Safety and Aesthetics of Autologous Dermis-Fat Graft after Parotidectomy: A Multidisciplinary Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. The Radiologist’s Perspective
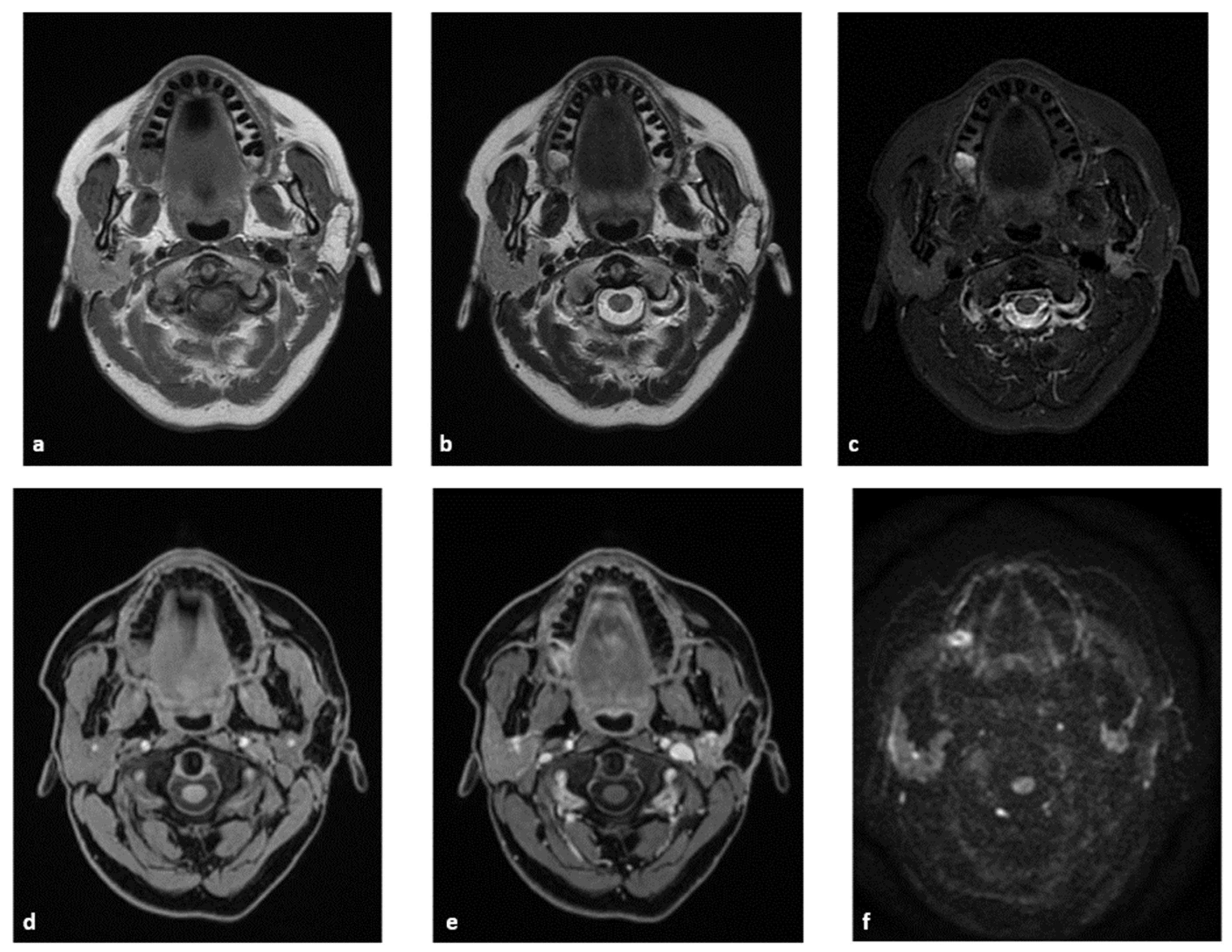
4.1.1. MRI Protocol
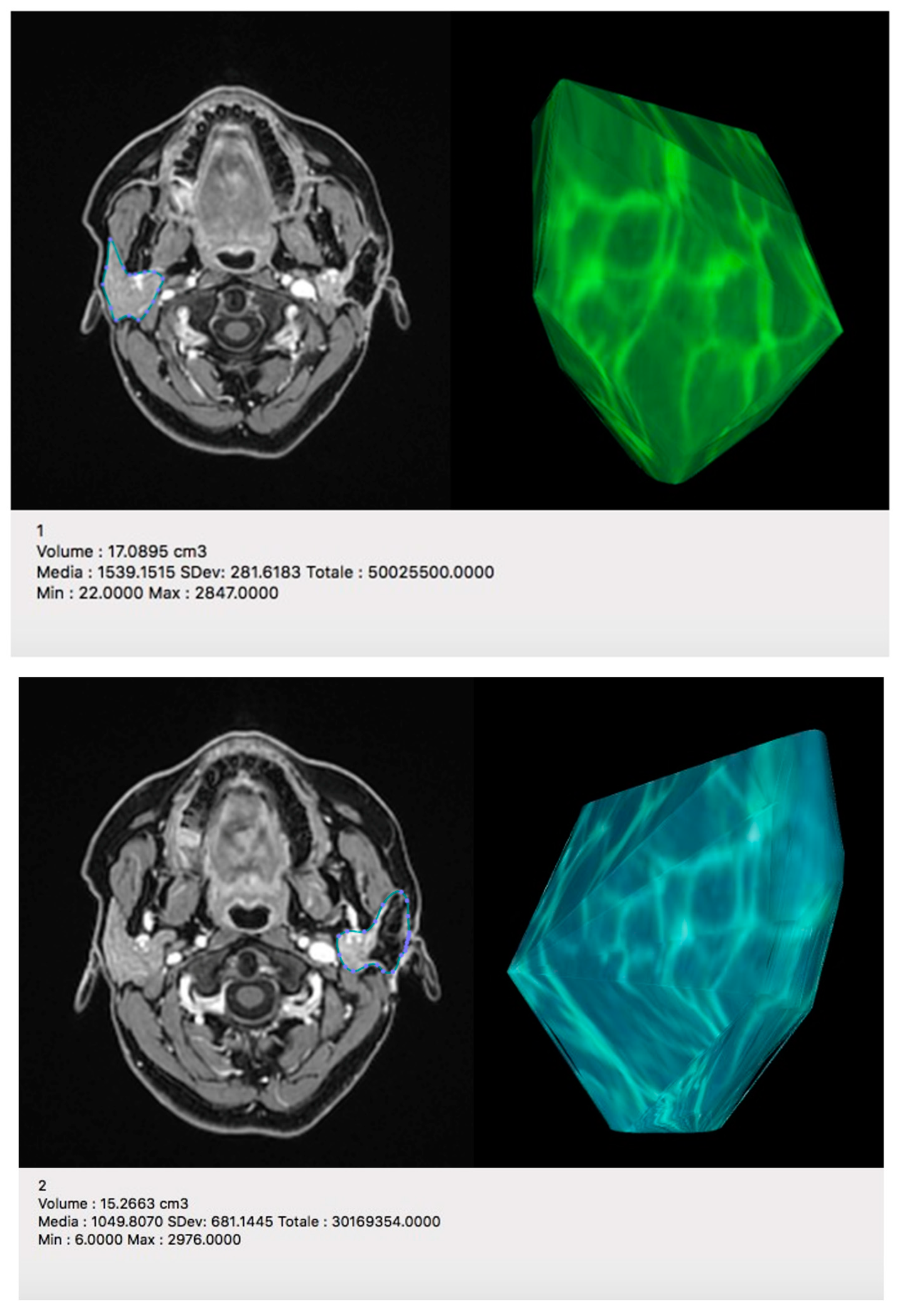
4.1.2. MRI Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Yang, Z.; Wang, W.; Xu, H.; Liu, F. Autologous Free Fat Graft for Repair of Concave Deformity after Total Parotidectomy. J. Craniofac. Surg. 2019, 30, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.R.; Miller, T.A.; Huang, C.; Roostaien, J.; Wasson, K.L.; Ashley, R.K.; Bradley, J.P. Autologous fat transfer for facial recontouring: Is there science behind the art? Plast. Reconstr. Surg. 2007, 119, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Tunca, M.; Süslü, N.S.; Karaosmanoğlu, A.A. Fat Transfer after Parotidectomy: Fat Resorption Rates, Aesthetic and Functional Outcomes of En-Bloc Fat Graft Versus Lipofilling Technique. Eur. Arch. Otorhinolaryngol. 2021, 278, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Bryant, L.M.; Cognetti, D.; Baker, A.; Roy, S.; Johnston, D.R.; Curry, J.; Krein, H. Esthetic and functional reconstruction after parotidectomy in pediatric patients—A case series. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2442–2445. [Google Scholar] [CrossRef]
- Gir, P.; Brown, S.A.; Oni, G.; Kashefi, N.; Mojallal, A.; Rohrich, R.J. Fat Grafting. Plast. Reconstr. Surg. 2012, 130, 249–258. [Google Scholar] [CrossRef]
- Brooker, J.E.; Rubin, J.P.; Marra, K.G. The Future of Facial Fat Grafting. J. Craniofac. Surg. 2019, 30, 644–651. [Google Scholar] [CrossRef]
- Nofal, A.A.; Mohamed, M. Sternocleidomastoid Muscle Flap after Parotidectomy. Int. Arch. Otorhinolaryngol. 2015, 19, 319–324. [Google Scholar] [CrossRef]
- Ambro, B.T.; Goodstein, L.A.; Morales, R.E.; Taylor, R.J. Evaluation of superficial musculoaponeurotic system flap and fat graft outcomes for benign and malignant parotid disease. Otolaryngol. Head Neck Surg. 2013, 148, 949–954. [Google Scholar] [CrossRef]
- Irvine, L.E.; Larian, B.; Azizzadeh, B. Locoregional Parotid Reconstruction. Otolaryngol. Clin. N. Am. 2016, 49, 435–446. [Google Scholar] [CrossRef]
- Keles, M.K.; Horoz, U.; Cepni, H. A simple method for breast de-epithelialization: The monobloc method. Trichol. Cosmetol. Open J. 2016, 1, 19–20. [Google Scholar] [CrossRef]
- Boschetti, C.E.; Lo Giudice, G.; Spuntarelli, C.; Apice, C.; Rauso, R.; Santagata, M.; Tartaro, G.; Colella, G. Kabat Rehabilitation in Facial Nerve Palsy after Parotid Gland Tumor Surgery: A Case-Control Study. Diagnostics 2022, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Garro, A.; Nigrovic, L.E. Managing Peripheral Facial Palsy. Ann. Emerg. Med. 2018, 71, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Conger, B.T.; Gourin, C.G. Free abdominal fat transfer for reconstruction of the total parotidectomy defect. Laryngoscope 2008, 118, 1186–1190. [Google Scholar] [CrossRef]
- Guijarro-Martínez, R.; Alba, L.M.; Mateo, M.M.; Torres, M.P.; Gil, J.V.P. Autologous fat transfer to the cranio-maxillofacial region: Updates and controversies. J. Craniomaxillofac. Surg. 2011, 39, 359–363. [Google Scholar] [CrossRef]
- Neuber, G. Fat transplantation. Chir. Klongr. Verhandl. Dtsch. Gesellsch. Chir. 1893, 22, 66. [Google Scholar]
- De Cicco, D.; Tartaro, G.; Colella, G.; Dell’Aversana Orabona, G.; Santagata, M.; Ferrieri, I.; Troiano, A.; Staglianò, S.; Volgare, A.S.; D’Amato, S. Fat Graft in Surgical Treatment of Medication-Related Osteonecrosis of the Jaws (MRONJ). Appl. Sci. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef]
- Colella, G.; Rauso, R.; De Cicco, D.; Boschetti, C.E.; Iorio, B.; Spuntarelli, C.; Franco, R.; Tartaro, G. Clinical management of squamous cell carcinoma of the tongue: Patients not eligible for free flaps, a systematic review of the literature. Expert Rev. Anticancer. Ther. 2021, 21, 9–22. [Google Scholar] [CrossRef]
- Baum, S.; Prörtner, R.; Ladwein, F.; Schmeling, C.; Rieger, G.; Mohr, C. Use of dermis-fat grafts in the prevention of Frey’s syndrome after parotidectomy. J. Craniomaxillofac. Surg. 2016, 44, 301–308. [Google Scholar] [CrossRef]
- Chan, L.S.; Barakate, M.S.; Havas, T.E. Free fat grafting in superficial parotid surgery to prevent Frey-s syndrome and improve aesthetic outcome. J. Laryngol. Otol. 2014, 128 (Suppl. S1), S44–S49. [Google Scholar] [CrossRef]
- Boschetti, C.E.; Vitagliano, R.; Staglianò, S.; Pollice, A.; Giudice, G.L.; Apice, C.; Santagata, M.; Tartaro, G.; Colella, G. Development of an application for mobile phones (App) capable to predict the improvement of the degree House Brackmann scale in patients suffering from iatrogenic facial palsy. Adv. Oral Maxillofac. Surg. 2022, 8, 100356. [Google Scholar] [CrossRef]
- Dehdashtian, A.; Bratley, J.V.; Svientek, S.R.; Kung, T.A.; Awan, T.M.; Cederna, P.S.; Kemp, S.W. Autologous fat grafting for nerve regeneration and neuropathic pain: Current state from bench-to-bedside. Regen. Med. 2020, 15, 2209–2228. [Google Scholar] [CrossRef] [PubMed]
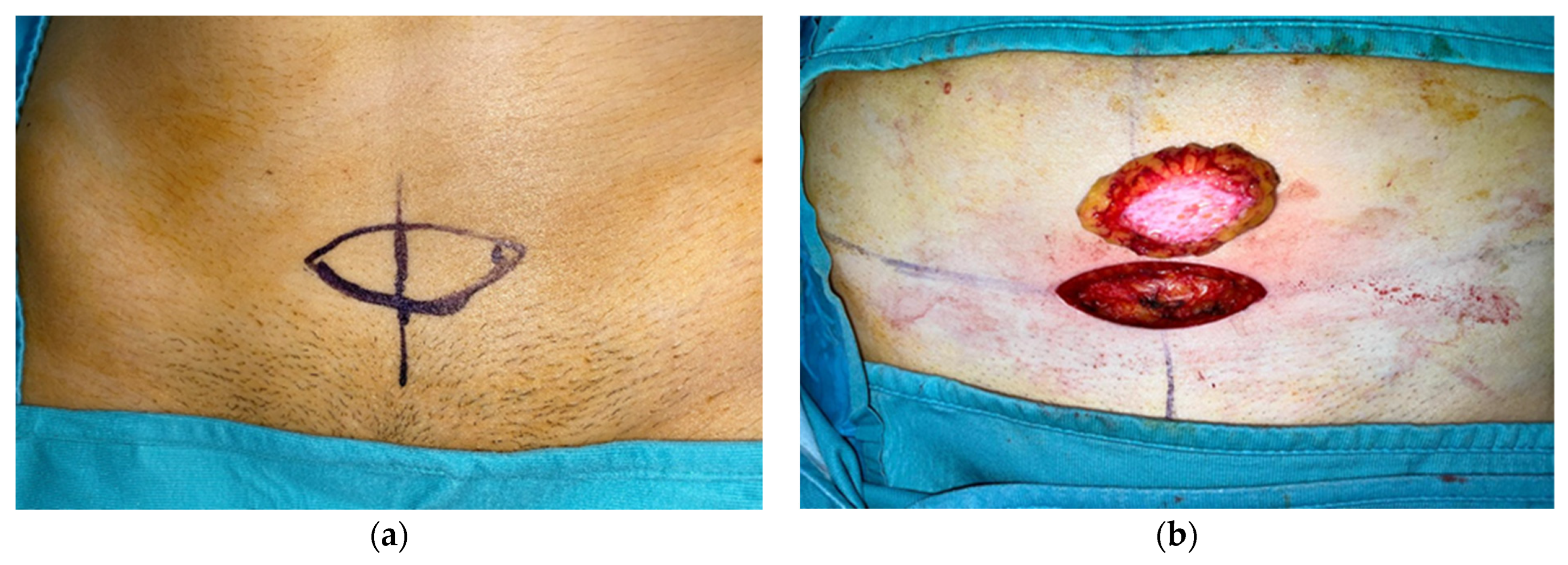
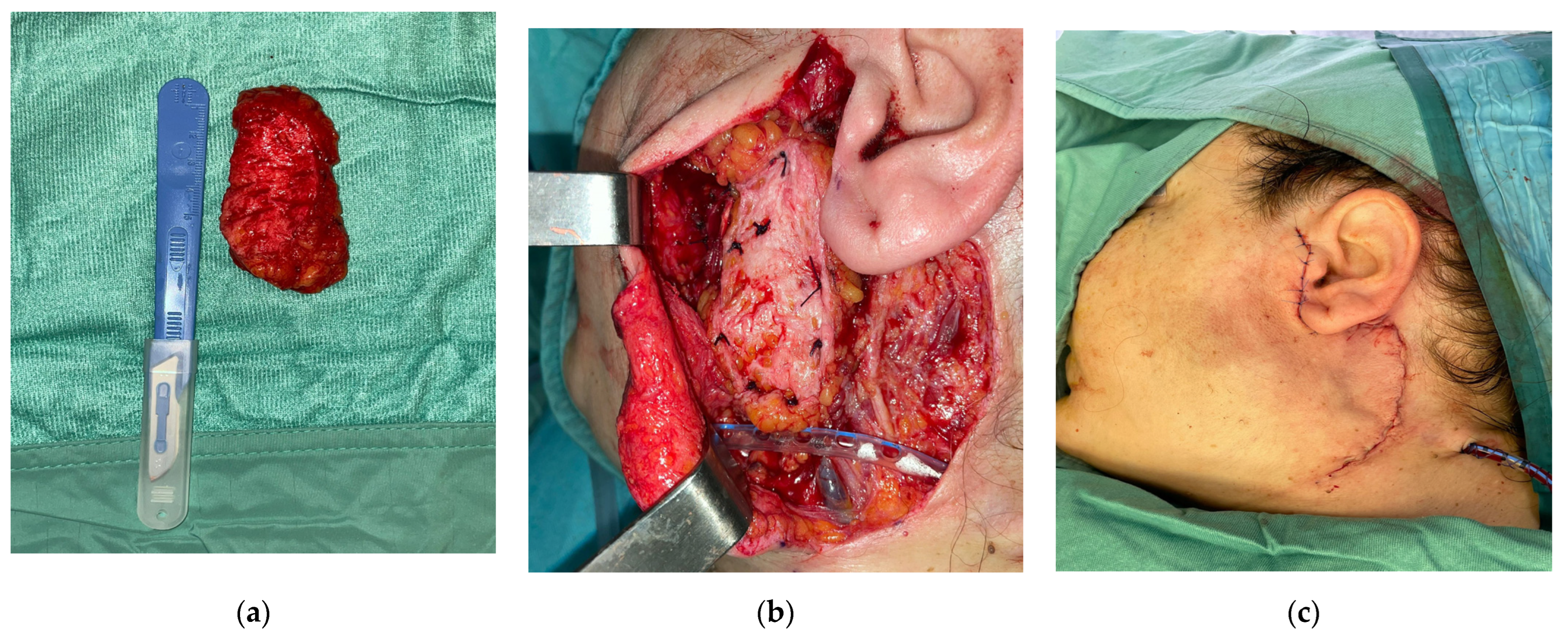

| Patient | Gender | Age | Extent of Surgery (P.P. 1, S.P. 2, T.P. 3) | Final Histologic Diagnosis |
|---|---|---|---|---|
| 1 | F | 49 | P.P. | Pleomorphic adenoma |
| 2 | F | 28 | P.P. | Pleomorphic adenoma |
| 3 | F | 14 | P.P. | Pleomorphic adenoma |
| 4 | F | 73 | P.P. | Warthin tumour |
| 5 | M | 54 | T.P. | Mucoepidermoid carcinoma |
| 6 | M | 78 | T.P. | Salivary duct carcinoma |
| 7 | M | 69 | T.P. | Metastatic squamous cell carcinoma |
| 8 | F | 21 | T.P. | Acinic cell carcinoma |
| 9 | M | 51 | P.P. | Warthin tumour |
| 10 | M | 21 | P.P. | Pleomorphic adenoma |
| 11 | F | 64 | P.P. | Warthin tumour |
| 12 | F | 48 | P.P. | Warthin tumour |
| 13 | F | 56 | S.P. | Carcinoma ex pleomorphic adenoma |
| 14 | F | 33 | S.P. | Myoepithelioma |
| 15 | M | 51 | T.P. | Salivary duct carcinoma |
| 16 | F | 37 | S.P. | Pleomorphic adenoma (recurrency) |
| 17 | M | 67 | T.P. | Pleomorphic adenoma (recurrency) |
| 18 | F | 55 | T.P. | Pleomorphic adenoma |
| 19 | F | 59 | S.P. | Mucoepidermoid carcinoma (low grade) |
| 20 | M | 65 | P.P. | Solitary Extrapleural Fibrous Tumour |
| 21 | F | 53 | S.P. | Myoepithelioma |
| 22 | F | 75 | S.P. | Myoepithelioma |
| 23 | M | 63 | S.P. | Basal cells adenoma |
| 24 | F | 37 | P.P. | Warthin tumour |
| 25 | F | 76 | S.P. | Myoepithelioma |
| 26 | M | 45 | S.P. | Pleomorphic adenoma |
| 27 | F | 62 | P.P. | Pleomorphic adenoma |
| 28 | F | 52 | P.P. | Warthin tumour |
| 29 | F | 44 | S.P. | Pleomorphic adenoma |
| 30 | F | 42 | S.P. | Acinic cell carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschetti, C.E.; Vitagliano, R.; Cornacchini, N.; Santagata, M.; Caliendo, V.; Belfiore, M.P.; Colella, G.; Tartaro, G.; Cappabianca, S. Safety and Aesthetics of Autologous Dermis-Fat Graft after Parotidectomy: A Multidisciplinary Retrospective Study. J. Pers. Med. 2023, 13, 1200. https://doi.org/10.3390/jpm13081200
Boschetti CE, Vitagliano R, Cornacchini N, Santagata M, Caliendo V, Belfiore MP, Colella G, Tartaro G, Cappabianca S. Safety and Aesthetics of Autologous Dermis-Fat Graft after Parotidectomy: A Multidisciplinary Retrospective Study. Journal of Personalized Medicine. 2023; 13(8):1200. https://doi.org/10.3390/jpm13081200
Chicago/Turabian StyleBoschetti, Ciro Emiliano, Rita Vitagliano, Nicola Cornacchini, Mario Santagata, Valentina Caliendo, Maria Paola Belfiore, Giuseppe Colella, Gianpaolo Tartaro, and Salvatore Cappabianca. 2023. "Safety and Aesthetics of Autologous Dermis-Fat Graft after Parotidectomy: A Multidisciplinary Retrospective Study" Journal of Personalized Medicine 13, no. 8: 1200. https://doi.org/10.3390/jpm13081200
APA StyleBoschetti, C. E., Vitagliano, R., Cornacchini, N., Santagata, M., Caliendo, V., Belfiore, M. P., Colella, G., Tartaro, G., & Cappabianca, S. (2023). Safety and Aesthetics of Autologous Dermis-Fat Graft after Parotidectomy: A Multidisciplinary Retrospective Study. Journal of Personalized Medicine, 13(8), 1200. https://doi.org/10.3390/jpm13081200








