Validation of Gait Measurements on Short-Distance Walkways Using Azure Kinect DK in Patients Receiving Chronic Hemodialysis
Abstract
1. Introduction
2. Gait Analysis for CMUH Patients
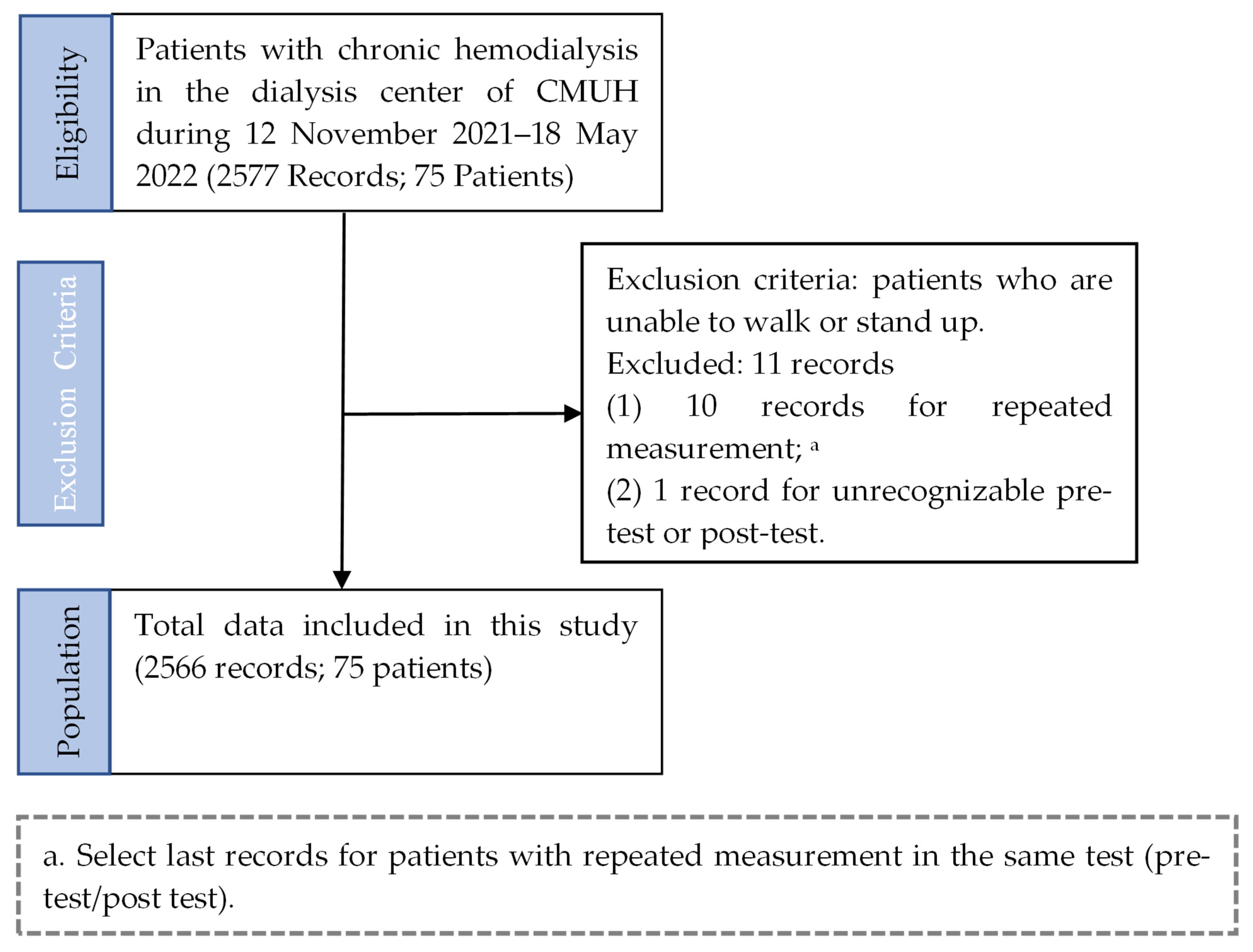
2.1. Subjects
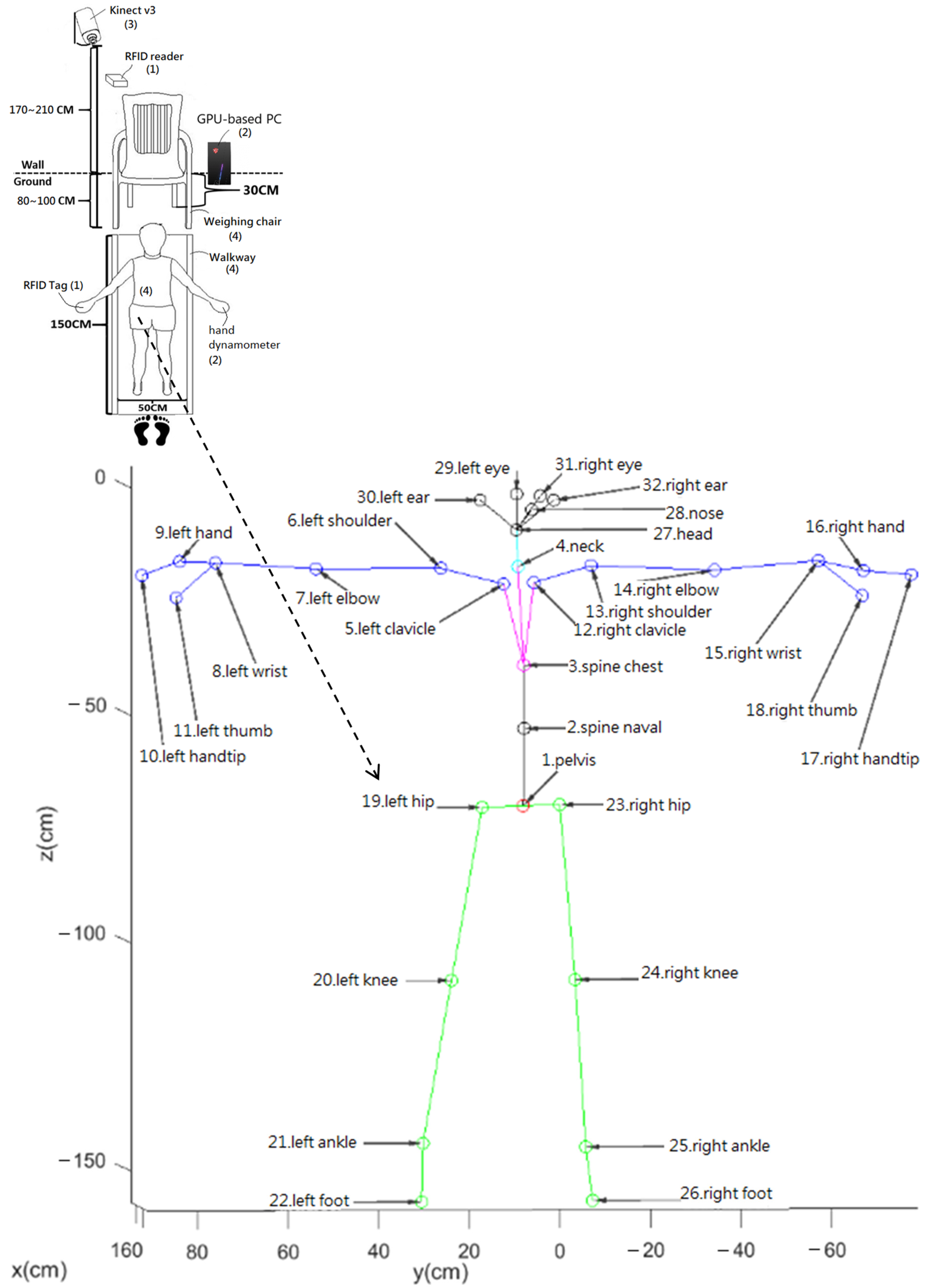
2.2. Study Procedures
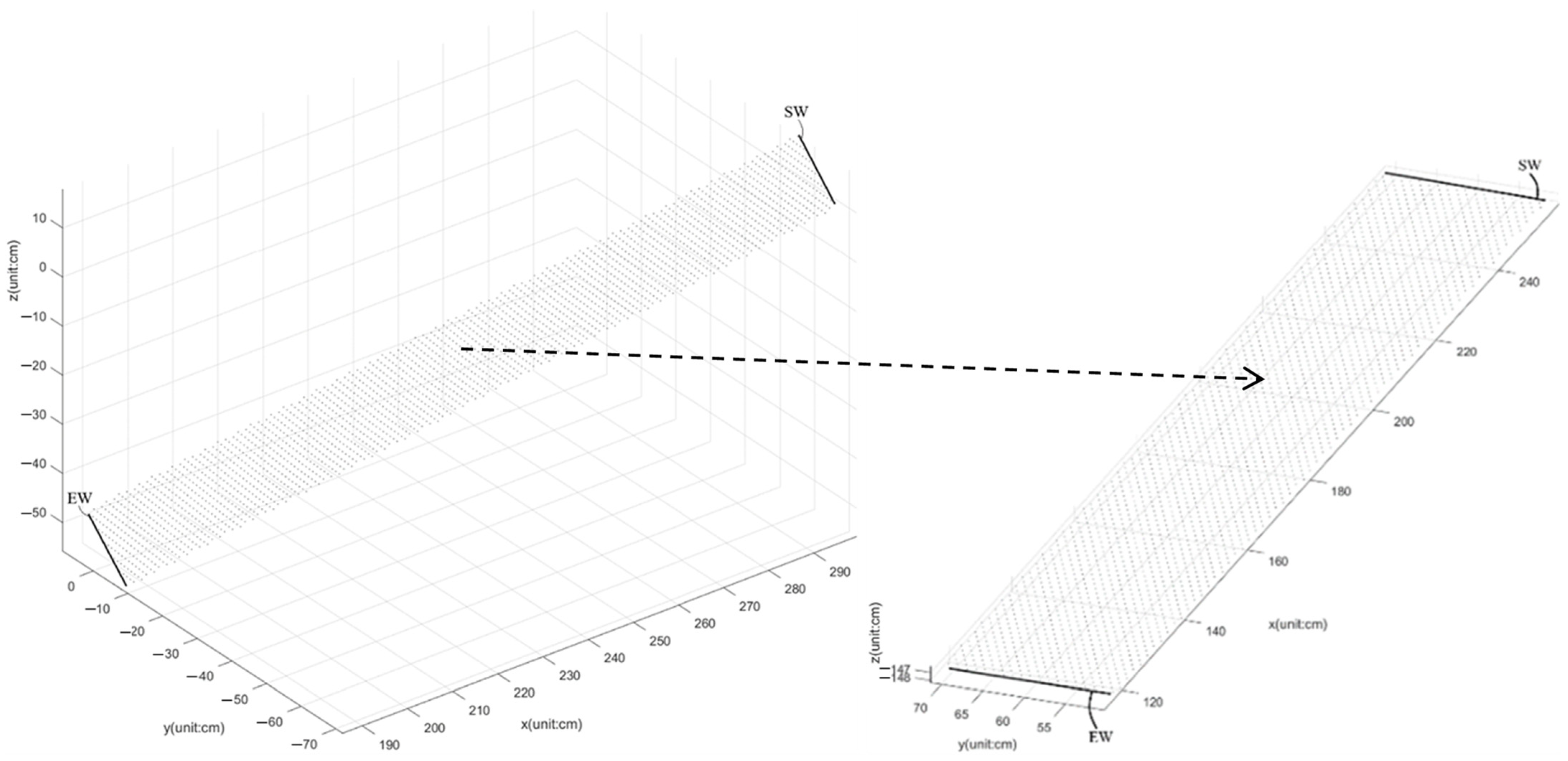
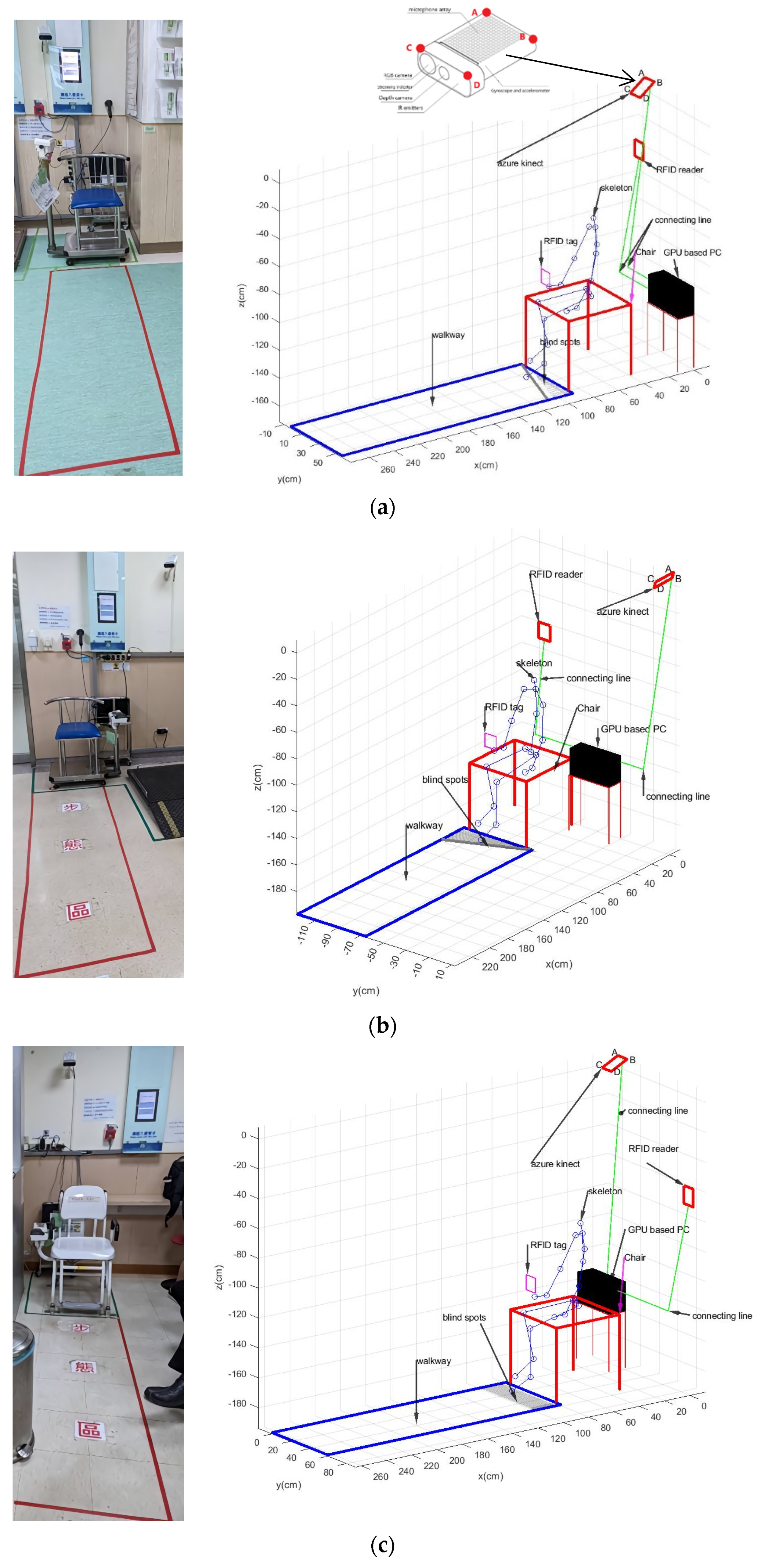
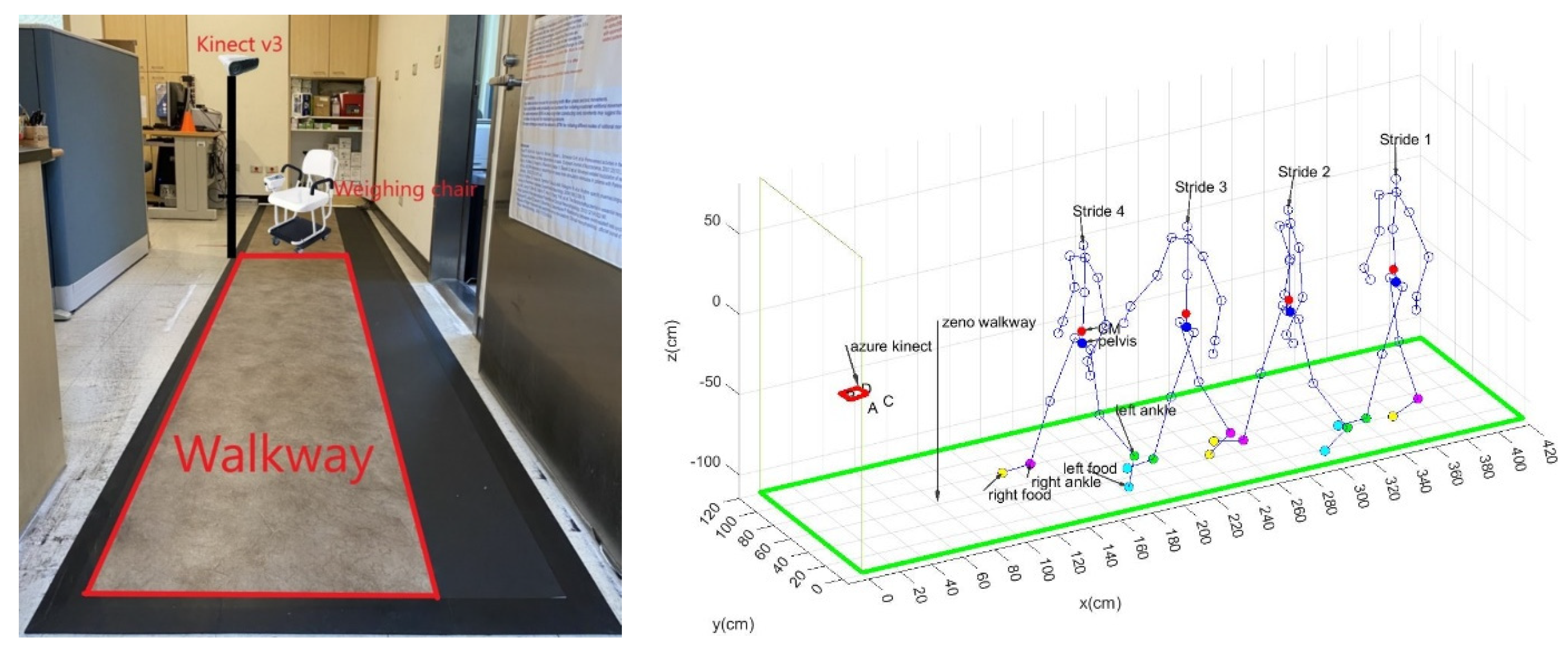
2.3. Calibration of Walkways
- Kinect v3 captures the skeleton frames of the participant walking from the starting point to the ending point on a short-distance 1.5 m walkway and goes to Step 2;
- The 2D grid coordinates are based on the coordinates contained in vectors x and y of the foot joints, which are in these skeleton frames. X is a matrix where each row is a copy of x, and Y is a matrix where each column is a copy of y. The grid represented by the coordinates X and Y has length (y) rows and length (x) columns. The grid size is 1 cm. Go to Step 3;
- Calculate z^ to fit a surface of the form z = f(x, y) to the scattered data in the vectors (x, y, z) of foot joints in these skeleton frames while the function f interpolates the surface at the query points specified by (X, Y) and returns the interpolated values, z^. The surface always passes through the data points defined by x and y. Go to Step 4;
- Compute the angle θy = atan((max(z) − min(z))/(max(x) − min(x))) · 80/π·sign(z(arg[min(x)]) − z(arg[max(x)]));
- 5.
- (b, a) ← the maximum difference of (No.22 (x, y)+ No.26 (x, y))/2. Go to Step 6;
- 6.
- θz = atan(a/b); z11 = cos(θz); z12 = −sin(θz); z21 = sin(θz); z22 = cos(θz);
2.4. Detection and Correction of Unreasonable Gait Movement
- If the height of the skeleton is <61 cm, then stop this algorithm or else, go to Step 2;
- If the length of skeleton frames >31, then a unit of sampling time = 0.04 s and a suitable moving window (units of sampling time × the range of ankle(x) cm) are used to find the longest segment (LS) of the skeleton frames (SF) and calculate the average pace (AP) = the length of pelvis(x) trajectory of LS/the duration of LS, then go to Step 3. Otherwise, stop this algorithm;
- Calculate Mm21 ← Mm(No.21(x)), Mm25 ← Mm(No.25(x)), a1 ← (Mm21 + Mm25)/2, go to Step 4;
- (b1 ← arg(a3)) ← (a3 ← max(a1)), go to Step 5;
- (b2 ← arg(a4)) ← (a4 ← min(a1)), go to Step 6;
- If (b1 > b2), then (c1 ← 0), (s1 ← b1), (a5 ← a1(b1)), go to Steps 7, 8, and 9;
- For i ∈ [b1,b2] do:
- If (a1(i) ≤ a5), then (a5 ← a1(i)), (s1 ← i), (c1 ← 0), else (c1 ← c1 + 1);
- If (c1 > 10), then break the for-loop, (a6 ← No.21(x([s1,b1]))), (a7 ← No.25(x([s1,b1])));
- Else (c1 ← 0), (s1 ← b1), (a5 ← a1(b1)), go to Steps 11, 12, and 13;
- For i ∈ [b1,b2] do:
- If (a1(i) ≤ a5), then (a5 ← a1(i)), (s1 ← i), (c1 ← 0), else (c1 ← c1 + 1);
- If (c1 > 10), then break the for-loop, (a6 ← No.21(x([b1,s1]))), (a7 ← No.25(x([b1,s1])));
- a2 ← a6-a7; a8 ← max{|diff(a6)|,|diff(a7)|};
- If a8 > 50, then stop this algorithm or else, go to Step 16;
- Calculate the maximum stride (MS), then stop this algorithm.
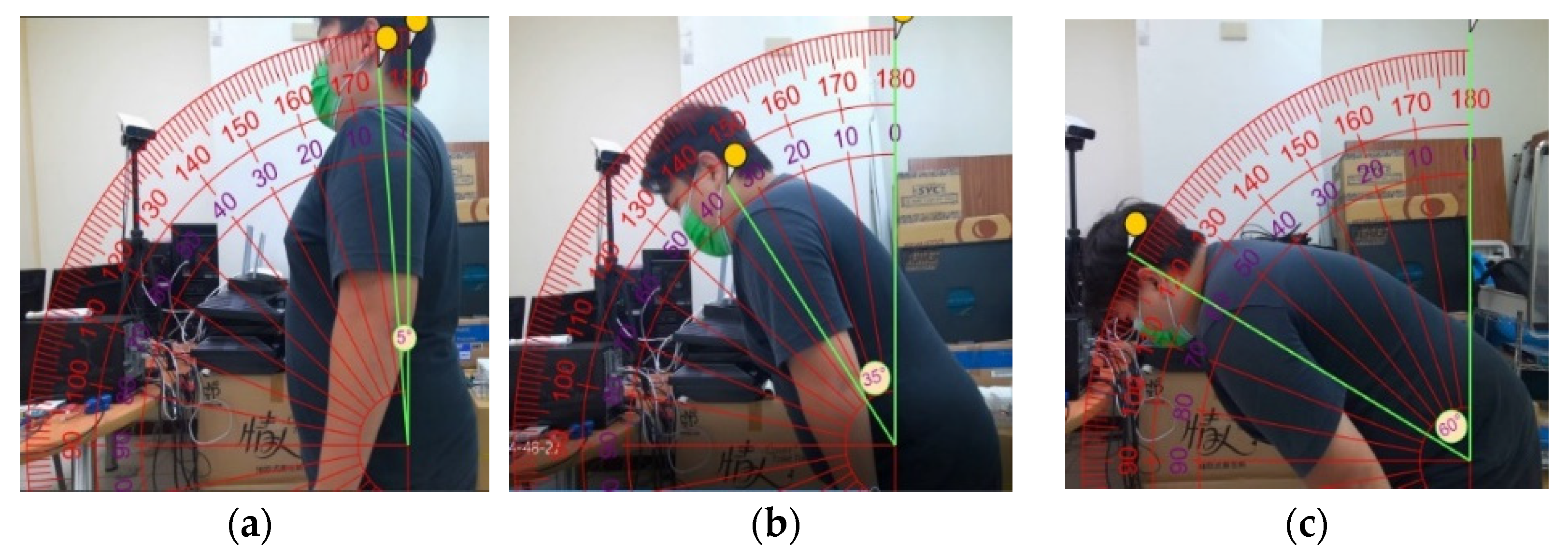
2.5. Calibration of the Forward Angle
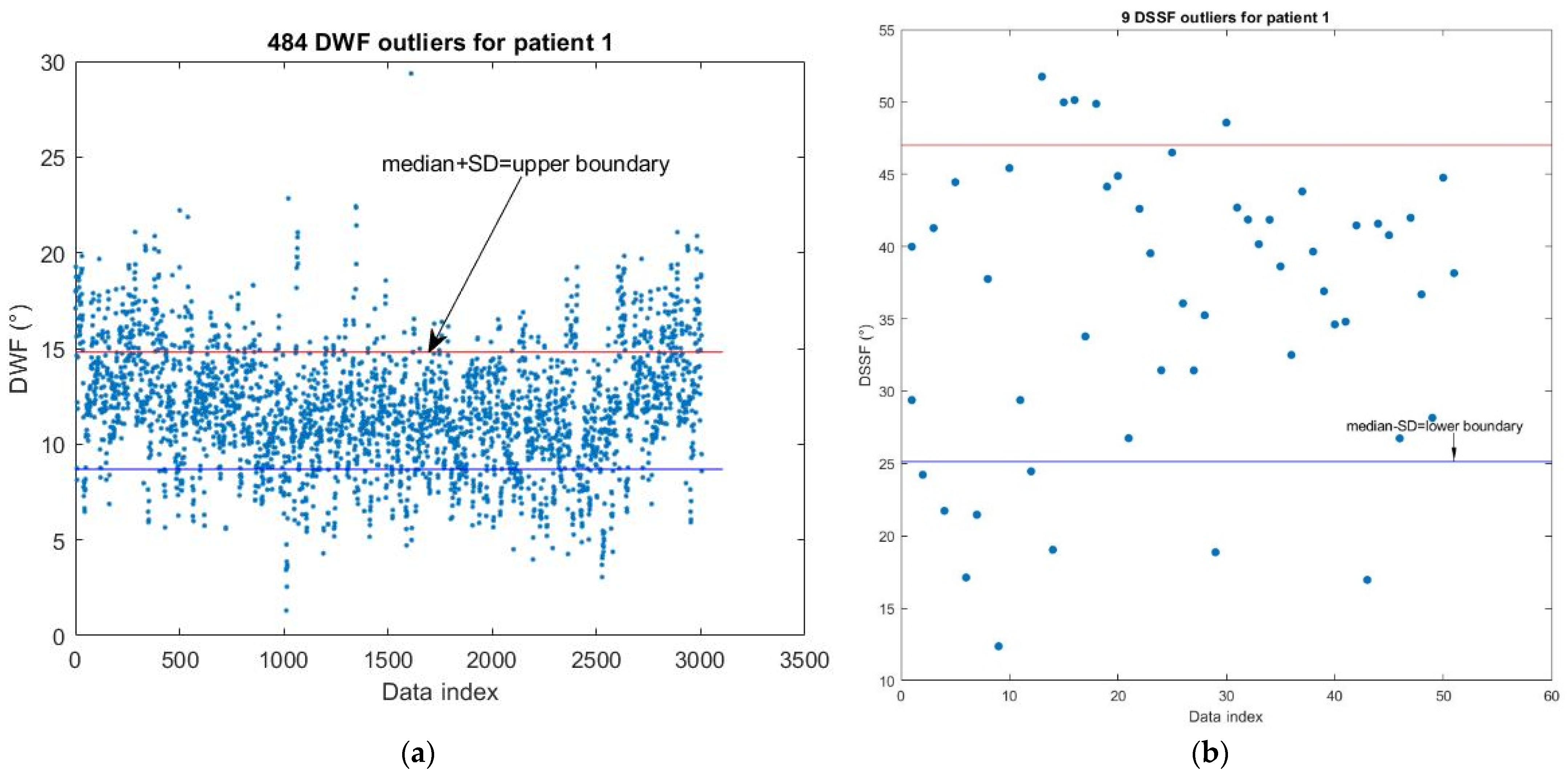
2.6. Detection of Personal DWF and DSSF Outliers
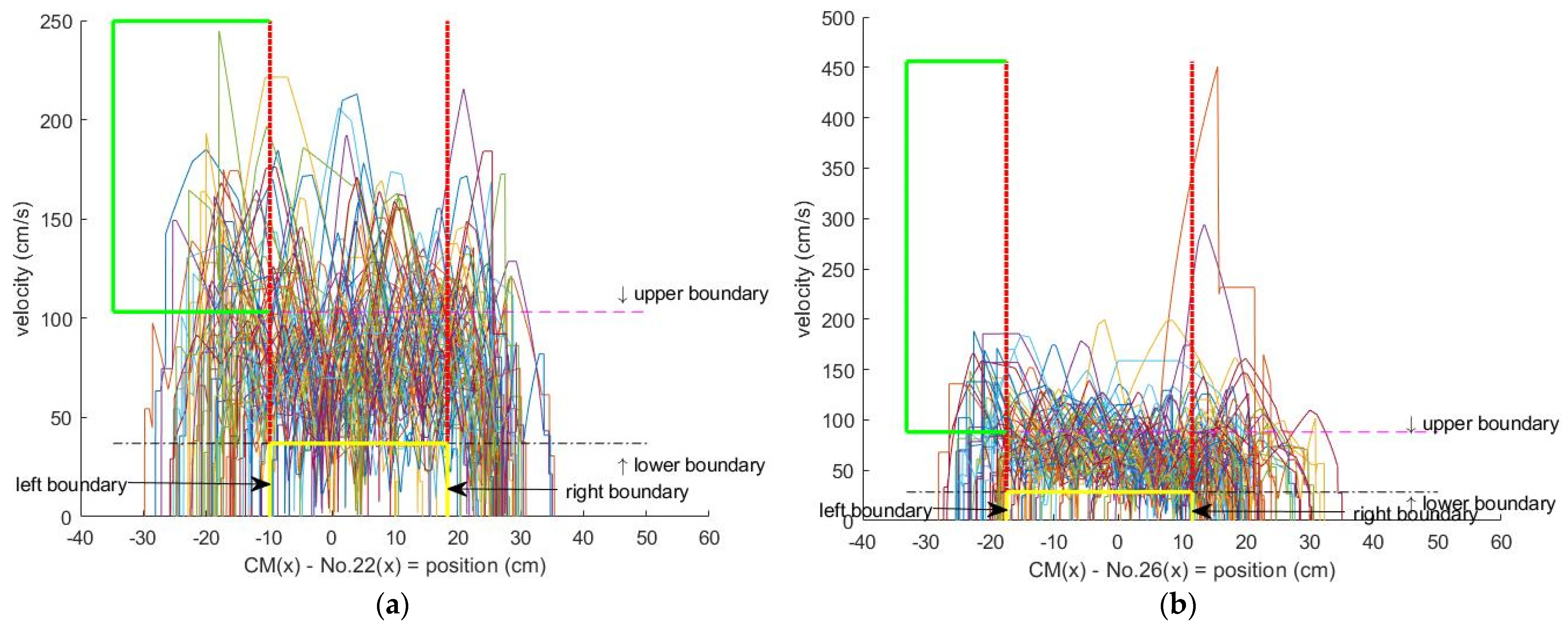
2.7. Data of Personal Unbalanced Gait
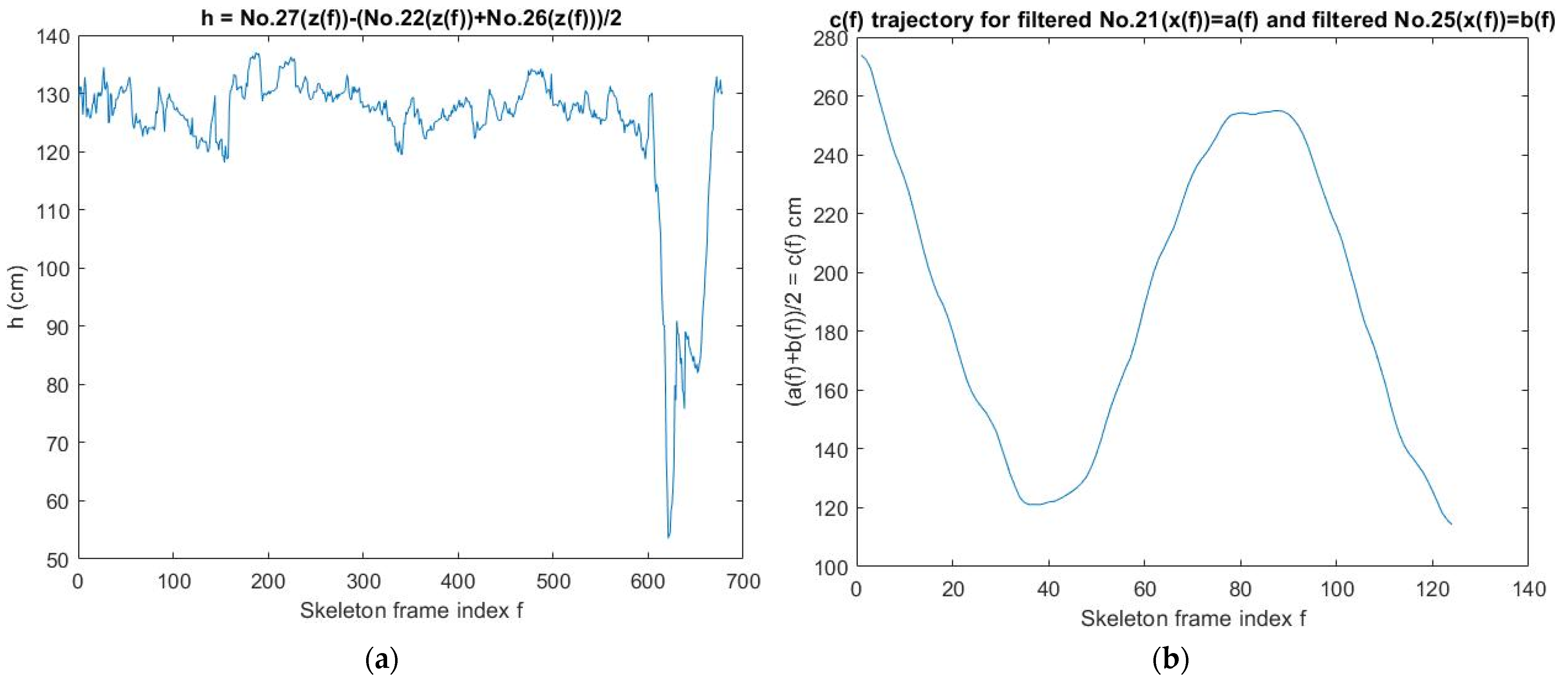
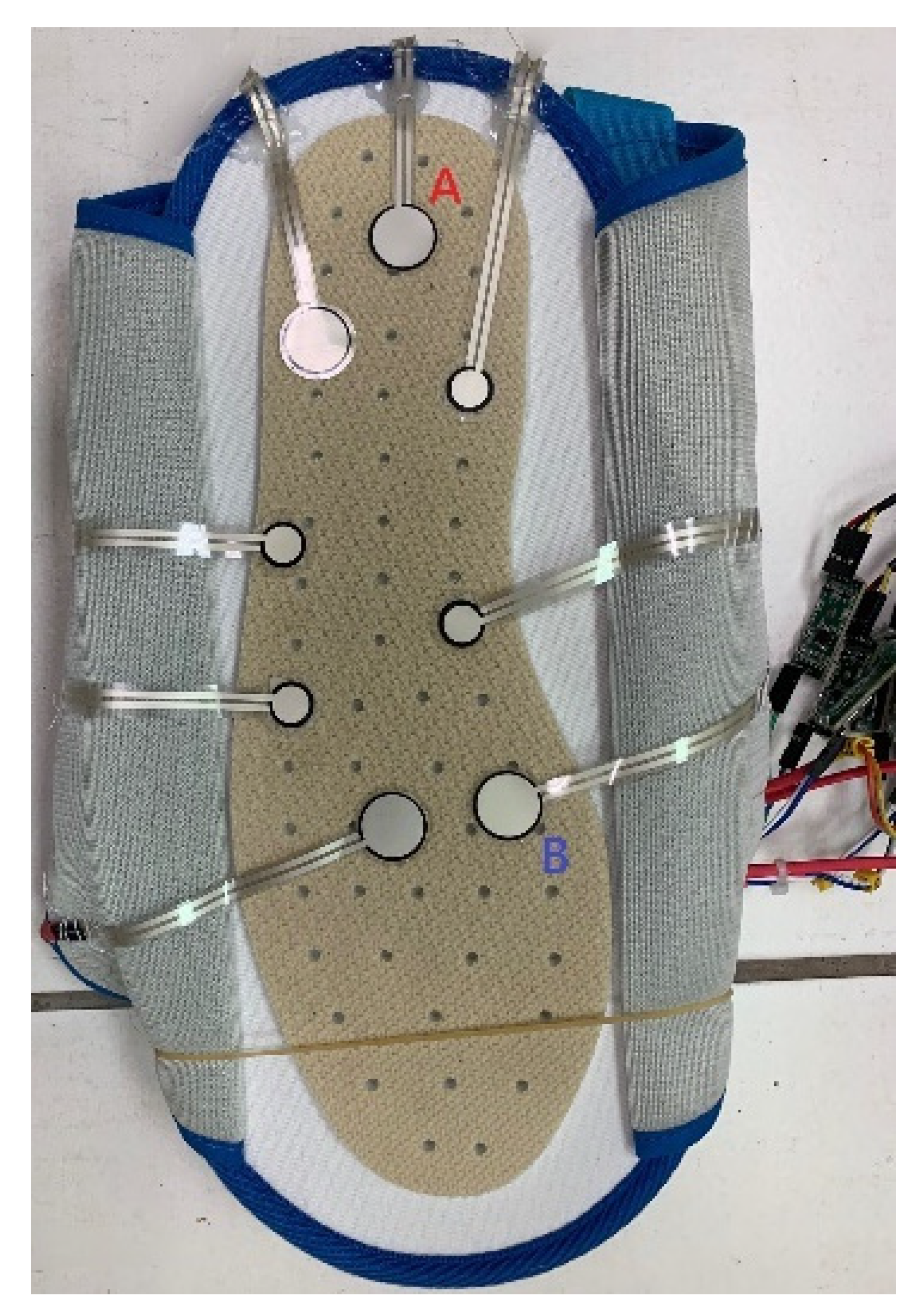
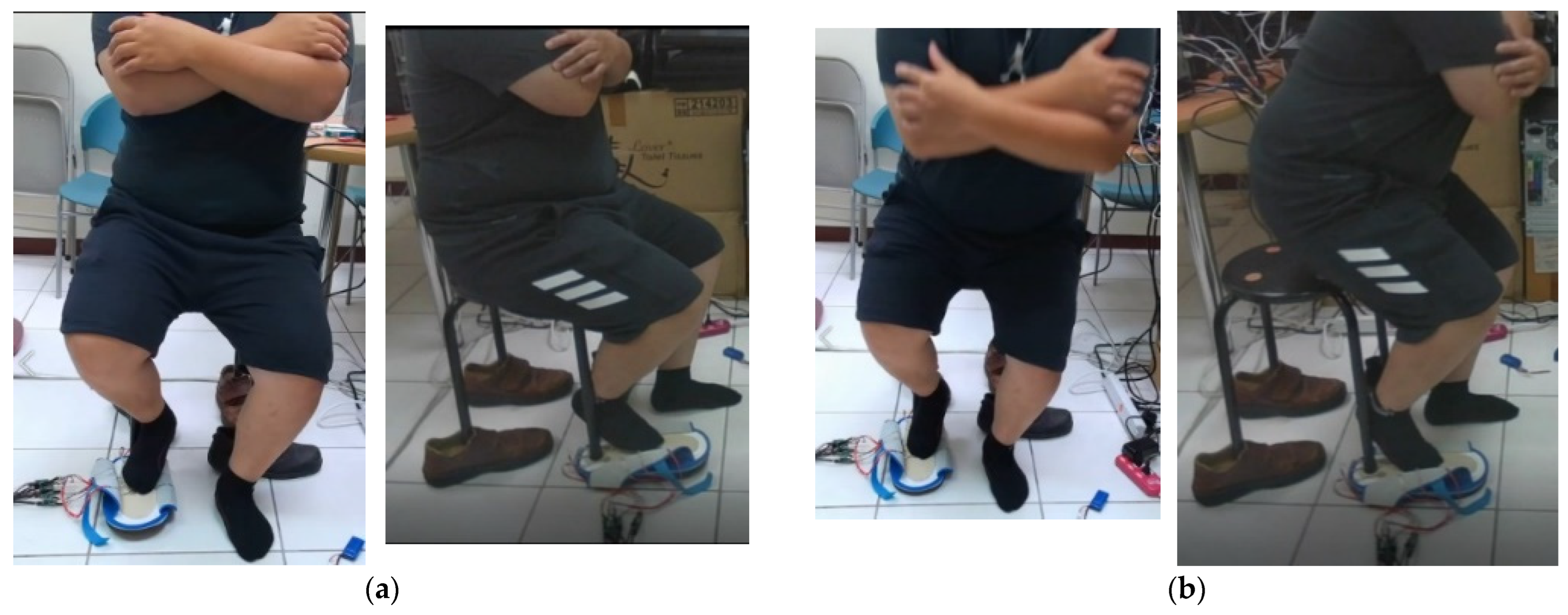
2.8. Calibration of the Sit-and-Stand Time (SST) Algorithm of K3S
- If the height of the skeleton is <61 cm, then stop this algorithm or else, go to Step 2;
- If the length of skeleton frames >31, then go to Step 3. Otherwise, stop this algorithm;
- If 0 < No.1(x) < 90 cm, then define that as the range of calculating SST. Go to Step 4.
- A unit of sampling time = 0.04 s and a suitable moving window (units of sampling time × the range of pelvis(z) cm) are used to find the longest segment of the skeleton frames (SF) to calculate the SST. Go to Step 5;
- If this segment of SF number <33, then stop this algorithm, or else, go to Step 6;
- Use the moving mean method (Mm) to filter pelvis(z) trajectory No. 1(z) of SF. Go to Step 7: Find local maxima points and local minima points of No. 1(z). Go to Step 8;
- If there are more than two neighboring local maxima points, then select the leftmost local maxima point to be reserved. Go to Step 9;
- If there are more than two neighboring local minima points, then reserve the rightmost local minima point t1, which has to satisfy the condition No. 1(z) of this point ≤ min(No. 1(z)) + 10 cm, and find its neighboring local maxima point t2. Go to Step 10;
- If there is only one local minima point and it is the initial point of No. 1(z), then find the reasonable corner point t1 from the range between the initial point and the neighbor local maxima point t2. Go to Step 11;
- Select the most reasonable data range No. 1(z*) that has the maximum difference of No. 1(x), which is at least 1.5 cm long. Go to Step 12;
- If the difference in No. 1(z*) > 6 cm, then SST≡t2 − t1; otherwise, SST does not exist.
3. Results
3.1. K3S Calibration Results
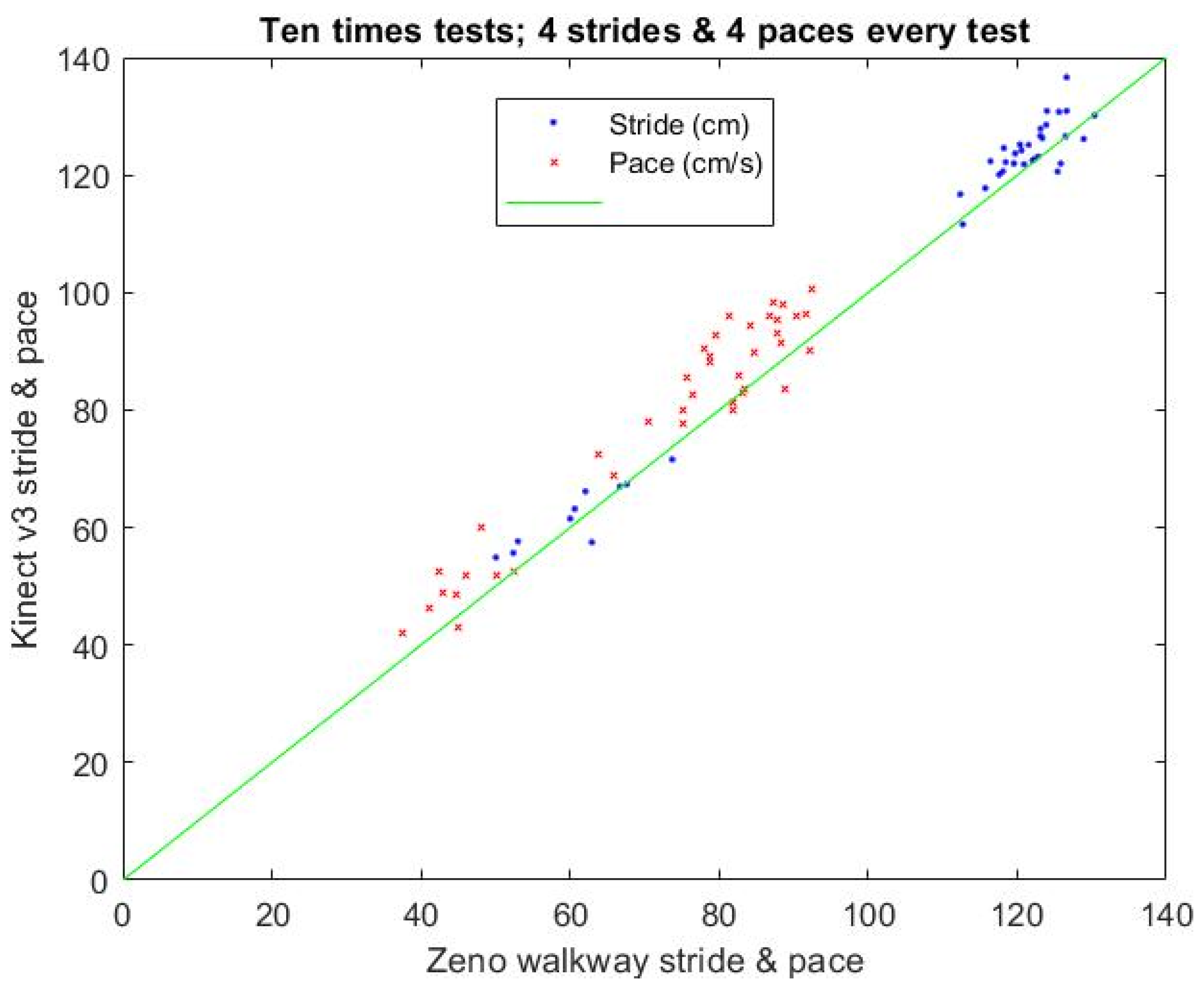
3.2. Calibration Results of the Stride and Pace
3.3. Stability Results of the Main Virtual Joints
3.4. Analysis of Reliability and Validity of Forward Angle
3.5. Analysis of Reliability and Validity of SST
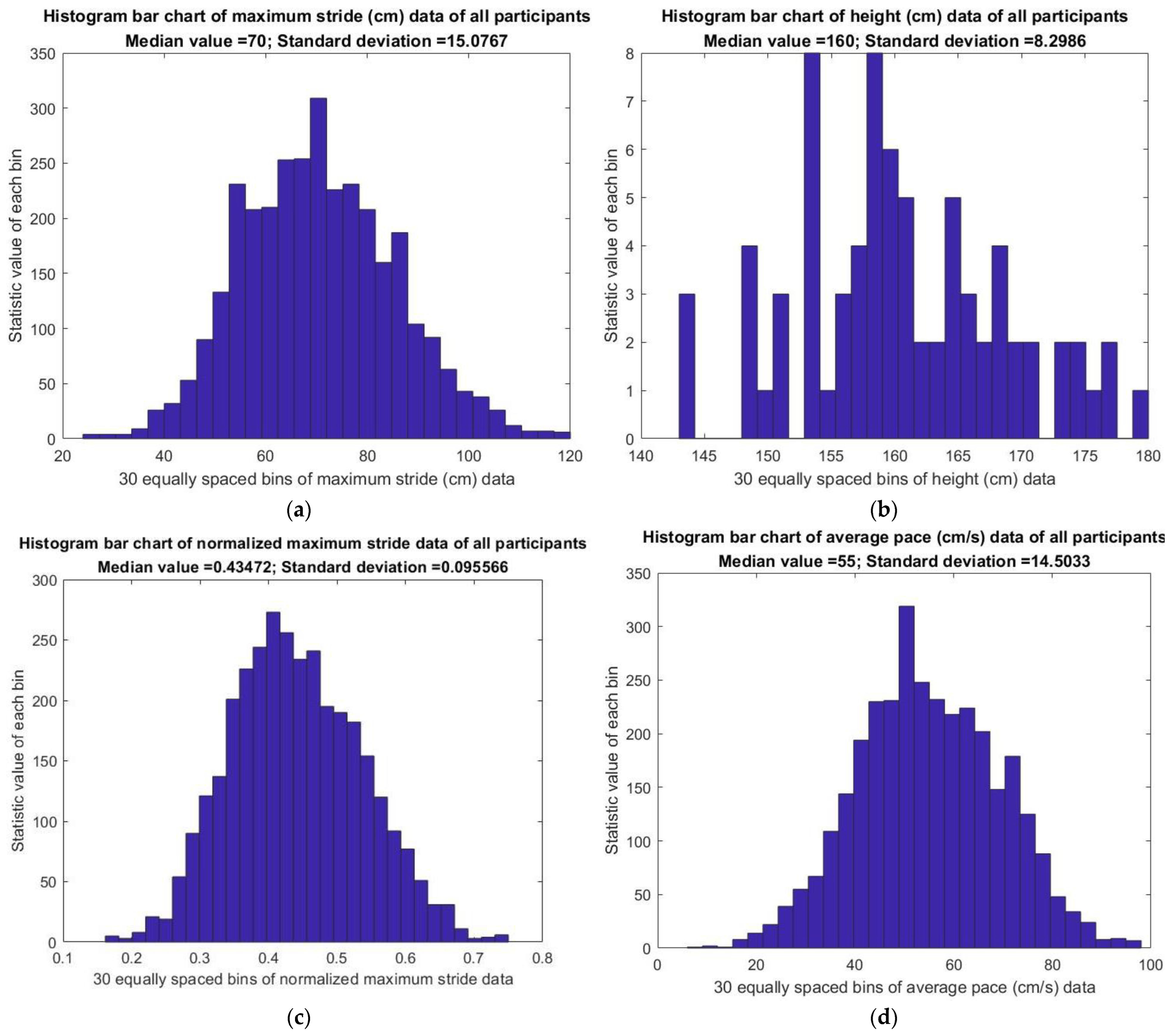
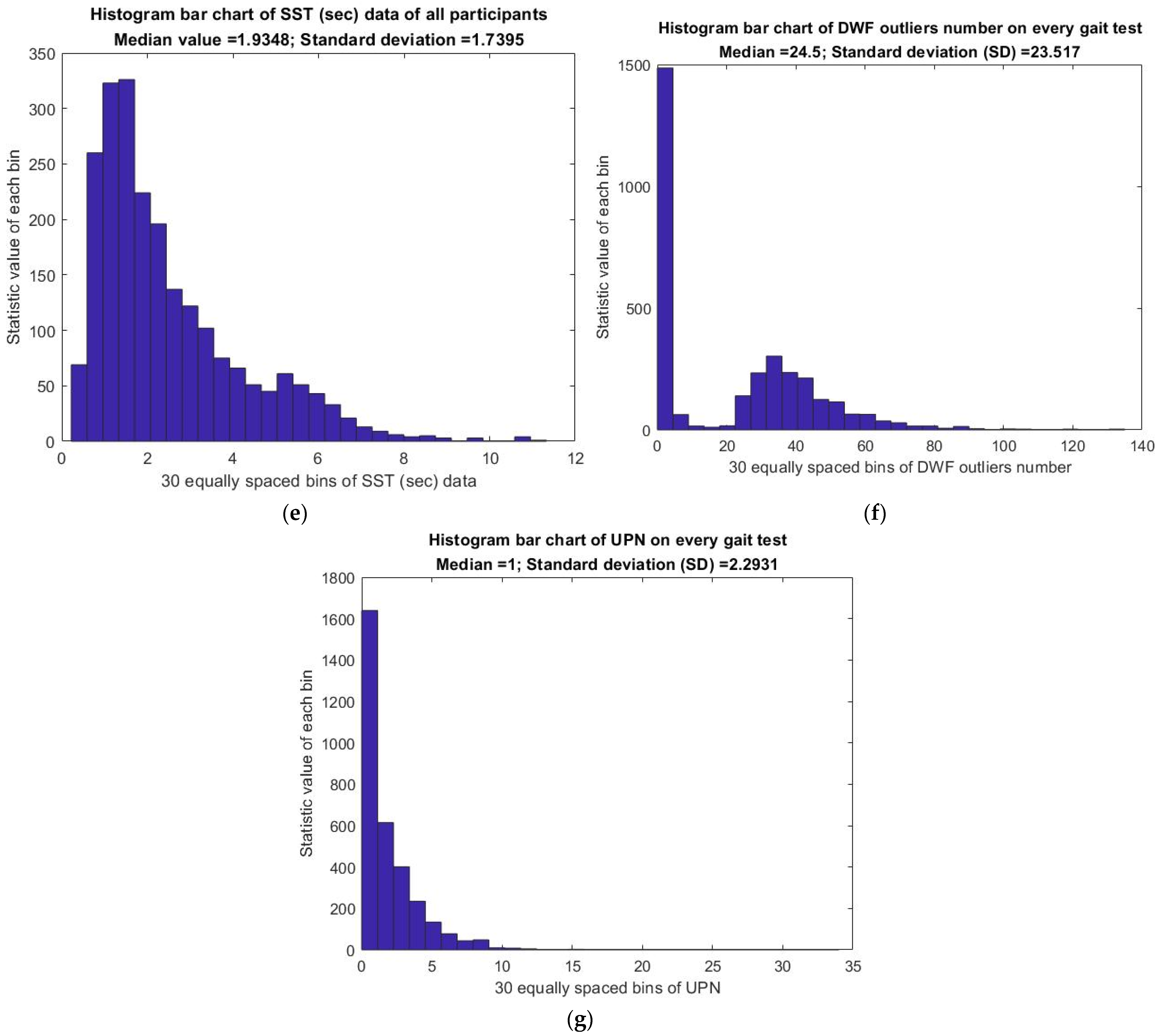
3.6. Analysis of Gait Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.K.; Leng, X.; Hsu, F.C.; Kritchevsky, S.B.; Ding, J.; Earnest, C.P.; Ferrucci, L.; Goodpaster, B.H.; Guralnik, J.M.; Lenchik, L.; et al. The impact of sarcopenia on a physical activity intervention: The Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P). J. Nutr. Health Aging 2014, 18, 59–64. [Google Scholar] [CrossRef]
- Zemp, D.D.; Giannini, O.; Quadri, P.; Rabuffetti, M.; Tettamanti, M.; de Bruin, E.D. Gait disorders in CKD patients: Muscle wasting or cognitive impairment? A cross-sectional pilot study to investigate gait signatures in Stage 1–5 CKD patients. BMC Nephrol. 2022, 23, 72. [Google Scholar]
- Kadi, F.A.; Yuniati, T.; Sribudiani, Y.; Rachmadi, D. The Association of rs187238, rs19465518 and rs1946519 IL-8 Polymorphisms with Acute Kidney Injury in Preterm Infants. BioMedicine 2021, 11, 43. [Google Scholar] [CrossRef]
- Kimura, A.; Paredes, W.; Pai, R.; Farooq, H.; Buttar, R.S.; Custodio, M.; Munugoti, S.; Kotwani, S.; Randhawa, L.S.; Dalezman, S.; et al. Step length and fall risk in adults with chronic kidney disease: A pilot study. BMC Nephrol. 2022, 23, 74. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.S.; Jung, S.W.; Hwang, H.S.; Moon, J.Y.; Jeong, K.H.; Lee, S.H.; Lee, S.Y.; Ko, G.J.; Lee, D.Y.; et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020, 21, 166. [Google Scholar] [CrossRef]
- Koren, M.J.; Blumen, H.M.; Ayers, E.I.; Verghese, J.; Abramowitz, M.K. Cognitive Dysfunction and Gait Abnormalities in CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef]
- Abramowitz, M.K.; Paredes, W.; Zhang, K.; Brightwell, C.R.; Newsom, J.N.; Kwon, H.J.; Custodio, M.; Buttar, R.S.; Farooq, H.; Zaidi, B.; et al. Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2018, 315, F1658–F1669. [Google Scholar] [CrossRef]
- Gage, J.R. Gait analysis. An essential tool in the treatment of cerebral palsy. Clin. Orthop. Relat. Res. 1993, 288, 126–134. [Google Scholar] [CrossRef]
- Hynes, A.; Czarnuch, S.; Kirkland, M.C.; Ploughman, M. Spatiotemporal gait measurement with a side-view depth sensor using human joint proposals. IEEE J. Biomed. Health Inform. 2020, 25, 1758–1769. [Google Scholar] [CrossRef]
- Kirkland, M.C.; Wallack, E.M.; Rancourt, S.N.; Ploughman, M. Comparing three dual-task methods and the relationship to physical and cognitive impairment in people with multiple sclerosis and controls. Mult. Scler. Int. 2015, 2015, 650645. [Google Scholar] [CrossRef]
- Prajapati, N.; Kaur, A.; Sethi, D. June, A Review on Clinical Gait Analysis. In Proceedings of the 2021 5th International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 3–5 June 2021; IEEE: Piscataway, NJ, USA, 2019; pp. 967–974. [Google Scholar]
- Lamine, H.; Bennour, S.; Laribi, M.; Romdhane, L.; Zaghloul, S. Evaluation of calibrated kinect gait kinematics using a vicon motion capture system. Comput. Methods Biomech. Biomed. Eng. 2017, 20 (Suppl. 1), S111–S112. [Google Scholar] [CrossRef] [PubMed]
- Pfister, A.; West, A.M.; Bronner, S.; Noah, J.A. Comparative abilities of Microsoft Kinect and Vicon 3D motion capture for gait analysis. J. Med. Eng. Technol. 2014, 38, 274–280. [Google Scholar] [CrossRef]
- Tamborini, R.; Novotny, E.; Prabhu, S.; Hofer, M.; Grall, C.; Klebig, B.; Bente, G. The effect of behavioral synchrony with black or white virtual agents on outgroup trust. Comput. Hum. Behav. 2018, 83, 176–183. [Google Scholar] [CrossRef]
- Huang, C.L.; Hsu, C.W.; Tsai, Z.R. Real-Time Human Body Motion Capturing System. J. Electron. Sci. Technol. 2017, 15, 115–122. [Google Scholar]
- Cho, A.B.; Otte, K.; Baskow, I.; Ehlen, F.; Maslahati, T.; Mansow-Model, S.; Schmitz-Hübsch, T.; Behnia, B.; Roepke, S. Motor signature of autism spectrum disorder in adults without intellectual impairment. Sci. Rep. 2022, 12, 7670. [Google Scholar] [CrossRef] [PubMed]
- Sabo, A. Vision-Based Assessment of Parkinsonism Severity in Gait. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2021. [Google Scholar]
- Zhang, H. Machine Learning Models for Accurate Ambulatory Gait Analysis with Instrumented Footwear. Ph.D. Thesis, Stevens Institute of Technology, Hoboken, NJ, USA, 2021. [Google Scholar]
- Shih, M.; Zhou, J.; Yang, Y.R.; Chen, C. Validation of an Adaptive Algorithm Used in Cost-Effective Kinect-Based System for Gait Analysis. Arch. Phys. Med. Rehabil. 2019, 100, e141–e142. [Google Scholar] [CrossRef]
- Gholami, F.; Trojan, D.A.; Kövecses, J.; Haddad, W.M.; Gholami, B. A microsoft kinect-based point-of-care gait assessment framework for multiple sclerosis patients. IEEE J. Biomed. Health Inform. 2016, 21, 1376–1385. [Google Scholar] [CrossRef]
- Hsieh, K.L.; Mirelman, A.; Shema-Shiratzky, S.; Galperin, I.; Regev, K.; Shen, S.; Schmitz-Hübsch, T.; Karni, A.; Paul, F.; Devos, H.; et al. A multi-modal virtual reality treadmill intervention for enhancing mobility and cognitive function in people with multiple sclerosis: Protocol for a randomized controlled trial. Contemp. Clin. Trials 2020, 97, 106122. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- Sharon, T.; Kurz, I.; Bernad-Elazari, H.; Shustak, S.; Galperin, I.; Giladi, N.; Mirelman, A.; Hausdorff, J.M.; Maidan, I. Which obstacle attributes place additional demands on higher-level cognitive function in patients with Parkinson’s disease? Park. Relat. Disord. 2020, 78, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Maidan, I.; Eyal, S.; Kurz, I.; Geffen, N.; Gazit, E.; Ravid, L.; Giladi, N.; Mirelman, A.; Hausdorff, J.M. Age-associated changes in obstacle negotiation strategies: Does size and timing matter? Gait Posture 2018, 59, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Maidan, I.; Shustak, S.; Sharon, T.; Bernad-Elazari, H.; Geffen, N.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. Prefrontal cortex activation during obstacle negotiation: What’s the effect size and timing? Brain Cogn. 2018, 122, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Craddock, I.; Tonkin, E.L.; Kinnunen, K.M.; McNaney, R.; Whitehouse, S.; Mirmehdi, M.; Heidarivincheh, F.; McConville, R.; Carey, J.; et al. Protocol for PD SENSORS: Parkinson’s Disease Symptom Evaluation in a Naturalistic Setting producing Outcome measuRes using SPHERE technology. An observational feasibility study of multi-modal multi-sensor technology to measure symptoms and activities of daily living in Parkinson’s disease. BMJ Open 2020, 10, e041303. [Google Scholar] [PubMed]
- Mahana, B. Does Use of a Dual Task Cognitive Game Based Treadmill Platform Improve Balance and Gait in Parkinson Disease? A Feasibility Study. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2019. [Google Scholar]
- Tölgyessy, M.; Dekan, M.; Chovanec, Ľ.; Hubinský, P. Evaluation of the azure Kinect and its comparison to Kinect V1 and Kinect V2. Sensors 2021, 21, 413. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.R.; Lien, C.Y.; Huang, C.C.; Lin, W.C.; Chen, Y.S.; Yu, C.C.; Cheng, B.C.; Kung, C.T.; Kung, C.F.; Chiang, Y.F.; et al. Clinical Disease Severity Mediates the Relationship between Stride Length and Speed and the Risk of Falling in Parkinson’s Disease. J. Pers. Med. 2022, 12, 192. [Google Scholar] [CrossRef]
- Pai, Y.C.; Patton, J. Center of mass velocity-position predictions for balance control. J. Biomech. 1997, 30, 347–354. [Google Scholar] [CrossRef]
- Mingxin, L.; Aiguo, S.; Yi, Y.; Yinghui, K. June, Intelligent Orthopedic Vest Based on Flexible Tactile Sensor. In Proceedings of the 2019 3rd International Conference on Circuits, System and Simulation (ICCSS), Nanjing, China, 13–15 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 33–36. [Google Scholar]




| Characteristics a | Total Patients = 75 |
|---|---|
| Age (years), median (IQR) | 68.0 (59.5~72.3) |
| Age < 55 years, no. (%) | 12 (16.0%) |
| 55 ≤ Age <65 years, no. (%) | 18 (24.0%) |
| 65 ≤ Age <70 years, no. (%) | 14 (18.7%) |
| Age ≥ 70 years, no. (%) | 31 (41.3%) |
| Male, no. (%) | 49 (65.3%) |
| Pre-test | |
| Total records, no. | 1461 |
| Frequency per patient b, median (IQR) | 13.0 (10.0~22.0) |
| Post-test | |
| Total records, no. | 1115 |
| Frequency per patient b, median (IQR) | 12.5 (8.0~20.5) |
| Ai | Joint No. | Bi | Joint No. | Di | Di Value |
| A1 | 27 | B1 | 4 | D1 | 47.10% |
| A2 | 4 | B2 | 3 | D2 | 48.55% |
| A3 | 3 | B3 | 1 | D3 | 57.55% |
| A4 | 23 | B4 | 24 | D4 | 55.25% |
| A5 | 19 | B5 | 20 | D5 | 55.25% |
| A6 | 24 | B6 | 25 | D6 | 59.10% |
| A7 | 20 | B7 | 21 | D7 | 59.10% |
| A8 | 25 | B8 | 26 | D8 | 52.75% |
| A9 | 21 | B9 | 22 | D9 | 52.75% |
| A10 | 13 | B10 | 14 | D10 | 56.15% |
| A11 | 6 | B11 | 7 | D11 | 56.15% |
| A12 | 14 | B12 | 15 | D12 | 64.25% |
| A13 | 7 | B13 | 8 | D13 | 64.25% |
| A14 | 15 | B14 | 16 | D14 | 53.15% |
| A15 | 8 | B15 | 9 | D15 | 53.15% |
| Link Name | Start Joint No. | End Joint No. | Gi | Ci | Ci Value |
| Head and neck | 27 | 4 | g1 | C1 | 8.41% |
| Upper trunk | 4 | 3 | g2 | C2 | 16.67% |
| Lower trunk | 3 | 1 | g3 | C3 | 27.35% |
| Thigh (right) | 23 | 24 | g4 | C4 | 14.14% |
| Thigh (left) | 19 | 20 | g5 | C5 | 14.14% |
| Shank (right) | 24 | 25 | g6 | C6 | 4.05% |
| Shank (left) | 20 | 21 | g7 | C7 | 4.05% |
| Foot (right) | 25 | 26 | g8 | C8 | 1.36% |
| Foot (left) | 21 | 22 | g9 | C9 | 1.36% |
| Upper arm (right) | 13 | 14 | g10 | C10 | 2.54% |
| Upper arm (left) | 6 | 7 | g11 | C11 | 2.54% |
| Forearm (right) | 14 | 15 | g12 | C12 | 1.19% |
| Forearm (left) | 7 | 8 | g13 | C13 | 1.19% |
| Hand (right) | 15 | 16 | g14 | C14 | 1.06% |
| Hand (left) | 8 | 9 | g15 | C15 | 1.06% |
| Parameters | Maximum Stride (cm) Total (N = 2566 Records) | Average Pace (cm/s) Total (N = 2566 Records) | SST (s) Total (N = 2566 Records) |
|---|---|---|---|
| Mean ± SD | 87.9 ± 16.3 | 56.0 ± 17.4 | 4.1 ± 3.6 |
| Fifth and 95th percentage | |||
| Median (P5, P95), cm | 86.7 (63.7, 116.1) | 56.7 (27.6, 83.4) | 3.2 (1.2, 10.2) |
| No. of records ≤P5 a | 130 | 130 | 129 |
| No. of records ≥P95 a | 132 | 132 | 129 |
| First and 99th percentage | |||
| Median (P1, P99), cm | 86.7 (48.1, 129.9) | 56.7 (3.9, 94.2) | 3.2 (0.8, 17.6) |
| No. of records ≤P1 a | 26 | 27 | 26 |
| No. of records ≥P99 a | 26 | 26 | 26 |
| Characteristics | Total Records a | Parameters ≤P5 or ≥P95 Records (%) b | Parameters ≤P1 or ≥P99 Records (%) b |
|---|---|---|---|
| Total | 2566 | 858 (33.3%) | 192 (7.5%) |
| Dialysis month | |||
| November 2021 | 21 | 10 (47.6%) | 1 (4.8%) |
| December 2021 | 178 | 51 (28.7%) | 16 (9.0%) |
| January 2022 | 262 | 89 (34.0%) | 11 (4.2%) |
| February 2022 | 243 | 64 (26.3%) | 12 (4.9%) |
| March 2022 | 299 | 102 (34.1%) | 26 (8.7%) |
| April 2022 | 845 | 289 (34.2%) | 65 (7.7%) |
| May 2022 | 718 | 253 (35.2%) | 61 (8.5%) |
| Dialysis section | |||
| Morning | 1538 | 483 (31.4%) | 108 (7.0%) |
| Afternoon | 1028 | 375 (36.5%) | 84 (8.2%) |
| Characteristics | Pre-Test | Post-Test | p Value a |
|---|---|---|---|
| (Total Records = 1454) | (Total Records = 1112) | ||
| Maximum stride (cm) | <0.001 | ||
| Median (IRQ) | 88.8 (79.7, 99.8) | 83.5 (74.3, 96.1) | |
| Mean (SD) | 90.2 (15.7) | 85.0 (16.7) | |
| Min | 29.3 | 24.5 | |
| Max | 146.3 | 138.2 | |
| Average pace (cm/s) | 0.409 | ||
| Median (IRQ) | 57.1 (45.0, 68.1) | 55.8 (44.7, 67.5) | |
| Mean (SD) | 56.2 (17.8) | 55.7 (16.9) | |
| Min | 0 | 0 | |
| Max | 116.8 | 102.7 | |
| SST (s) | <0.001 | ||
| Median (IRQ) | 2.8 (1.9, 4.5) | 3.6 (2.3, 5.7) | |
| Mean (SD) | 3.8 (3.4) | 4.6 (3.7) | |
| Min | 0.1 | 0.1 | |
| Max | 42.4 | 59.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Z.-R.; Kuo, C.-C.; Wang, C.-J.; Tsai, J.J.P.; Chou, H.-H. Validation of Gait Measurements on Short-Distance Walkways Using Azure Kinect DK in Patients Receiving Chronic Hemodialysis. J. Pers. Med. 2023, 13, 1181. https://doi.org/10.3390/jpm13071181
Tsai Z-R, Kuo C-C, Wang C-J, Tsai JJP, Chou H-H. Validation of Gait Measurements on Short-Distance Walkways Using Azure Kinect DK in Patients Receiving Chronic Hemodialysis. Journal of Personalized Medicine. 2023; 13(7):1181. https://doi.org/10.3390/jpm13071181
Chicago/Turabian StyleTsai, Zhi-Ren, Chin-Chi Kuo, Cheng-Jui Wang, Jeffrey J. P. Tsai, and Hsin-Hsu Chou. 2023. "Validation of Gait Measurements on Short-Distance Walkways Using Azure Kinect DK in Patients Receiving Chronic Hemodialysis" Journal of Personalized Medicine 13, no. 7: 1181. https://doi.org/10.3390/jpm13071181
APA StyleTsai, Z.-R., Kuo, C.-C., Wang, C.-J., Tsai, J. J. P., & Chou, H.-H. (2023). Validation of Gait Measurements on Short-Distance Walkways Using Azure Kinect DK in Patients Receiving Chronic Hemodialysis. Journal of Personalized Medicine, 13(7), 1181. https://doi.org/10.3390/jpm13071181






