.3.1. PBM Improves Thyroid Tissue and Functionality in HT
1.3.2. PBM Immunomodulates Inflammatory Cytokines Induced by HT
1.4. Rationale in Conducting This Study
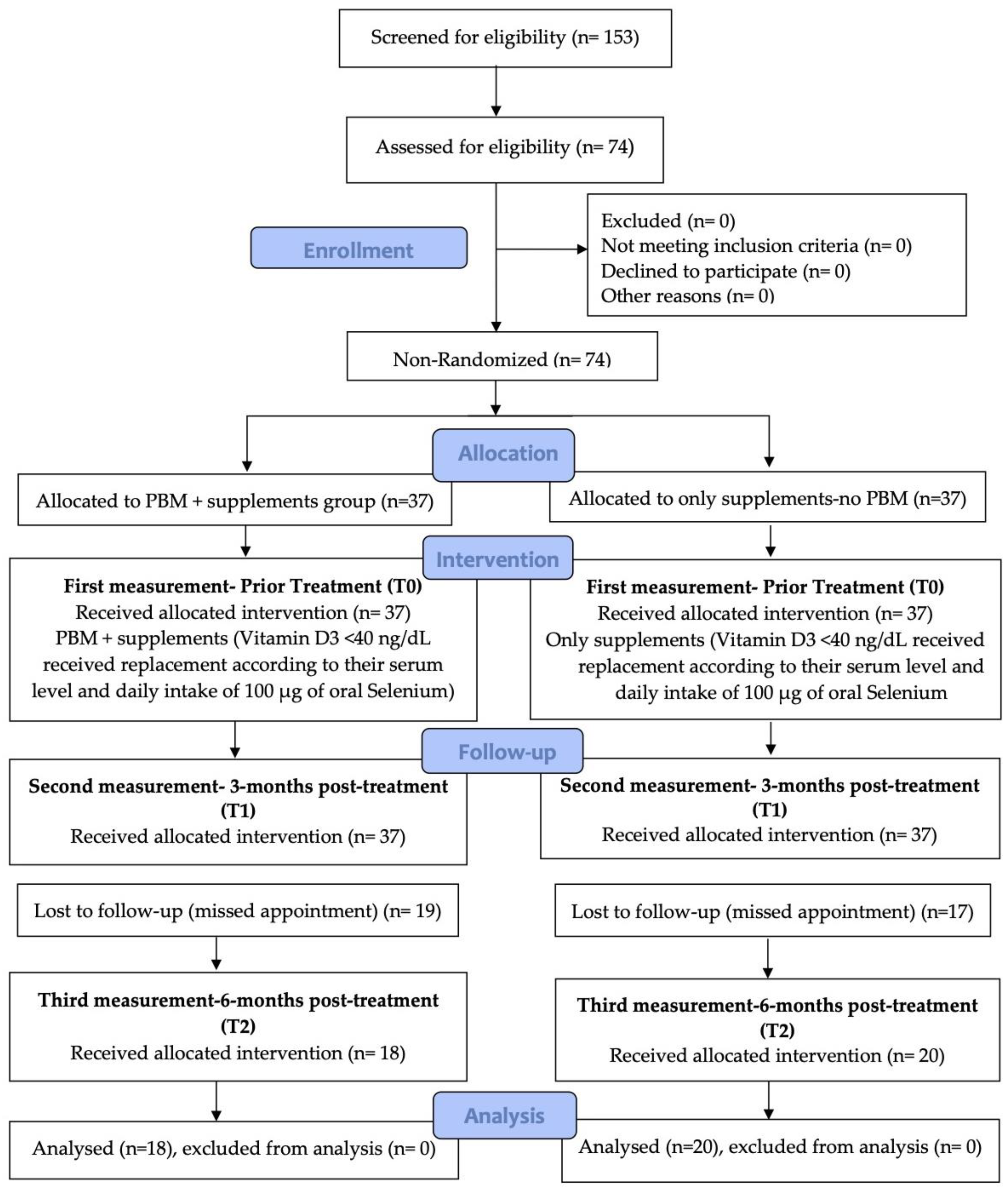
2. Materials and Methods
2.1. Study Design
2.1.1. Population (P), Intervention (I), Comparison (C) and Outcome (O)—PICO
2.1.2. Eligibility Criteria
Inclusion Criteria
Exclusion Criteria
- Patients with any known autoimmune diseases except HT or any other treatment except LT4.
- Adult female aged <20 and >50 years old.
- Adult male of any age group.
- Patients previously treated with radioiodine.
- Patients taking immunosuppressants, immunostimulants and any drug that could interfere with the production, transport and metabolism of thyroid hormones (e.g., corticosteroids, lithium, amiodarone).
- Subjects with thyroid nodules or ectopic thyroid or thyroid hypoplasia.
- Hypothyroidism stemming from postpartum thyroiditis (up to 18 months after gestation).
- A history of Graves’ disease.
- Tracheal stenosis.
- Pregnant women.
- Subjects with a history of exposure to ionizing irradiation and/or neoplasia in the cervical region.
- Patients with previous thyroid surgery.
- Patients with a serious illness (e.g., kidney and liver failure, cancer, stroke).
2.1.3. Patient Cohort
2.1.4. Treatment Protocols
Ultrasound
PBM Protocol
2.2. Outcomes Measures
2.2.1. Primary Outcomes Measurement
2.2.2. Secondary Outcomes Measurement
2.3. Assessment Tools Utilized to Evaluate Outcome Variables
2.3.1. Biochemical Measurement
2.3.2. Lifestyle Factors
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajjan, R.A.; Weetman, A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in Our Understanding. Horm. Metab. Res. 2015, 47, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, K.L.; Mooij, C.F.; van Trotsenburg, A.S.P. Persisting symptoms in patients with Hashimoto’s disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review. J. Transl. Autoimmun. 2021, 4, 100101. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Fountoulakis, S.; Tsatsoulis, A. On the pathogenesis of autoimmune thyroid disease: A unifying hypothesis. Clin. Endocrinol. 2004, 60, 397–409. [Google Scholar] [CrossRef]
- Weetman, A.P. Autoimmune thyroid disease. Autoimmunity 2004, 37, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Fu, D.G. Autoimmune Thyroid Disease: Mechanism, Genetics and Current Knowledge. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3611–3618. [Google Scholar] [PubMed]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-Analysis of the Association between Vitamin D and Autoimmune Thyroid Disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Wang, S.; Liu, J.; Zhao, N.; Huang, M.; Qin, J.; Li, Y.; Shan, Z.; Teng, W. Elevated MicroRNA-326 Levels Regulate the IL-23/IL-23R/Th17 Cell Axis in Hashimoto’s Thyroiditis by Targeting a Disintegrin and Metalloprotease 17. Thyroid 2020, 30, 1327–1337. [Google Scholar] [CrossRef]
- Li, G.; He, L.; Huang, J.; Liu, J.; Chen, W.; Zhong, J.; Wei, T.; Li, Z.; Zhu, J.; Lei, J. miR-142-3p encapsulated in T lymphocyte-derived tissue small extracellular vesicles induces Treg function defect and thyrocyte destruction in Hashimoto’s thyroiditis. BMC Med. 2023, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Zosin, I.; Balaş, M. Clinical, ultrasonographical and histopathological aspects in Hashimoto’s thyroiditis associated with malignant and benign thyroid nodules. Endokrynol. Pol. 2013, 64, 255–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and Thyroid Disorders: A Systematic Review and Meta-Analysis of Observational Studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef]
- Cvek, M.; Kalicanin, D.; Baric, A.; Vuletic, M.; Gunjaca, I.; Torlak Lovric, V.; Škrabic, V.; Punda, A.; Boraska Perica, V. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, P.; Materazi, G.; Rossi, L. Levothyroxine Therapy in Thyrodectomized Patients. Front. Endocrinol. 2021, 11, 626268. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, D.; Vainer, I.; Amir, I.; Robenshtok, E.; Hirsch, D.; Watt, T.; Hilly, O.; Shkedy, Y.; Shpitzer, T.; Bachar, G.; et al. Quality of life following lobectomy versus total thyroidectomy is significantly related to hypothyroidism. J. Surg. Oncol. 2022, 126, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Piticchio, T.; Frasca, F.; Malandrino, P.; Trimboli, P.; Carrubba, N.; Tumminia, A.; Vinciguerra, F.; Frittitta, L. Effect of gluten-free diet on autoimmune thyroiditis progression in patients with no symptoms or histology of celiac disease: A meta-analysis. Front. Endocrinol. 2023, 14, 1200372. [Google Scholar] [CrossRef]
- Štefanic, M.; Tokic, S. Serum 25 Hydoxyvitamin D Concentrationsin Relation to Hashimoto’s Thyroiditis: A SystematicReview, Meta-Analysis and Meta-Regression of Observational Studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef]
- Fang, F.; Chai, Y.; Wei, H.; Wang, K.; Tan, L.; Zhang, W.; Fan, Y.; Li, F.; Shan, Z.; Zhu, M. Vitamin D Deficiency Is Associated with Thyroid Autoimmunity: Results from an Epidemiological Survey in Tianjin, China. Endocrine 2021, 73, 447–454. [Google Scholar] [CrossRef]
- Mele, C.; Caputo, M.; Bisceglia, A.; Samà, M.T.; Zavattaro, M.; Aimaretti, G.; Pagano, L.; Prodam, F.; Marzullo, P. Immunomodulatory Effects of Vitamin D in Thyroid Diseases. Nutrients 2020, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Lebiedziński, F.; Lisowska, K.A. Impact of Vitamin D on Immunopathology of Hashimoto’s Thyroiditis: From Theory to Practice. Nutrients 2023, 15, 3174. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Zhu, Y.; Fang, L. Correlation Between Hashimoto’s Thyroiditis–Related Thyroid Hormone Levels and 25-Hydroxyvitamin D. Front. Endocrinol. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, N.; Su, R.; Dai, C.; Zhang, R. Selenium Supplementation May Decrease Thyroid Peroxidase Antibody Titer via Reducing Oxidative Stress in Euthyroid Patients with Autoimmune Thyroiditis. Int. J. Endocrinol. 2020, 2020, 9210572. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Piana, S.; Riganti, F.; Froio, E.; Andrioli, M.; Pacella, C.M.; Valcavi, R. Pathological findings of thyroid nodules after percutaneous laser ablation. Endocr Pathol. 2012, 23, 94–100. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Sălăgean, T.; Pop, I.D.; Bordea, I.R.; Benedicenti, S. Understanding COVID-19 Pandemic: Molecular Mechanisms and Potential Therapeutic Strategies. An Evidence-Based Review. J. Inflamm. Res. 2021, 14, 13–56. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Sălăgean, T.; Bordea, I.R.; Benedicenti, S. Phototherapy as a rational antioxidant treatment modality in COVID-19 management; new concept and strategic approach: Critical review. Antioxidants 2020, 9, 875. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Benedicenti, S. Role of Photobiomodulation Therapy in Modulating Oxidative Stress in Temporomandibular Disorders. A Systematic Review and Meta-Analysis of Human Randomised Controlled Trials. Antioxidants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Hanna, R.; Bensadoun, R.J.; Beken, S.V.; Burton, P.; Carroll, J.; Benedicenti, S. Outpatient Oral Neuropathic Pain Management with Photobiomodulation Therapy: A Prospective Analgesic Pharmacotherapy-Paralleled Feasibility Trial. Antioxidants 2022, 11, 533. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Raber-Durlacher, J.E.; Benedicenti, S. Role of Photobiomodulation Therapy in Neurological Primary Burning Mouth Syndrome. A Systematic Review and Meta-Analysis of Human Randomised Controlled Clinical Trials. Pharmaceutics 2021, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, J.; Williams-Lockhart, S.; Bensadoun, R.J.; Hanna, R. A Novel Approach of Combining Methylene Blue Photodynamic Inactivation, Photobiomodulation and Oral Ingested Methylene Blue in COVID-19 Management: A Pilot Clinical Study with 12-Month Follow-Up. Antioxidants 2022, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Panhoca, V.H.; Ferreira, L.T.; de Souza, V.B.; Ferreira, S.A.; Simão, G.; de Aquino Junior, A.E.; Bagnato, V.S.; Hanna, R. Can Photobiomodulation Restore Anosmia and Ageusia Induced by COVID-19? A Pilot Clinical Study. J. Biophotonics 2023, 6, e202300003. [Google Scholar] [CrossRef] [PubMed]
- Yavas, A.D.; Degirmenci, A.N.; Berkan, F.; Oner, C. Low Level Laser Therapy in Rheumatoid Arthritis: Ultrasonographic and Clinical Assessment of Efficacy. J. Immunol. Clin. Microbiol. 2021, 6, 69–80. [Google Scholar]
- Souza, N.H.C.; Ferrari, R.A.M.; Silva, D.F.T.; Nunes, F.D.; Bussadori, S.K.; Fernandes, K.P.S. Effect of low-level laser therapy on the modulation of the mitochondrial activity of macrophages. Braz. J. Phys. Ther. 2014, 18, 308–314. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Camastra, S.; Paparo, S.R.; Giusti, C.; Gonnella, D.; Ruffilli, I.; Shoenfeld, Y.; et al. Novel therapies for thyroid autoimmune diseases: An update. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101366. [Google Scholar] [CrossRef]
- Höfling, D.B.; Chavantes, M.C.; Juliano, A.G.; Cerri, G.G.; Knobel, M.; Yoshimura, E.M.; Chammas, M.C. Low-level laser in the treatment of patients with hypothyroidism induced by chronic autoimmune thyroiditis: A randomized, placebo-controlled clinical trial. Lasers Med. Sci. 2013, 28, 743–753. [Google Scholar] [CrossRef]
- Höfling, D.B.; Chavantes, M.C.; Juliano, A.G.; Cerri, G.G.; Romao, R.; Yoshimura, E.M.; Chammas, M.C. Low-level laser therapy in chronic autoimmune thyroiditis: A pilot study. Lasers Surg. Med. 2010, 42, 589–596. [Google Scholar] [CrossRef]
- Vidal, L.; Ortiz, M.; Perez de Vargas, I. Ultrastructural changes in thyroid perifollicular capillaries during normal postnatal development and after infrared laser radiation. Lasers Med. Sci. 2002, 17, 187–197. [Google Scholar] [CrossRef]
- Serra, C.; Silveira, L. Evaluation of structural and ultrastructural changes in thyroid and parathyroid glands after near infrared irradiation: Study on an animal model. PeerJ 2021, 9, e11891. [Google Scholar] [CrossRef] [PubMed]
- Mun, I.K.; Yoo, W.S.; Lee, S.J.; Chung, P.-S.; Woo, S.H. Effect of Low-level Laser Therapy on Propylthiouracil-induced Hypothyroidism Model Mice: A Pilot Study. Med. Lasers 2021, 10, 37–44. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Kazem-Arki, M.; Keramatinia, L.; Rezaei-Tavirani, M. Low-Level Laser Therapy in the Treatment of Autoimmune Thyroiditis. J. Lasers Med. Sci. 2022, 13, e34. [Google Scholar] [CrossRef] [PubMed]
- Valcavi, R.; Riganti, F.; Bertani, A.; Formisano, D.; Pacella, C.M. Percutaneous laser ablation of cold benign thyroid nodules: A 3-year follow-up study in 122 patients. Thyroid 2010, 20, 1253–1261. [Google Scholar] [CrossRef]
- Azevedo, L.H.; Aranha, A.C.; Stolf, S.F.; Eduardo Cde, P.; Vieira, M.M. Evaluation of low intensity laser effects on the thyroid gland of male mice. Photomed. Laser Surg. 2005, 23, 567–570–570. [Google Scholar] [CrossRef]
- Fronza, B.; Somacal, T.; Mayer, L.; De Moraes, J.; De Oliveira, M.; Weber, J. Assessment of the systemic effects of low-level laser therapy (LLLT) on thyroid hormone function in a rabbit model. Int. J. Oral Maxillofac. Surg. 2013, 42, 26–30. [Google Scholar] [CrossRef]
- Morcos, N.; Omran, M.; Ghanem, H.; Elahdal, M.; Kamel, N.; Attia, E. Phototherapeutic Effect of Low-Level Laser on Thyroid Gland of Gamma-Irradiated Rats. Photochem. Photobiol. 2015, 91, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Smelova, I.; Golovneva, E. The study of morphological and functional changes in the thyroid follicles of healthy rats and rats with experimentally induced hypothyroidism following exposure to medium-power laser radiation. Bull. Russ. State Med Univ. 2018, 3, 65–71. [Google Scholar] [CrossRef]
- Luty, J.; Ruckemann-Dziurdzińska, K.; Witkowski, J.M.; Bryl, E. Immunological aspects of autoimmune thyroid disease–Complex interplay between cells and cytokines. Cytokine 2019, 116, 128–133. [Google Scholar] [CrossRef]
- Colombo, F.; Neto, A.A.P.V.; de Sousa, A.P.C.; Marchionni, A.M.T.; Pinheiro, A.L.B.; de Almeida Reis, S.R. Effect of Low-Level Laser Therapy (l660 nm) on Angiogenesis in Wound Healing: A Immunohistochemical Study in a Rodent Model. Braz. Dent. J. 2013, 24, 308–312. [Google Scholar] [CrossRef]
- Hamblin, M.R.; de Sousa, M.V.P.; Arany, P.R.; Carroll, J.D.; Patthoff, D. Low level laser (light) therapy and photobiomodulation: The path forward. In Mechanisms for Low-Light Therapy X; International Society for Optics and Photonics: Bellingham, WA, USA, 2015. [Google Scholar]
- Höfling, D.B.; Chavantes, M.C.; Acencio, M.M.; Cerri, G.G.; Marui, S.; Yoshimura, E.M.; Chammas, M.C. Effects of low-level laser therapy on the serum TGF-β1 concentrations in individuals with autoimmune thyroiditis. Photomed. Laser Surg. 2014, 32, 444–449. [Google Scholar] [CrossRef]
- Wickenheisser, V.A.; Zywot, E.M.; Rabjohns, E.M.; Lee, H.H.; Lawrence, D.S.; Tarrant, T.K. Laser Light Therapy in Inflammatory, Musculoskeletal, and Autoimmune Disease. Curr. Allergy Asthma Rep. 2019, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.B.; Mayer, L.; Cenci, R.A.; Baraldi, C.E.; Ponzoni, D.; Gerhardt de Oliveira, M. Effect of three different protocols of low-level laser therapy on thyroid hormone production after dental implant placement in an experimental rabbit model. Photomed. Laser Surg. 2014, 32, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Ercetin, C.; Sahbaz, N.A.; Acar, S.; Tutal, F.; Erbil, Y. Impact of Photobiomodulation on T3/T4 Ratio and Quality of Life in Hashimoto Thyroiditis. Photobiomodul. Photomed. Laser Surg. 2020, 38, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque-Pontes, G.M.; de Paula Vieira, R.; Tomazoni, S.S.; Caires, C.O.; Nemeth, V.; Vanin, A.A.; Santos, L.A.; Pinto, H.D.; Marcos, R.L.; Leal-Junior, E.C. Effect of pre-irradiation with different doses, wavelengths, and application intervals of low-level laser therapy on cytochrome c oxidase activity in intact skeletal muscle of rats. Lasers Med. Sci. 2015, 30, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Höfling, D.B.; Chavantes, M.C.; Juliano, A.G.; Cerri, G.G.; Knobel, M.; Yoshimura, E.M.; Chammas, M.C. Safety and Efficacy of Low-Level Laser Therapy in Autoimmune thyroiditis: Long-Term Follow-Up Study. Int. J. Endocrinol. 2018, 4, 8387530. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Khannazer, N.; Kazem Arki, M.; Keramatinia, A.; Rezaei-Tavirani, M. Low-Level Laser Therapy for Rheumatoid Arthritis: A Review of Experimental Approaches. J. Lasers Med. Sci. 2022, 13, e62. [Google Scholar] [CrossRef]
- Boschi, E.S.; Leite, C.E.; Saciura, V.C.; Caaberlon, E.; Lunaardelli, A.; Bitencourt, S.; Melo, D.A.; Oliveria, J.R. Anti-inflammatory effects of low-level laser therapy (660nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg. Med. 2008, 40, 500–508. [Google Scholar] [CrossRef]
- Singh, H.L.; Chandra, A.K.; Yumnam, S.D.; Sarkar, D.; Manglem, R.K.; Dhabali, T.; Mookerjee, S.; Ray, I. Thiocyanate in excess develops goiter followed by auto immune thyroid diseases even after effective salt iodization in a rural community of north east India. Ecotoxicol. Environ. Saf. 2021, 208, 111711. [Google Scholar] [CrossRef]
- De Vasconcelos, J.C.; de Siqueira, I.B.; Maia, F.F.R.; Parisi, M.C.R.; Zantut-Wittmann, D.E. Influence of thyroid hormone in the expression of the marker pro-apoptosis BID, in spite of the predominance of anti-apoptosis activation in intratiroidal lymphocytic infiltration in Hashimoto’s thyroiditis. Mol. Cell. Endocrinol. 2021, 537, 111421. [Google Scholar] [CrossRef]
- Fung, A.W.S.; Knauer, M.J.; Blasutig, I.M.; Colantonio, D.A.; Kulasingam, V. Evaluation of electrochemiluminescence immunoassays for immunosuppressive drugs on the Roche cobas e411 analyzer. F1000Research 2017, 6, 1832. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.A.; Carroll, J.D. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed. Laser Surg. 2011, 29, 785–787. [Google Scholar] [CrossRef] [PubMed]
| Manufacturer | Omega XP |
|---|---|
| Semiconductor materials (emitter type) | GaAIAs |
| Probe design | Single probe |
| Beam delivery system | 3B laser |
| Laser-aiming beam | None |
| Wavelength | 820 nm |
| Operating emission mode | Continuous wave (CW) |
| Polarization | Linear |
| Therapeutic power output | 200 mW |
| Fluence (dose) | 32 J/cm2 per point |
| Irradiation time per point | 20 s per point |
| Total number of irradiated points around thyroid gland | 8 points |
| Total of fluence per session | 256 J/cm2 per session |
| Total irradiation time per session | 160 s |
| Time interval | Relatively two days, excluding weekends |
| Treatment frequency | Twice a week |
| Total treatment sessions | Six sessions |
| Treatment duration | Three consecutive weeks |
| Scanning technique | Stationary application |
| Light-tissue distance | In contact with the skin |
| Variables | Group 1 (n = 18) Mean ± SD or Median (IQR) | Group 2 (n = 20) Mean ± SD or Median (IQR) | p Value |
|---|---|---|---|
| Age (yrs) | 39.78 ± 4.40 | 37.30 ± 5.91 | t = 1.452, p = 0.155 |
| Height (cm) | 1.64 ± 0.05 | 1.65 ± 0.05 | t = 0.588, p = 0.560 |
| Weight (kg) | 80.00 ± 9.63 | 83.57 ± 9.61 | t = 1.142, p = 0.261 |
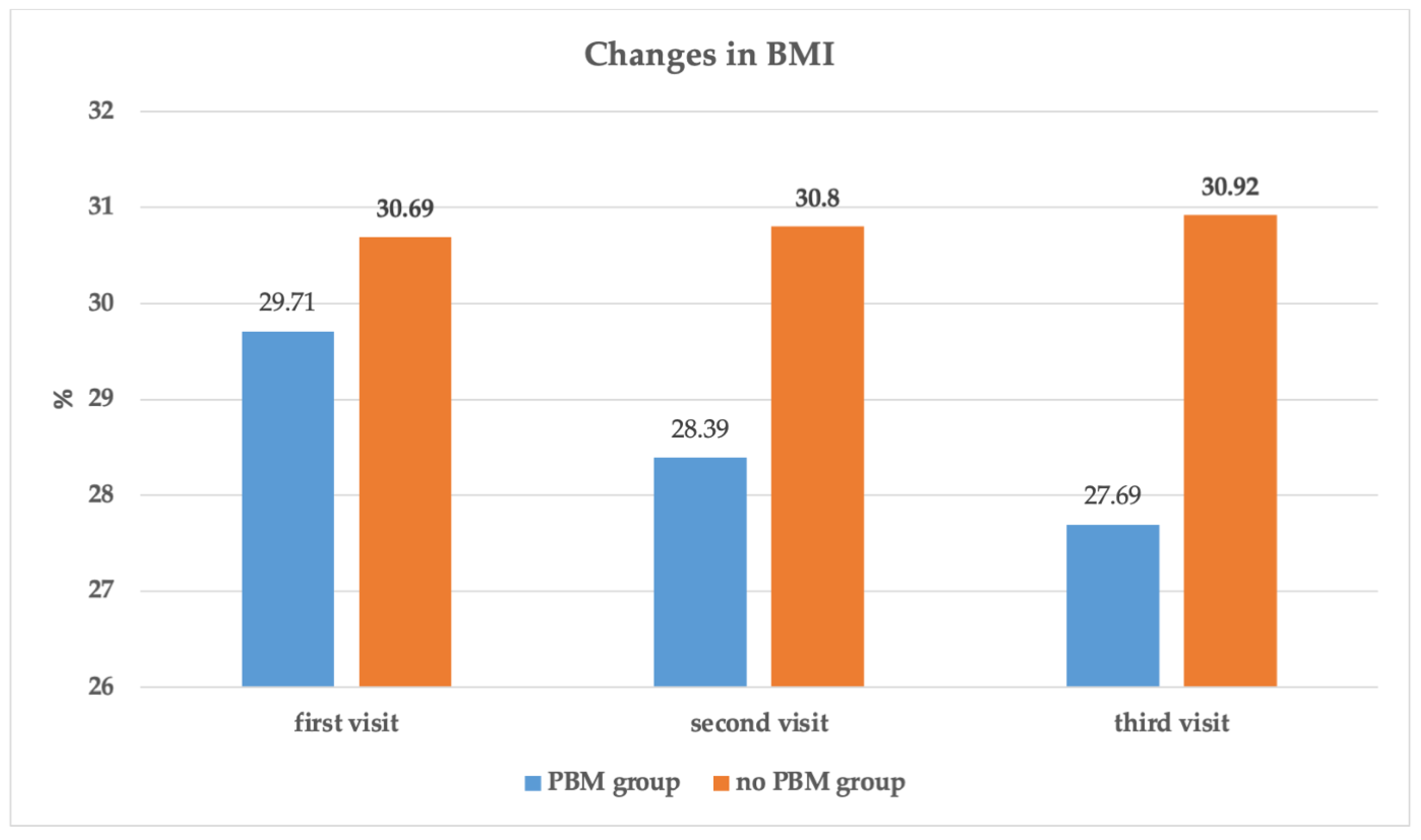
| BMI (kg/m2) | 29.71 ± 3.36 | 30.69 ± 3.13 | t = 0.991, p = 0.357 |
| Waist (cm) | 100.39 ± 9.54 | 105.45 ± 9.84 | t = 0.999, p = 0.117 |
| Hip (cm) | 112.22 ± 7.82 | 116.55 ± 6.71 | t = 0.780, p = 0.075 |
| Waist/Hip | 0.90 ± 0.06 | 0.90 ± 0.06 | t = 0.681, p = 1.000 |
| TSH | 3.50(1.94–4.65) | 2.92 (1.38–3.80) | U = 134.00, p = 0.186 |
| FT4 | 1.34 ± 0.27 | 1.53 ± 0.37 | t = 1.722, p = 0.094 |
| FT3 | 3.70 (2.48–4.21) | 4.10 (3.55–5.08) | U = 247.50, p = 0.048 |
| antiTPO | 523.60 (291.75–652.55) | 219.75 (70.45–538.97) | U = 111.50, p = 0.044 |
| antiTG | 58.00 (9.05–349.75) | 39.28 (18.04–198.25) | U = 166.00, p = 0.916 |
| Dose of LT4 prior to treatment (T0) | 75.00 (50.00–131.25) | 62.50 (50.00–100.00) | U = 126.00, p = 0.119 |
| Interventional Groups | Variables | Measurements | Statistical Analysis Significant/Statistically Insignificance | |||||
|---|---|---|---|---|---|---|---|---|
| First Mean ± SD or Median (IQR) | Second Mean ± SD or Median (IQR) | Third Mean ± SD or Median (IQR) | p a | p b | p c | |||
| Group 1 | Weight (kg) | 80.00 ± 9.63 | 76.41 ± 9.16 | 74.59 ± 8.57 | F = 51.479, p < 0.0001 | <0.0001 | <0.0001 | <0.0001 |
| BMI (kg/m2) | 29.71 ± 3.36 | 28.39 ± 3.24 | 27.69 ± 2.96 | F = 48.097, p < 0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Waist (cm) | 100.39 ± 9.54 | 94.50 ± 9.79 | 93.44 ± 9.44 | F = 24.262, p < 0.0001 | <0.0001 | 0.030 | <0.0001 | |
| Hip (cm) | 112.22 ± 7.82 | 109.61 ± 7.00 | 108.89 ± 7.14 | F = 18.953, p < 0.0001 | 0.001 | 0.061 | <0.0001 | |
| Waist/Hip | 0.90 ± 0.06 | 0.87 ± 0.07 | 0.87 ± 0.07 | F = 5.667, p = 0.029 | 0.029 | NA | 0.029 | |
| TSH | 3.50 (1.94–4.65) | 0.85 (0.10–1.27) | 1.25 (0.46–1.51) | x2 = 24.602, p < 0.0001 | <0.0001 | 0.147 | 0.001 | |
| FT4 | 1.34 ± 0.27 | 3.08 ± 1.74 | 1.85 ± 0.62 | F = 11.908, p = 0.001 | <0.0001 | 0.007 | 0.011 | |
| FT3 | 3.70 (2.48–4.21) | 5.8 (5.35–6.95) | 5.15 (4.77–5.20) | x2 = 27.070, p < 0.0001 | <0.0001 | 0.001 | 0.001 | |
| antiTPO | 523.60 (291.75–652.55) | 100.15 (78.00–127.95) | 88.25 (62.25–129.50) | x2 = 23.111, p < 0.0001 | <0.0001 | 0.102 | <0.0001 | |
| antiTG | 58.00 (9.05–349.75) | 36.60 (20.00–143.77) | 44.15 (22.85–89.47) | x2 = 5.765, p = 0.056 | 0.025 | 0.433 | 0.044 | |
| Dose of LT4 | 75.00 (50.00–131.25) | 75.00 (50.00–131.25) | 75.00 (50.00–106.25) | x2 = 16.000, p < 0.0001 | 1.000 | 0.005 | 0.005 | |
| Group 2 | Weight (kg) | 83.57 ± 9.61 | 83.86 ± 9.94 | 84.17 ± 9.99 | F = 2.502, p = 0.095 | 0.293 | 0.084 | 0.092 |
| BMI (kg/m2) | 30.69 ± 3.13 | 30.80 ± 3.25 | 30.92 ± 3.23 | F = 2.801, p = 0.073 | 0.286 | 0.059 | 0.074 | |
| Waist (cm) | 105.45 ± 9.84 | 105.70 ± 9.96 | 105.95 ± 10.15 | F = 1.727, p = 0.204 | 0.398 | 0.056 | 0.163 | |
| Hip (cm) | 116.55 ± 6.71 | 116.85 ± 6.81 | 116.90 ± 6.83 | F = 2.424, p = 0.133 | 0.163 | 0.330 | 0.110 | |
| Waist/Hip | 0.90 ± 0.06 | 0.90 ± 0.06 | 0.90 ± 0.06 | F = 0.000, p = 1.000 | 1.000 | NA | 1.000 | |
| TSH | 2.92 (1.38–3.80) | 3.35 (2.45–4.25) | 3.05 (2.50–4.05) | x2 = 4.785, p = 0.091 | 0.025 | 0.926 | 0.059 | |
| FT4 | 1.53 ± 0.37 | 1.36 ± 0.38 | 1.28 ± 0.20 | F = 3.328, p = 0.053 | 0.018 | 0.519 | 0.024 | |
| FT3 | 4.10(3.55–5.08) | 3.90 (3.1–4.44) | 3.80 (2.84–4.25) | x2 = 7.620, p = 0.022 | 0.012 | 0.687 | 0.028 | |
| antiTPO | 219.75 (70.45–538.97) | 167.50 (92.35–360.75) | 171.85 (87.12–443.60) | x2 = 0.700, p = 0.705 | 0.263 | 1.000 | 0.478 | |
| antiTG | 39.28 (18.04–198.25) | 44.10 (17.92–276.62) | 37.55 (17.95–341.75) | x2 = 1.200, p = 0.549 | 0.467 | 0.970 | 0.737 | |
| Dose of LT4 | 62.50 (50.00–100.00) | 62.50 (50.00–100.00) | 75.00 (56.25–100.00) | x2 = 14.000, p = 0.001 | 1.000 | 0.005 | 0.008 | |
| Variables | Time*Group Factor | |
|---|---|---|
| F | p Value | |
| Weight (kg) | 54.024 | <0.0001 |
| BMI (kg/m2) | 52.073 | <0.0001 |
| Waist (cm) | 28.310 | <0.0001 |
| Hip (cm) | 23.284 | <0.0001 |
| Waist/Hip | 1.038 | 0.315 |
| TSH | 22.829 | <0.0001 |
| FT4 | 14.023 | <0.0001 |
| FT3 | 30.290 | <0.0001 |
| antiTPO | 19.083 | <0.0001 |
| antiTG | 4.915 | 0.028 |
| Dose of LT4 | 23.932 | <0.0001 |
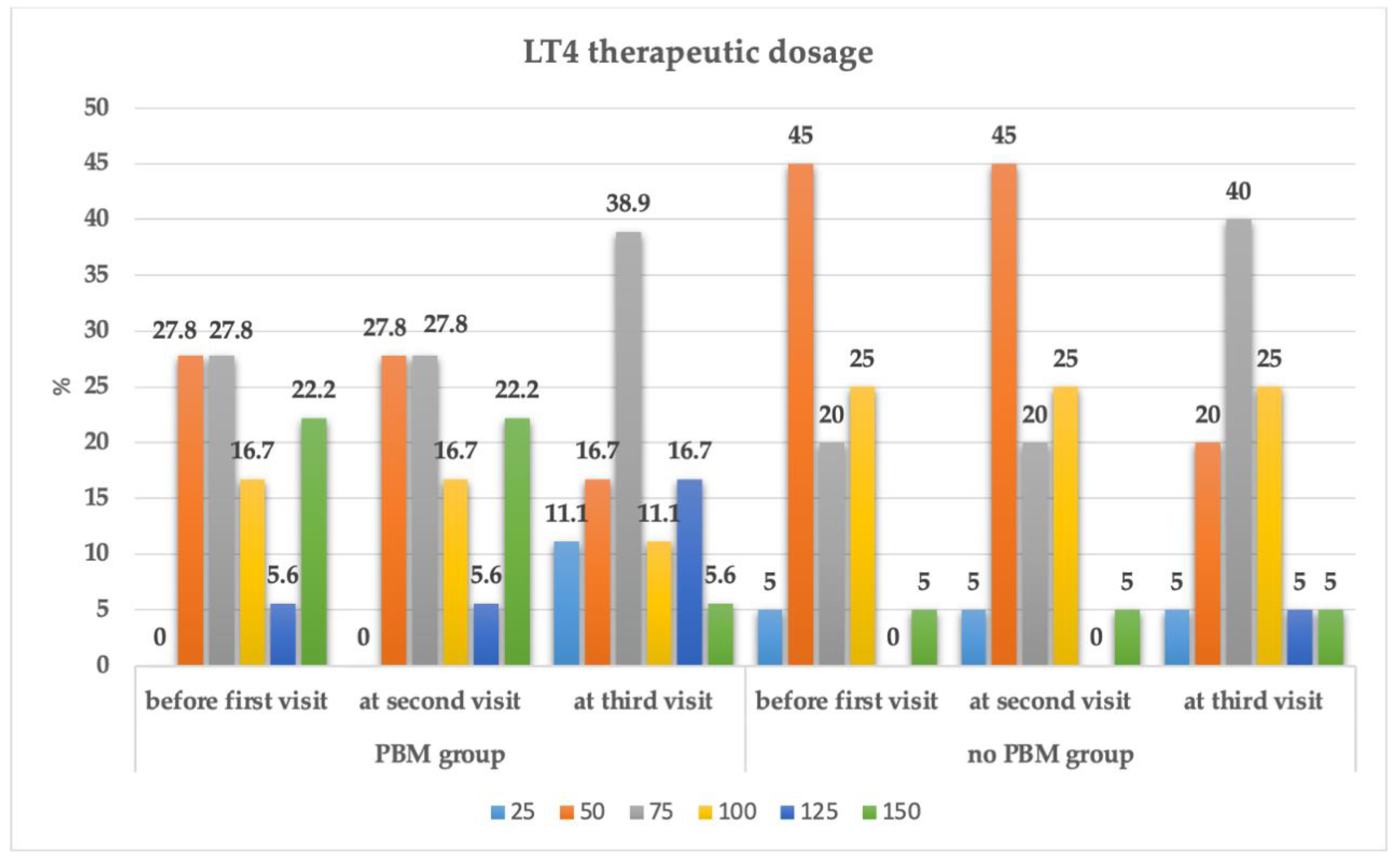
| LT4 at second visit (T1) | LT4 at Third Visit (T2) | ||||||||
| Interventional Group | Dose | 25 | 50 | 75 | 100 | 125 | 150 | Total | |
| Group 1 PBM + supplements | 50 | 2(11.1) | 3(16.3) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 5(27.8) | |
| 75 | 0(0.0) | 0(0.0) | 5(27.8) | 0(0.0) | 0(0.0) | 0(0.0) | 5(27.8) | ||
| 100 | 0(0.0) | 0(0.0) | 2(11.1) | 1(5.6) | 0(0.0) | 0(0.0) | 3(16.7) | ||
| 125 | 0(0.0) | 0(0.0) | 0(0.0) | 1(5.6) | 0(0.0) | 0(0.0) | 1(5.6) | ||
| 150 | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 3(16.7) | 1(5.6) | 4(22.2) | ||
| Total | 2(11.1) | 3(16.7) | 7(38.9) | 2(11.1) | 3(16.7) | 1(5.6) | 18(100.0) | ||
| Group 2 Only supplements, no PBM | 25 | 1(5.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(5.0) | |
| 50 | 0(0.0) | 4(20.0) | 5(25.0) | 0(0.0) | 0(0.0) | 0(0.0) | 9(45.0) | ||
| 75 | 0(0.0) | 0(0.0) | 3(15.0) | 1(5.0) | 0(0.0) | 0(0.0) | 4(20.0) | ||
| 100 | 0(0.0) | 0(0.0) | 0(0.0) | 4(20.0) | 1(5.0) | 0(0.0) | 5(25.0) | ||
| 150 | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(5.0) | 1(5.0) | ||
| Total | 1(5.0) | 4(20.0) | 8(40.0) | 5(25.0) | 1(5.0) | 1(5.0) | 20(100.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berisha-Muharremi, V.; Tahirbegolli, B.; Phypers, R.; Hanna, R. Efficacy of Combined Photobiomodulation Therapy with Supplements versus Supplements alone in Restoring Thyroid Gland Homeostasis in Hashimoto Thyroiditis: A Clinical Feasibility Parallel Trial with 6-Months Follow-Up. J. Pers. Med. 2023, 13, 1274. https://doi.org/10.3390/jpm13081274
Berisha-Muharremi V, Tahirbegolli B, Phypers R, Hanna R. Efficacy of Combined Photobiomodulation Therapy with Supplements versus Supplements alone in Restoring Thyroid Gland Homeostasis in Hashimoto Thyroiditis: A Clinical Feasibility Parallel Trial with 6-Months Follow-Up. Journal of Personalized Medicine. 2023; 13(8):1274. https://doi.org/10.3390/jpm13081274
Chicago/Turabian StyleBerisha-Muharremi, Venera, Bernard Tahirbegolli, Ruth Phypers, and Reem Hanna. 2023. "Efficacy of Combined Photobiomodulation Therapy with Supplements versus Supplements alone in Restoring Thyroid Gland Homeostasis in Hashimoto Thyroiditis: A Clinical Feasibility Parallel Trial with 6-Months Follow-Up" Journal of Personalized Medicine 13, no. 8: 1274. https://doi.org/10.3390/jpm13081274
APA StyleBerisha-Muharremi, V., Tahirbegolli, B., Phypers, R., & Hanna, R. (2023). Efficacy of Combined Photobiomodulation Therapy with Supplements versus Supplements alone in Restoring Thyroid Gland Homeostasis in Hashimoto Thyroiditis: A Clinical Feasibility Parallel Trial with 6-Months Follow-Up. Journal of Personalized Medicine, 13(8), 1274. https://doi.org/10.3390/jpm13081274