mTOR Inhibition via Low-Dose, Pulsed Rapamycin with Intraovarian Condensed Platelet Cytokines: An Individualized Protocol to Recover Diminished Reserve?
Abstract
1. Introduction
2. PRP and Its Cytokine Constituents
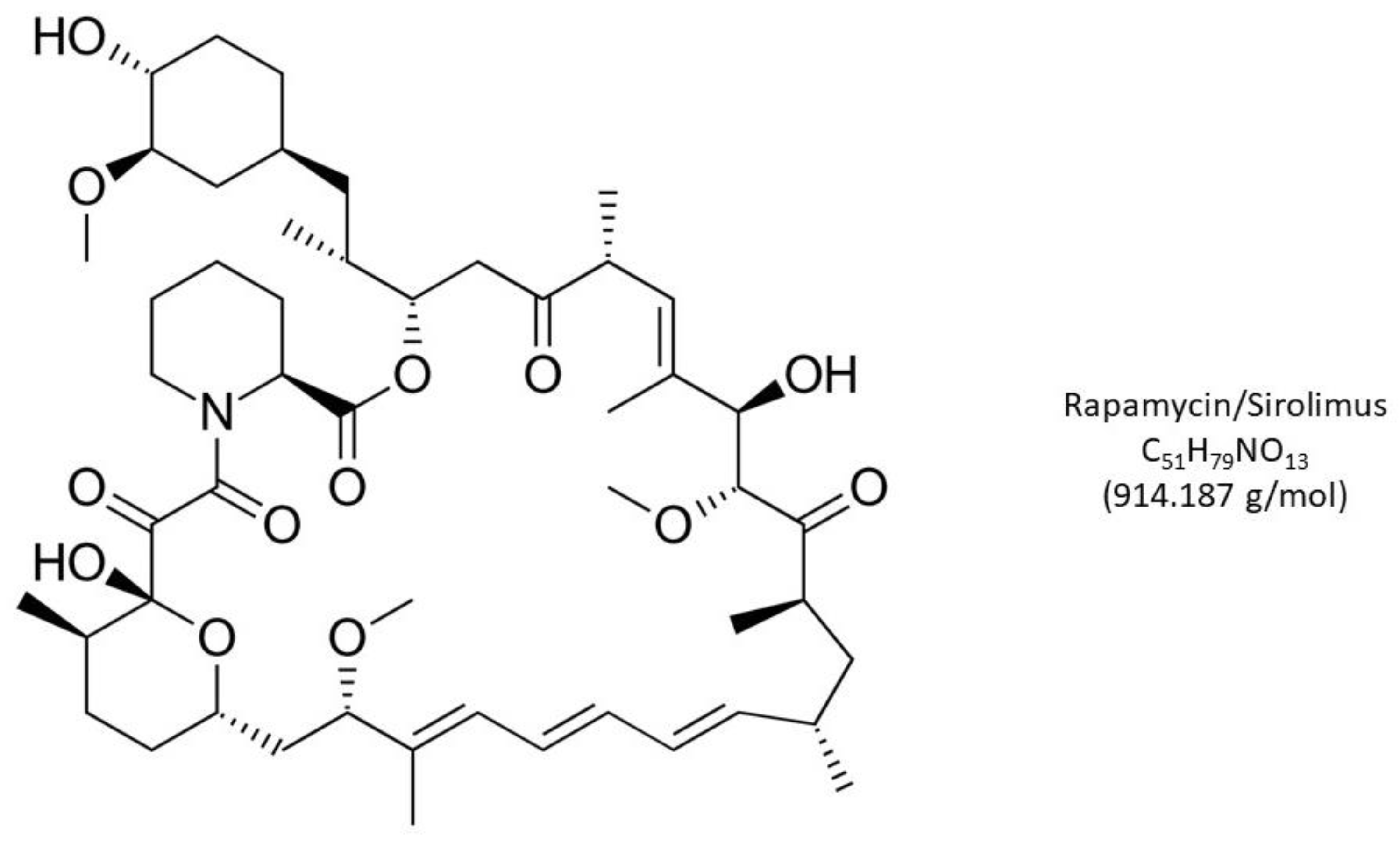
3. Rapamycin, mTOR and Reproductive Biology
4. PLT Cytokine Augmentation by Rapamycin?
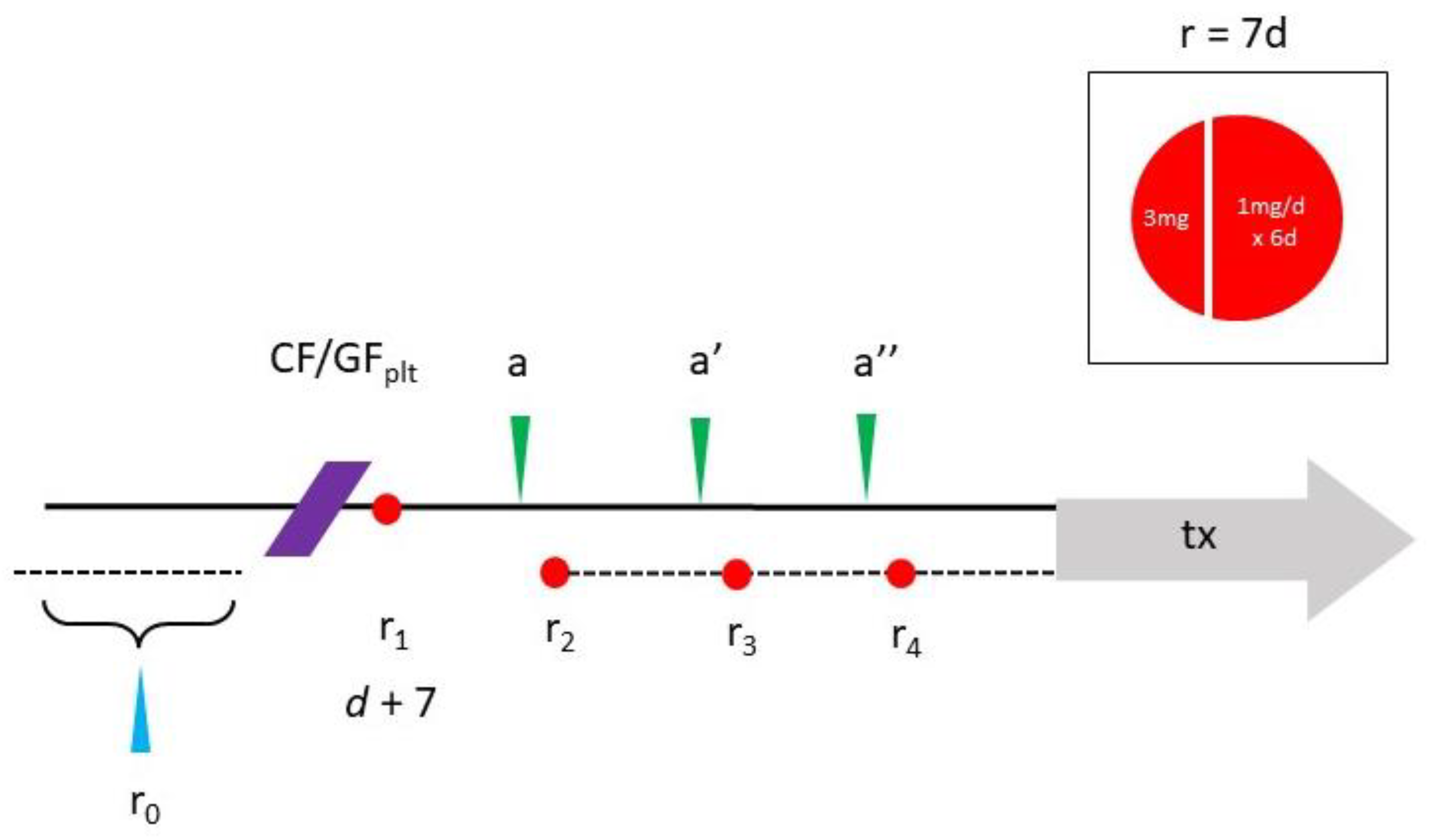
5. Rapamycin—Scheduling and Toxicity Issues
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kallen, A.; Polotsky, A.J.; Johnson, J. Untapped Reserves: Controlling Primordial Follicle Growth Activation. Trends Mol. Med. 2018, 24, 319–331. [Google Scholar] [CrossRef]
- Ford, E.A.; Beckett, E.L.; Roman, S.; McLaughlin, E.A.; Sutherland, J. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2020, 159, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; La, X.; Feng, H.L. Comparison of different stimulation protocols used in in vitro fertilization: A review. Ann. Transl. Med. 2015, 3, 137. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.; Nath, N.; Chimote, N.; Chimote, M.; Chimote, N. Follicular fluid insulin like growth factor-1 (FF IGF-1) is a biochemical marker of embryo quality and implantation rates in in vitro fertilization cycles. J. Hum. Reprod. Sci. 2013, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Quan, X.; Lan, Y.; Zhao, M.; Tian, X.; Yang, X. Gonadotropin versus Follicle-Stimulating Hormone for Ovarian Response in Patients Undergoing in vitro Fertilization: A Retrospective Cohort Comparison. Curr. Ther. Res. 2019, 92, 100572. [Google Scholar] [CrossRef]
- Banu, J.; Tarique, M.; Jahan, N.; Lasker, N.; Sultana, N.; Alamgir, C.F.; Darmoni, M.; Munira, S. Efficacy of autologous platelet rich plasma for ovarian rejuvenation in infertile women having poor ovarian reserve. Int. J. Reprod. Contracept. Obstet. Gynecol. 2022, 11, 2948–2953. [Google Scholar] [CrossRef]
- Cakiroglu, Y.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Korun, Z.E.U.; Herlihy, N.; Scott, R.T.; Tiras, B.; Seli, E. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging 2022, 14, 2513–2523. [Google Scholar] [CrossRef]
- Herlihy, N.S.; Seli, E. The use of intraovarian injection of autologous platelet rich plasma (PRP) in patients with poor ovarian response and premature ovarian insufficiency. Curr. Opin. Obstet. Gynecol. 2022, 34, 133–137. [Google Scholar] [CrossRef]
- Grosbois, J.; Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum. Reprod. 2018, 33, 1705–1714. [Google Scholar] [CrossRef]
- Gareis, N.; Huber, E.; Hein, G.; Rodríguez, F.; Salvetti, N.; Angeli, E.; Ortega, H.; Rey, F. Impaired insulin signaling pathways affect ovarian steroidogenesis in cows with COD. Anim. Reprod. Sci. 2020, 192, 298–312. [Google Scholar] [CrossRef]
- Papageorgiou, K.; Mastora, E.; Zikopoulos, A.; Grigoriou, M.E.; Georgiou, I.; Michaelidis, T.M. Interplay Between mTOR and Hippo Signaling in the Ovary: Clinical Choice Guidance Between Different Gonadotropin Preparations for Better IVF. Front. Endocrinol. 2021, 12, 702446. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Zubova, S.G.; Bukreeva, E.I.; Pospelov, V.A.; Pospelova, T.V.; Blagosklonny, M.V. Rapamycin decelerates cellular senescence. Cell Cycle 2009, 8, 1888–1895. [Google Scholar] [CrossRef]
- Hambright, W.S.; Philippon, M.J.; Huard, J. Rapamycin for aging stem cells. Aging 2020, 12, 15184–15185. [Google Scholar] [CrossRef] [PubMed]
- Shindyapina, A.V.; Cho, Y.; Kaya, A.; Tyshkovskiy, A.; Castro, J.P.; Deik, A.; Gordevicius, J.; Poganik, J.R.; Clish, C.B.; Horvath, S.; et al. Rapamycin treatment during development extends life span and health span of male mice and Daphnia magna. Sci. Adv. 2022, 8, eabo5482. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.A.J.; Janssens, G.E.; Goddijn, M.; Hamer, G.; Houtkooper, R.H.; Mastenbroek, S. Longevity pathways are associated with human ovarian ageing. Hum. Reprod. Open 2021, 2021, hoab020. [Google Scholar] [CrossRef] [PubMed]
- Rosenwaks, Z.; Veeck, L.L.; Liu, H.-C. Pregnancy following transfer of in vitro fertilized donated oocytes. Fertil. Steril. 1986, 45, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Khadivi, F.; Koruji, M.; Akbari, M.; Jabari, A.; Talebi, A.; Movassagh, S.A.; Boroujeni, A.P.; Feizollahi, N.; Nikmahzar, A.; Pourahmadi, M.; et al. Application of platelet-rich plasma (PRP) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems. Acta Histochem. 2020, 122, 151627. [Google Scholar] [CrossRef]
- Parte, S.C.; Bhartiya, D.; Telang, J.; Daithankar, V.; Salvi, V.; Zaveri, K.; Hinduja, I.; Sánchez-Maldonado, B.; Galicia, M.d.L.; Rojo, C.; et al. Detection, Characterization, and Spontaneous Differentiation In Vitro of Very Small Embryonic-Like Putative Stem Cells in Adult Mammalian Ovary. Stem Cells Dev. 2011, 20, 1451–1464. [Google Scholar] [CrossRef]
- Bhartiya, D.; Singh, P.; Sharma, D.; Kaushik, A. Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues. Stem Cell Rev. Rep. 2022, 18, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Skutella, T. Stem cells in aged mammalian ovaries. Aging 2010, 2, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lu, Z.; Zhu, Q.; Ma, L.; Xue, L.; Li, Y.; Zhou, S.; Yan, W.; Ye, W.; Zhang, J.; et al. DDX04+ Stem Cells in the Ovaries of Postmenopausal Women: Existence and Differentiation Potential. Stem Cells 2022, 40, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.C.; Tilly, J.L. Revisiting Claims of the Continued Absence of Functional Germline Stem Cells in Adult Ovaries. Stem Cells 2023, 41, 200–204. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, X.; Peng, Y.; Zhang, C.; Liu, W.; Yang, W.; Dou, X.; Jiang, Y.; Wang, Y.; Yang, S.; et al. Macrophage-derived extracellular vesicles regulate follicular activation and improve ovarian function in old mice by modulating local environment. Clin. Transl. Med. 2022, 12, e1071. [Google Scholar] [CrossRef]
- Cruz-Guilloty, F.; Saeed, A.M.; Echegaray, J.J.; Duffort, S.; Ballmick, A.; Tan, Y.; Betancourt, M.; Viteri, E.; Ramkhellawan, G.C.; Ewald, E.; et al. Infiltration of Proinflammatory M1 Macrophages into the Outer Retina Precedes Damage in a Mouse Model of Age-Related Macular Degeneration. Int. J. Inflamm. 2013, 2013, 503725. [Google Scholar] [CrossRef]
- Peltier, J.; O’Neill, A.; Schaffer, D.V. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev. Neurobiol. 2007, 67, 1348–1361. [Google Scholar] [CrossRef]
- Ojeda, L.; Gao, J.; Hooten, K.G.; Wang, E.; Thonhoff, J.R.; Dunn, T.J.; Gao, T.; Wu, P. Critical Role of PI3K/Akt/GSK3β in Motoneuron Specification from Human Neural Stem Cells in Response to FGF2 and EGF. PLoS ONE 2011, 6, e23414. [Google Scholar] [CrossRef]
- Marck, R.E.; Gardien, K.L.M.; Vlig, M.; Breederveld, R.S.; Middelkoop, E. Growth Factor Quantification of Platelet-Rich Plasma in Burn Patients Compared to Matched Healthy Volunteers. Int. J. Mol. Sci. 2019, 20, 288. [Google Scholar] [CrossRef]
- Pantos, K.; Nitsos, N.; Kokkali, G.; Vaxevanoglou, T.; Markomichali, C.; Pantou, A.; Grammatis, M.; Lazaros, L.; Sfakianoudis, K. Ovarian Rejuvenation and Folliculogenesis Reactivation in Peri-Menopausal Women after Autologous Platelet-Rich Plasma Treatment (Abstract). In Proceedings of the ESHRE 32nd Annual Meeting, Helsinki, Finland, 3–6 July 2016. [Google Scholar]
- Vézina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975, 28, 721–726. [Google Scholar] [CrossRef]
- Chung, J.; Kuo, C.J.; Crabtree, G.R.; Blenis, J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 1992, 69, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for Cell Cycle Arrest by the Immunosuppressant Rapamycin in Yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M.; Erdjument-Bromage, H.; Lui, M.; Tempst, P.; Snyder, S.H. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 1994, 78, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Śledź, K.M.; Moore, S.F.; Durrant, T.N.; Blair, T.A.; Hunter, R.W.; Hers, I. Rapamycin restrains platelet procoagulant responses via FKBP-mediated protection of mitochondrial integrity. Biochem. Pharmacol. 2020, 177, 113975. [Google Scholar] [CrossRef]
- Meng, D.; Frank, A.R.; Jewell, J.L. mTOR signaling in stem and progenitor cells. Development 2018, 145, dev152595. [Google Scholar] [CrossRef]
- Murakami, M.; Ichisaka, T.; Maeda, M.; Oshiro, N.; Hara, K.; Edenhofer, F.; Kiyama, H.; Yonezawa, K.; Yamanaka, S. mTOR Is Essential for Growth and Proliferation in Early Mouse Embryos and Embryonic Stem Cells. Mol. Cell. Biol. 2004, 24, 6710–6718. [Google Scholar] [CrossRef]
- Alhasan, B.A.; Gordeev, S.A.; Knyazeva, A.R.; Aleksandrova, K.V.; Margulis, B.A.; Guzhova, I.V.; Suvorova, I.I. The mTOR Pathway in Pluripotent Stem Cells: Lessons for Understanding Cancer Cell Dormancy. Membranes 2021, 11, 858. [Google Scholar] [CrossRef]
- Garbern, J.C.; Helman, A.; Sereda, R.; Sarikhani, M.; Ahmed, A.; Escalante, G.O.; Ogurlu, R.; Kim, S.L.; Zimmerman, J.F.; Cho, A.; et al. Inhibition of mTOR Signaling Enhances Maturation of Cardiomyocytes Derived From Human-Induced Pluripotent Stem Cells via p53-Induced Quiescence. Circulation 2020, 141, 285–300. [Google Scholar] [CrossRef]
- Takayama, K.; Kawakami, Y.; Lavasani, M.; Mu, X.; Cummins, J.H.; Yurube, T.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Robbins, P.D.; et al. mTOR signaling plays a critical role in the defects observed in muscle-derived stem/progenitor cells isolated from a murine model of accelerated aging. J. Orthop. Res. 2017, 35, 1375–1382. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.A.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Hambright, W.S.; Takayama, K.; Mu, X.; Lu, A.; Cummins, J.H.; Matsumoto, T.; Yurube, T.; Kuroda, R.; Kurosaka, M.; et al. Rapamycin Rescues Age-Related Changes in Muscle-Derived Stem/Progenitor Cells from Progeroid Mice. Mol. Ther.-Methods Clin. Dev. 2019, 14, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zeng, X.; Wang, W.; Wang, Z.; Zhang, Y.; Wang, Y. Protective effect of rapamycin on endothelial-to-mesenchymal transition in HUVECs through the Notch signaling pathway. Vasc. Pharmacol. 2019, 113, 20–26. [Google Scholar] [CrossRef]
- Gordon, L.B.; Norris, W.; Hamren, S.; Goodson, R.; LeClair, J.; Massaro, J.; Lyass, A.; D’agostino, R.B.; Tuminelli, K.; Kieran, M.W.; et al. Plasma Progerin in Patients With Hutchinson-Gilford Progeria Syndrome: Immunoassay Development and Clinical Evaluation. Circulation 2023, 147, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Mosevitsky, M.I. Progerin and Its role in accelerated and natural aging. Mol. Biol. 2022, 56, 181–205. [Google Scholar] [CrossRef]
- Neupane, B.; Pradhan, K.; Ortega-Ramirez, A.M.; Aidery, P.; Kucikas, V.; Marks, M.; van Zandvoort, M.A.M.J.; Klingel, K.; Witte, K.K.; Gründer, S.; et al. Personalized Medicine Approach in a DCM Patient with LMNA Mutation Reveals Dysregulation of mTOR Signaling. J. Pers. Med. 2022, 12, 1149. [Google Scholar] [CrossRef]
- Palozzi, J.M.; Hurd, T.R. The role of programmed mitophagy in germline mitochondrial DNA quality control. Autophagy 2023, 1–2. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Kim, E.; Beemiller, P.; Wang, C.-Y.; Swanson, J.; You, M.; Guan, K.-L. Bnip3 Mediates the Hypoxia-induced Inhibition on Mammalian Target of Rapamycin by Interacting with Rheb. J. Biol. Chem. 2007, 282, 35803–35813. [Google Scholar] [CrossRef]
- Bar, D.Z.; Charar, C.; Dorfman, J.; Yadid, T.; Tafforeau, L.; Lafontaine, D.L.J.; Gruenbaum, Y. Cell size and fat content of dietary-restricted Caenorhabditis elegans are regulated by ATX-2, an mTOR repressor. Proc. Natl. Acad. Sci. USA 2016, 113, E4620–E4629. [Google Scholar] [CrossRef]
- Silva, E.; Rosario, F.J.; Powell, T.L.; Jansson, T. Mechanistic Target of Rapamycin Is a Novel Molecular Mechanism Linking Folate Availability and Cell Function. J. Nutr. 2017, 147, 1237–1242. [Google Scholar] [CrossRef]
- Park, H.; Heo, G.; Yang, S.; Koo, D. Rapamycin encourages the maintenance of mitochondrial dynamic balance and mitophagy activity for improving developmental competence of blastocysts in porcine embryos in vitro. Mol. Reprod. Dev. 2023, 90, 236–247. [Google Scholar] [CrossRef]
- Yang, Q.; Xi, Q.; Wang, M.; Liu, J.; Li, Z.; Hu, J.; Jin, L.; Zhu, L. Rapamycin improves the developmental competence of human oocytes by alleviating DNA damage during IVM. Hum. Reprod. Open 2022, 2022, hoac050. [Google Scholar] [CrossRef] [PubMed]
- Allan, S. Seeing mTOR in a new light. Nat. Rev. Immunol. 2008, 8, 904. [Google Scholar] [CrossRef]
- Sills, E.S.; Wood, S.H. Epigenetics, ovarian cell plasticity, and platelet-rich plasma: Mechanistic theories. Reprod. Fertil. 2022, 3, C44–C51. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.-H.; Lim, K.S.; Park, D.S.; Shim, J.-W.; Lee, S.-Y.; Jang, E.-J.; Park, J.-K.; Kim, J.-H.; Jeong, M.H. Sirolimus coating on heparinized stents prevents restenosis and thrombosis. J. Biomater. Appl. 2017, 31, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Babinska, A.; Markell, M.S.; Salifu, M.O.; Akoad, M.; Ehrlich, Y.H.; Kornecki, E. Enhancement of human platelet aggregation and secretion induced by rapamycin. Nephrol. Dial. Transplant. 1998, 13, 3153–3159. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, K.-S.; Chen, M.; Huang, D.-J. Rapamycin enhances platelet aggregation induced by adenosine diphosphatein vitro. Platelets 2009, 20, 428–431. [Google Scholar] [CrossRef]
- López, E.; Berna-Erro, A.; Bermejo, N.; Brull, J.M.; Martinez, R.; Pino, G.G.; Alvarado, R.; Salido, G.M.; Rosado, J.A.; Cubero, J.J.; et al. Long-term mTOR inhibitors administration evokes altered calcium homeostasis and platelet dysfunction in kidney transplant patients. J. Cell. Mol. Med. 2013, 17, 636–647. [Google Scholar] [CrossRef]
- Sills, E.S.; Rickers, N.S.; Svid, C.S.; Rickers, J.M.; Wood, S.H. Normalized Ploidy Following 20 Consecutive Blastocysts with Chromosomal Error: Healthy 46, XY Pregnancy with IVF after Intraovarian Injection of Autologous Enriched Platelet-derived Growth Factors. Int. J. Mol. Cell. Med. 2019, 8, 84–90. [Google Scholar] [CrossRef]
- Goldman, K.N.; Chenette, D.; Arju, R.; Duncan, F.E.; Keefe, D.L.; Grifo, J.A.; Schneider, R.J. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, 3186–3191. [Google Scholar] [CrossRef]
- Escobar, K.A.; Cole, N.H.; Mermier, C.M.; VanDusseldorp, T.A. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell 2019, 18, e12876. [Google Scholar] [CrossRef]
- Lamming, D.W.; Ye, L.; Sabatini, D.M.; Baur, J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Investig. 2013, 123, 980–989. [Google Scholar] [CrossRef]
- Mannick, J.B.; Del Giudice, G.; Lattanzi, M.; Valiante, N.M.; Praestgaard, J.; Huang, B.; Lonetto, M.A.; Maecker, H.T.; Kovarik, J.; Carson, S.; et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014, 6, 268ra179. [Google Scholar] [CrossRef]
- Ceschi, A.; Heistermann, E.; Gros, S.; Reichert, C.; Kupferschmidt, H.; Banner, N.R.; Krähenbühl, S.; Taegtmeyer, A.B. Acute Sirolimus Overdose: A Multicenter Case Series. PLoS ONE 2015, 10, e0128033. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Rapamycin for longevity: Opinion article. Aging 2019, 11, 8048–8067. [Google Scholar] [CrossRef]
- Foster, D.A.; Toschi, A. Targeting mTOR with rapamycin: One dose does not fit all. Cell Cycle 2009, 8, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Sills, E.S.; Wood, S.H.; Walsh, A.P. Intraovarian condensed platelet cytokines for infertility and menopause—Mirage or miracle? Biochimie 2023, 204, 41–47. [Google Scholar] [CrossRef]
- Kennedy, B.; Lamming, D.W. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef]
- Mayo Clinic Health Information (Drugs & Supplements)—Sirolimus DRG-20068199. Mayo Foundation for Medical Education & Research, 1 March 2023. Available online: https://www.mayoclinic.org/drugs-supplements/sirolimus-oral-route/proper-use/drg-20068199 (accessed on 15 May 2023).
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov (accessed on 15 May 2023).
- Sills, E.S.; Wood, S.H. Progress in human ovarian rejuvenation: Current platelet-rich plasma and condensed cytokine research activity by scope and international origin. Clin. Exp. Reprod. Med. 2021, 48, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M. Rapamycin and ageing: When, for how long, and how much? J. Genet. Genomics 2014, 41, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Fasting and rapamycin: Diabetes versus benevolent glucose intolerance. Cell Death Dis. 2019, 10, 607. [Google Scholar] [CrossRef]
- Atkinson, L.; Martin, F.; Sturmey, R.G. Intraovarian injection of platelet-rich plasma in assisted reproduction: Too much too soon? Hum. Reprod. 2021, 36, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Sills, E.S. Ovarian recovery via autologous platelet-rich plasma: New benchmarks for condensed cytokine applications to reverse reproductive aging. Aging Med. 2022, 5, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Garavelas, A.; Mallis, P.; Michalopoulos, E.; Nikitos, E. Clinical Benefit of Autologous Platelet-Rich Plasma Infusion in Ovarian Function Rejuvenation: Evidence from a Before-After Prospective Pilot Study. Medicines 2023, 10, 19. [Google Scholar] [CrossRef]
- Papapanou, M.; Syristatidi, K.; Gazouli, M.; Eleftheriades, M.; Vlahos, N.; Siristatidis, C. The Effect of Stimulation Protocols (GnRH Agonist vs. Antagonist) on the Activity of mTOR and Hippo Pathways of Ovarian Granulosa Cells and Its Potential Correlation with the Outcomes of In Vitro Fertilization: A Hypothesis. J. Clin. Med. 2022, 11, 6131. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sills, E.S.; Harrity, C.; Wood, S.H.; Tan, S.L. mTOR Inhibition via Low-Dose, Pulsed Rapamycin with Intraovarian Condensed Platelet Cytokines: An Individualized Protocol to Recover Diminished Reserve? J. Pers. Med. 2023, 13, 1147. https://doi.org/10.3390/jpm13071147
Sills ES, Harrity C, Wood SH, Tan SL. mTOR Inhibition via Low-Dose, Pulsed Rapamycin with Intraovarian Condensed Platelet Cytokines: An Individualized Protocol to Recover Diminished Reserve? Journal of Personalized Medicine. 2023; 13(7):1147. https://doi.org/10.3390/jpm13071147
Chicago/Turabian StyleSills, E. Scott, Conor Harrity, Samuel H. Wood, and Seang Lin Tan. 2023. "mTOR Inhibition via Low-Dose, Pulsed Rapamycin with Intraovarian Condensed Platelet Cytokines: An Individualized Protocol to Recover Diminished Reserve?" Journal of Personalized Medicine 13, no. 7: 1147. https://doi.org/10.3390/jpm13071147
APA StyleSills, E. S., Harrity, C., Wood, S. H., & Tan, S. L. (2023). mTOR Inhibition via Low-Dose, Pulsed Rapamycin with Intraovarian Condensed Platelet Cytokines: An Individualized Protocol to Recover Diminished Reserve? Journal of Personalized Medicine, 13(7), 1147. https://doi.org/10.3390/jpm13071147






