Assessment of Pain in Osteoarthritis of the Knee
Abstract
1. Introduction
1.1. Epidemiology, Burden, and Impact of Osteoarthritis
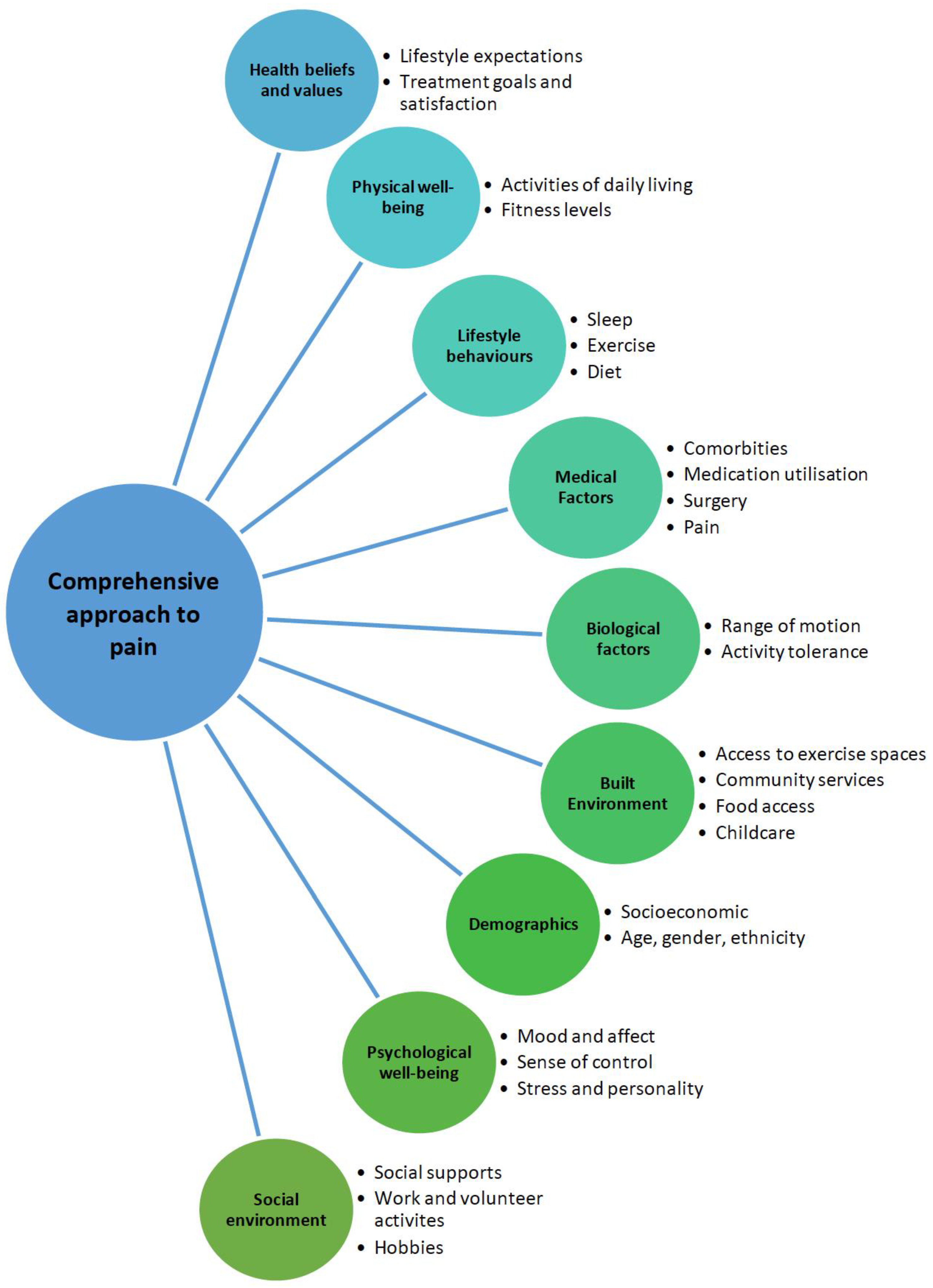
1.2. Holistic Assessment of the Person with Osteoarthritis
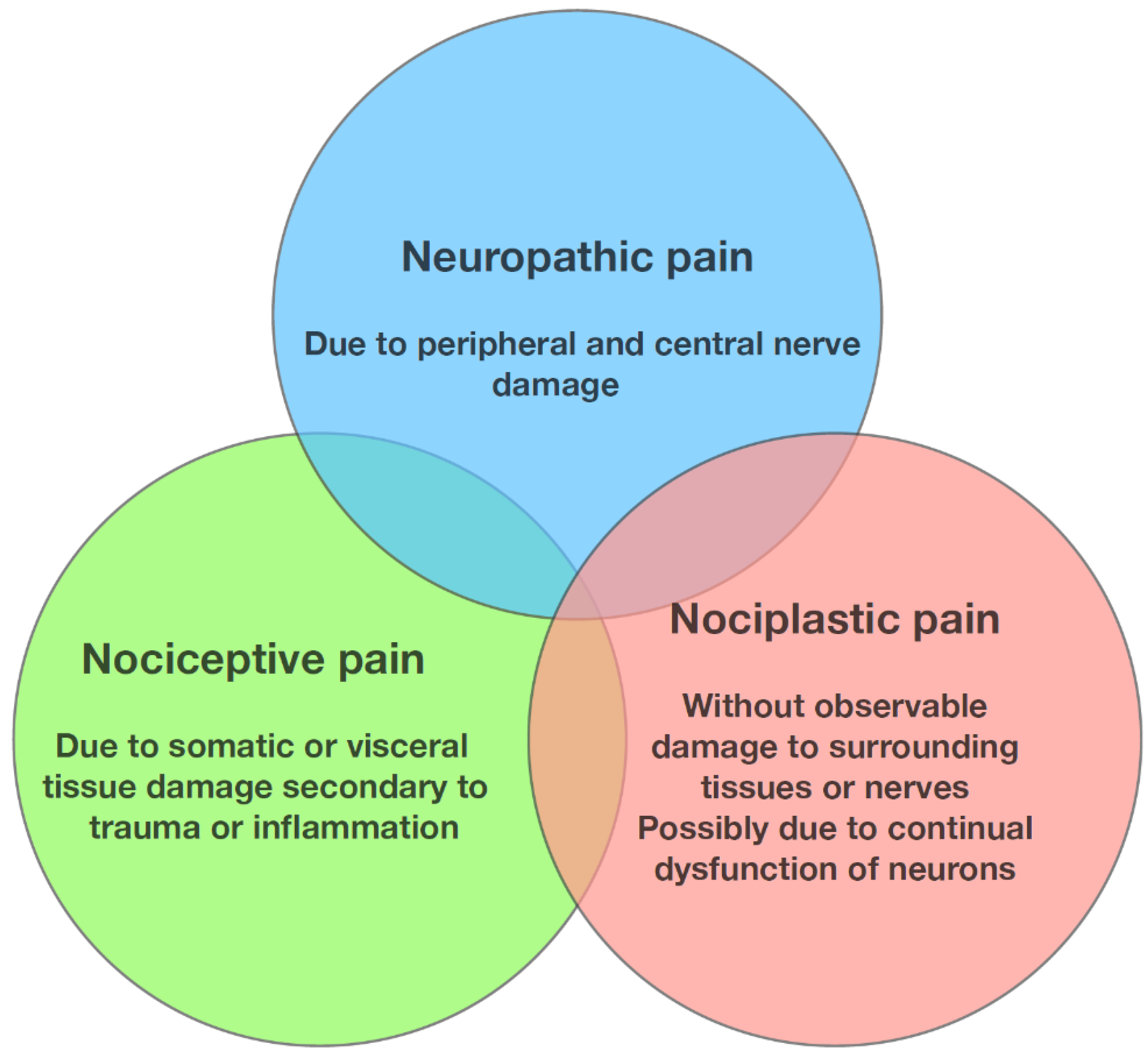
1.3. Nociceptive, Neuropathic, and Nociplastic Pain
1.4. Pain Sensitisation
2. Concept of Flares; What Causes Them and How to Assess Them
2.1. Pain Flares in KOA
2.2. Susceptibility to Flares
2.3. Assessment of Pain Flares
2.4. Preventing or Reducing Flares
3. Assessment of Pain
3.1. Knee Pain Map
3.2. Functional MRI
3.3. Quantitative Sensory Testing (QST)
3.3.1. Pressure Pain Thresholds
3.3.2. Conditioned Pain Modulation
4. Application in Clinical Practice
4.1. Role of Pain Assessment Tools
4.2. Role of Imaging and Laboratory Tests
4.3. Periarticular Causes of Pain
4.4. Role of Physical Examination
4.5. Compartment-Specific Symptoms—Patellofemoral vs. Tibiofemoral Osteoarthritis
4.6. Tailoring Treatments to Pain Characteristics
5. Application in Trials
5.1. Impact of Co-Administration of Analgesics
5.2. Regression to the Mean
5.3. Contextual Effects
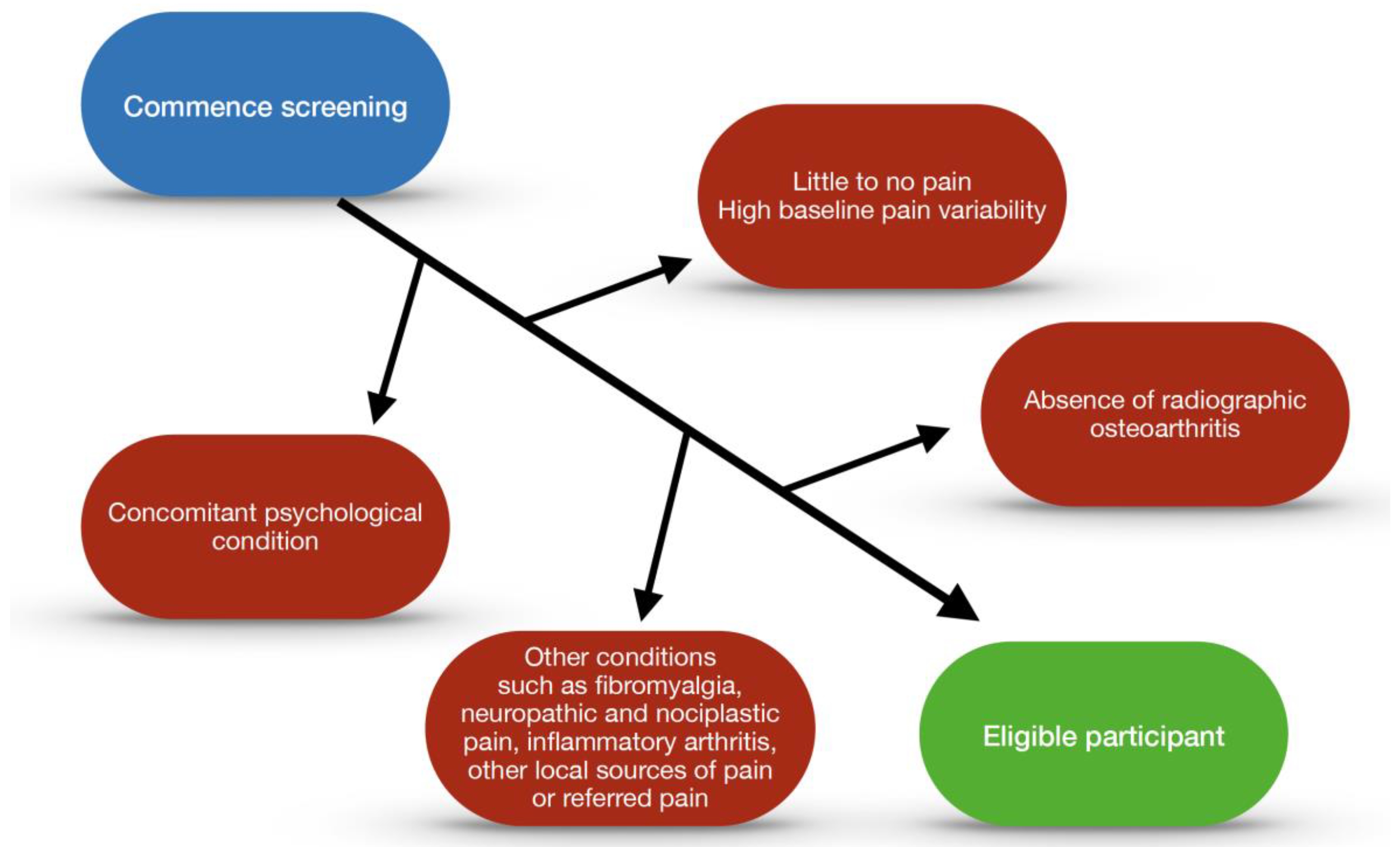
5.4. Eligibility Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahluwalia, S.C.; Giannitrapani, K.F.; Dobscha, S.K.; Cromer, R.; Lorenz, K.A. “Sometimes you wonder, is this really true?”: Clinician assessment of patients’ subjective experience of pain. J. Eval. Clin. Pract. 2020, 26, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Skou, S.T.; Roos, E.M.; Laursen, M.B.; Rathleff, M.S.; Arendt-Nielsen, L.; Rasmussen, S.; Simonsen, O. Total knee replacement and non-surgical treatment of knee osteoarthritis: 2-year outcome from two parallel randomized controlled trials. Osteoarthr. Cartil. 2018, 26, 1170–1180. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, S.; Zhang, F.; Zhang, X.; Zhang, X.; Li, T.; Li, D.; Zhu, W. Global Burden of osteoarthritis associated with high body mass index in 204 countries and territories, 1990–2019: Findings from the Global Burden of Disease Study 2019. Endocrine 2023, 79, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Perrot, S. Osteoarthritis pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, I.N.; Kemp, J.L.; Crossley, K.M.; Culvenor, A.G.; Hinman, R.S. Hip and Knee Osteoarthritis Affects Younger People, Too. J. Orthop. Sports Phys. Ther. 2017, 47, 67–79. [Google Scholar] [CrossRef]
- Petersen, K.K.-S. Predicting pain after standard pain therapy for knee osteoarthritis—The first steps towards personalized mechanistic-based pain medicine in osteoarthritis. Scand. J. Pain 2023, 23, 40–48. [Google Scholar] [CrossRef]
- Jordan, J.M. An Ongoing Assessment of Osteoarthritis in African Americans and Caucasians in North Carolina: The Johnston County Osteoarthritis Project. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 77–86. [Google Scholar]
- Lee, Y.; Lee, S.H.; Lim, S.M.; Baek, S.H.; Ha, I.H. Mental health and quality of life of patients with osteoarthritis pain: The sixth Korea National Health and Nutrition Examination Survey (2013–2015). PLoS ONE 2020, 15, e0242077. [Google Scholar] [CrossRef]
- Wise, B.L.; Niu, J.; Zhang, Y.; Wang, N.; Jordan, J.M.; Choy, E.; Hunter, D.J. Psychological factors and their relation to osteoarthritis pain. Osteoarthr. Cartil. 2010, 18, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Spector, T.D. Genetic epidemiology of hip and knee osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Petersson, I.F.; Björk, J.; Hawker, G.; Dahlberg, L.E.; Lohmander, L.S.; Englund, M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr. Cartil. 2014, 22, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis in over 16s: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022.
- Bonnin, M.; Chambat, P. Osteoarthritis of the Knee; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Hunter, D.J.; Eyles, J.P. Osteoarthritis Health Professional Training Manual; Academic Press: London, UK, 2023. [Google Scholar]
- Wilder, F.V.; Hall, B.J.; Barrett, J.P.; Lemrow, N.B. History of acute knee injury and osteoarthritis of the knee: A prospective epidemiological assessment: The Clearwater Osteoarthritis Study. Osteoarthr. Cartil. 2002, 10, 611–616. [Google Scholar] [CrossRef]
- Snoeker, B.; Turkiewicz, A.; Magnusson, K.; Frobell, R.; Yu, D.; Peat, G.; Englund, M. Risk of knee osteoarthritis after different types of knee injuries in young adults: A population-based cohort study. Br. J. Sports Med. 2020, 54, 725–730. [Google Scholar] [CrossRef]
- Marsh, J.D.; Degen, R.; Birmingham, T.B.; Giffin, J.R.; Getgood, A.; Litchfield, R.; Willits, K.; McClure, J.A.; Welk, B. The rate of unnecessary interventions for the management of knee osteoarthritis: A population-based cohort study. Can. J. Surg. 2022, 65, E114–E120. [Google Scholar] [CrossRef]
- Mease, P.J.; Hanna, S.; Frakes, E.P.; Altman, R.D. Pain Mechanisms in Osteoarthritis: Understanding the Role of Central Pain and Current Approaches to Its Treatment. J. Rheumatol. 2011, 38, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Ciapetti, A.; Carotti, M. The sources of pain in osteoarthritis: A pathophysiological review. Reumatismo 2014, 66, 57–71. [Google Scholar] [CrossRef]
- Bailly, F.; Cantagrel, A.; Bertin, P.; Perrot, S.; Thomas, T.; Lansaman, T.; Grange, L.; Wendling, D.; Dovico, C.; Anne-Priscille, T. Part of pain labelled neuropathic in rheumatic disease might be rather nociplastic. RMD Open 2020, 6, e001326. [Google Scholar] [CrossRef]
- van Eefje Martine, H.; Paco, M.J.W.; Mylène, P.J.; Willem Paul, G.; Marieke, L.; Margreet, K.; Francisco, B.; Ida, K.H.; Francis, B.; Anne, C.B.-J.; et al. Neuropathic pain in the IMI-APPROACH knee osteoarthritis cohort: Prevalence and phenotyping. RMD Open 2021, 7, e002025. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Nie, H.; Laursen, M.B.; Laursen, B.S.; Madeleine, P.; Simonsen, O.H.; Graven-Nielsen, T. Sensitization in patients with painful knee osteoarthritis. PAIN 2010, 149, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Stewart, L.; French, M.R.; Cibere, J.; Jordan, J.M.; March, L.; Suarez-Almazor, M.; Gooberman-Hill, R. Understanding the pain experience in hip and knee osteoarthritis—An OARSI/OMERACT initiative. Osteoarthr. Cartil. 2008, 16, 415–422. [Google Scholar] [CrossRef]
- Gwilym, S.E.; Keltner, J.R.; Warnaby, C.E.; Carr, A.J.; Chizh, B.; Chessell, I.; Tracey, I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Care Res. 2009, 61, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Dickenson, A.H.; Baron, R. Osteoarthritis pain: Nociceptive or neuropathic? Nat. Rev. Rheumatol. 2014, 10, 374–380. [Google Scholar] [CrossRef]
- Gebke, K.B.; McCarberg, B.; Shaw, E.; Turk, D.C.; Wright, W.L.; Semel, D. A practical guide to recognize, assess, treat and evaluate (RATE) primary care patients with chronic pain. Postgrad. Med. 2023, 135, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain (Primer). Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Orita, S.; Yamashita, M.; Ishikawa, T.; Ito, T.; Shigemura, T.; Nishiyama, H.; Konno, S.; Ohta, H.; Takaso, M.; et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med. J. 2012, 53, 801–805. [Google Scholar] [CrossRef]
- Hochman, J.R.; Davis, A.M.; Elkayam, J.; Gagliese, L.; Hawker, G.A. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1236–1242. [Google Scholar] [CrossRef]
- Bensen, G.P.; Rogers, A.C.; Leifer, V.P.; Edwards, R.R.; Neogi, T.; Kostic, A.M.; Paltiel, A.D.; Collins, J.E.; Hunter, D.J.; Katz, J.N.; et al. Does gabapentin provide benefit for patients with knee OA? A benefit-harm and cost-effectiveness analysis. Osteoarthr. Cartil. 2023, 31, 279–290. [Google Scholar] [CrossRef]
- Hochman, J.R.; Gagliese, L.; Davis, A.M.; Hawker, G.A. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthr. Cartil. 2011, 19, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Iida, S.; Miyashita, T.; Katou, K.; Kawarai, Y.; Nakamura, J.; Orita, S.; Ohtori, S. Mechanism of Chronic Pain of Symptomatic Hip Osteoarthritis by Association of its Distribution, Nociceptive, Neuropathic, Nociplastic, or Mixed-pain Screening, and the Prevalence of Lumbar Spinal Stenosis: A Cross-sectional Study. Clin. J. Pain 2021, 38, 77–87. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Moreton, B.J.; Tew, V.; das Nair, R.; Wheeler, M.; Walsh, D.A.; Lincoln, N.B. Pain Phenotype in Patients With Knee Osteoarthritis: Classification and Measurement Properties of painDETECT and Self-Report Leeds Assessment of Neuropathic Symptoms and Signs Scale in a Cross-Sectional Study. Arthritis Care Res. 2015, 67, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Previtali, D.; Capone, G.; Marchettini, P.; Candrian, C.; Zaffagnini, S.; Filardo, G. High Prevalence of Pain Sensitization in Knee Osteoarthritis: A Meta-Analysis with Meta-Regression. Cartilage 2022, 13, 19476035221087698. [Google Scholar] [CrossRef]
- Fingleton, C.; Smart, K.; Moloney, N.; Fullen, B.M.; Doody, C. Pain sensitization in people with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 1043–1056. [Google Scholar] [CrossRef]
- Thomas, M.J.; Neogi, T. Flare-ups of osteoarthritis: What do they mean in the short-term and the long-term? Osteoarthr. Cartil. 2020, 28, 870–873. [Google Scholar] [CrossRef]
- Liu, A.; Kendzerska, T.; Stanaitis, I.; Hawker, G. The relationship between knee pain characteristics and symptom state acceptability in people with knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 178–183. [Google Scholar] [CrossRef]
- Parry, E.; Ogollah, R.; Peat, G. Significant pain variability in persons with, or at high risk of, knee osteoarthritis: Preliminary investigation based on secondary analysis of cohort data. BMC Musculoskelet. Disord. 2017, 18, 80. [Google Scholar] [CrossRef]
- Murphy, S.L.; Lyden, A.K.; Kratz, A.L.; Fritz, H.; Williams, D.A.; Clauw, D.J.; Gammaitoni, A.R.; Phillips, K. Characterizing Pain Flares From the Perspective of Individuals With Symptomatic Knee Osteoarthritis. Arthritis Care Res. 2015, 67, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Guillemin, F.; Neogi, T. Osteoarthritis Flares. Clin. Geriatr. Med. 2022, 38, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Rathod-Mistry, T.; Parry, E.L.; Pope, C.; Neogi, T.; Peat, G. Triggers for acute flare in adults with, or at risk of, knee osteoarthritis: A web-based case-crossover study in community-dwelling adults. Osteoarthr. Cartil. 2021, 29, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Atukorala, I.; Pathmeswaran, A.; Batuwita, N.; Rajapaksha, N.; Ratnasiri, V.; Wijayaratne, L.; De Silva, M.; Chang, T.; Zhang, Y.; Hunter, D.J. Is being barefoot, wearing shoes and physical activity associated with knee osteoarthritis pain flares? Data from a usually barefoot Sri Lankan cohort. Int. J. Rheum. Dis. 2021, 24, 96–105. [Google Scholar] [CrossRef]
- Zobel, I.; Erfani, T.; Bennell, K.L.; Makovey, J.; Metcalf, B.; Chen, J.S.; March, L.; Zhang, Y.; Eckstein, F.; Hunter, D.J. Relationship of Buckling and Knee Injury to Pain Exacerbation in Knee Osteoarthritis: A Web-Based Case-Crossover Study. Interact. J. Med. Res. 2016, 5, e17. [Google Scholar] [CrossRef]
- Erfani, T.; Keefe, F.; Bennell, K.; Chen, J.; Makovey, J.; Metcalf, B.; March, L.; Williams, A.; Zhang, Y.; Hunter, D. Psychological Factors and Pain Exacerbation in Knee Osteoarthritis: A Web Based Case-Crossover Study. Rheumatology 2015, S6, 5. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Zhang, Y.; Metcalf, B.; Makovey, J.; Bennell, K.L.; March, L.; Hunter, D.J. The influence of weather on the risk of pain exacerbation in patients with knee osteoarthritis—A case-crossover study. Osteoarthr. Cartil. 2016, 24, 2042–2047. [Google Scholar] [CrossRef]
- Muraki, S.; Dennison, E.; Jameson, K.; Boucher, B.J.; Akune, T.; Yoshimura, N.; Judge, A.; Arden, N.K.; Javaid, K.; Cooper, C. Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1301–1306. [Google Scholar] [CrossRef]
- Cross, M.; Dubouis, L.; Mangin, M.; Hunter, D.J.; March, L.; Hawker, G.; Guillemin, F. Defining Flare in Osteoarthritis of the Hip and Knee: A Systematic Literature Review—OMERACT Virtual Special Interest Group. J. Rheumatol. 2017, 44, 1920–1927. [Google Scholar] [CrossRef]
- Marty, M.; Hilliquin, P.; Rozenberg, S.; Valat, J.P.; Vignon, E.; Coste, P.; Savarieau, B.; Allaert, F.A. Validation of the KOFUS (Knee Osteoarthritis Flare-Ups Score). Jt. Bone Spine Rev. Du Rhum. 2009, 76, 268–272. [Google Scholar] [CrossRef]
- Traore, Y.; Epstein, J.; Spitz, E.; March, L.; Maillefert, J.F.; Rutherford, C.; Ricatte, C.; Alleyrat, C.; Cross, M.; King, L.K.; et al. Development and validation of the Flare-OA questionnaire for measuring flare in knee and hip osteoarthritis. Osteoarthr. Cartil. 2022, 30, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Davis, A.M.; French, M.R.; Cibere, J.; Jordan, J.M.; March, L.; Suarez-Almazor, M.; Katz, J.N.; Dieppe, P. Development and preliminary psychometric testing of a new OA pain measure—An OARSI/OMERACT initiative. Osteoarthr. Cartil. 2008, 16, 409–414. [Google Scholar] [CrossRef] [PubMed]
- King, L.K.; Epstein, J.; Cross, M.; Buzzi, M.; Buttel, T.; Cembalo, S.M.; Spitz, E.; Adams, C.L.; Adebajo, A.; Bennell, K.; et al. Endorsement of the domains of knee and hip osteoarthritis (OA) flare: A report from the OMERACT 2020 inaugural virtual consensus vote from the flares in OA working group. Semin. Arthritis Rheum. 2021, 51, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.L.; Kobayashi, S.; Hunter, D.J.; Mills, K.; Peat, G.; Guillemin, F.; Parry, E.; Thomas, M.J.; Eyles, J.P. Best-practice clinical management of flares in people with osteoarthritis: A scoping review of behavioral, lifestyle and adjunctive treatments. Semin. Arthritis Rheum. 2021, 51, 749–760. [Google Scholar] [CrossRef]
- Bartholdy, C.; Klokker, L.; Bandak, E.; Bliddal, H.; Henriksen, M. A Standardized “Rescue” Exercise Program for Symptomatic Flare-up of Knee Osteoarthritis: Description and Safety Considerations. J. Orthop. Sports Phys. Ther. 2016, 46, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Gondhalekar, G.A.; Deo, M.V. Retrowalking as an adjunct to conventional treatment versus conventional treatment alone on pain and disability in patients with acute exacerbation of chronic knee osteoarthritis: A randomized clinical trial. N. Am. J. Med. Sci. 2013, 5, 108–112. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. S1), S17–S24. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar] [CrossRef]
- Nugent, S.M.; Lovejoy, T.I.; Shull, S.; Dobscha, S.K.; Morasco, B.J. Associations of Pain Numeric Rating Scale Scores Collected during Usual Care with Research Administered Patient Reported Pain Outcomes. Pain Med. 2021, 22, 2235–2241. [Google Scholar] [CrossRef]
- Bolognese, J.A.; Schnitzer, T.J.; Ehrich, E.W. Response relationship of VAS and Likert scales in osteoarthritis efficacy measurement. Osteoarthr. Cartil. 2003, 11, 499–507. [Google Scholar] [CrossRef]
- Harland, N.J.; Dawkin, M.J.; Martin, D. Relative utility of a visual analogue scale vs a six-point Likert scale in the measurement of global subject outcome in patients with low back pain receiving physiotherapy. Physiotherapy 2015, 101, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Atukorala, I.; Pathmeswaran, A.; Makovey, J.; Metcalf, B.; March, L.; Bennell, K.L.; Chang, T.; Zhang, Y.; Hunter, D.J. Is there a relationship between the Intermittent and Constant Osteoarthritis Pain score (ICOAP) and pain flares in knee osteoarthritis? Osteoarthr. Cartil. 2016, 24, S429–S430. [Google Scholar] [CrossRef]
- Fleisher, I.T.; Thompson, M.C.; Mensah, C.J.; Joseph, A.D.; McLawhorn, A.S.; Padgett, D.E.; Lyman, S. Development and Validation of Crosswalks Between the Western Ontario & McMaster Universities Osteoarthritis Index and Hip Disability and Osteoarthritis Outcome Score Joint Replacement/Knee Injury and Osteoarthritis Outcome Score Joint Replacement. J. Arthroplast. 2022, 37, 1034–1039.e1033. [Google Scholar] [CrossRef]
- Roos, M.K.; LS Lohmander, E.M. WOMAC Osteoarthritis Index: Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Scand. J. Rheumatol. 1999, 28, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Nadrian, H.; Moghimi, N.; Nadrian, E.; Moradzadeh, R.; Bahmanpour, K.; Iranpour, A.; Bellamy, N. Validity and reliability of the Persian versions of WOMAC Osteoarthritis Index and Lequesne Algofunctional Index. Clin. Rheumatol. 2012, 31, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a Self-Administered Outcome Measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Stevens-Lapsley, J.E.; Schenkman, M.L.; Dayton, M.R. Comparison of Self-Reported Knee Injury and Osteoarthritis Outcome Score to Performance Measures in Patients After Total Knee Arthroplasty. PM&R 2011, 3, 541–549. [Google Scholar] [CrossRef]
- Roos, E.M.; Toksvig-Larsen, S. Knee injury and Osteoarthritis Outcome Score (KOOS)—Validation and comparison to the WOMAC in total knee replacement. Health Qual. Life Outcomes 2003, 1, 17. [Google Scholar] [CrossRef]
- Picavet, H.S.; Hoeymans, N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann. Rheum. Dis. 2004, 63, 723–729. [Google Scholar] [CrossRef]
- Gusi, N.; Olivares, P.R.; Rajendram, R. The EQ-5D Health-Related Quality of Life Questionnaire. In Handbook of Disease Burdens and Quality of Life Measures; Springer: Berlin/Heidelberg, Germany, 2010; pp. 87–99. [Google Scholar] [CrossRef]
- Kontodimopoulos, N.; Pappa, E.; Niakas, D.; Yfantopoulos, J.; Dimitrakaki, C.; Tountas, Y. Validity of the EuroQoL (EQ-5D) Instrument in a Greek General Population. Value Health 2008, 11, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Fishbain, D.A.; Lewis, J.E.; Cutler, R.; Cole, B.; Rosomoff, H.L.; Rosomoff, R.S. Can the Neuropathic Pain Scale Discriminate Between Non-neuropathic and Neuropathic Pain? Pain Med. 2007, 9, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Galer, B.S.; Jensen, M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology 1997, 48, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Gray, P. Acute neuropathic pain: Diagnosis and treatment. Curr. Opin. Anaesthesiol. 2008, 21, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The Development and Psychometric Validation of the Central Sensitization Inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain 2013, 14, 438–445. [Google Scholar] [CrossRef]
- Elson, D.W.; Jones, S.; Caplan, N.; Stewart, S.; St Clair Gibson, A.; Kader, D.F. The photographic knee pain map: Locating knee pain with an instrument developed for diagnostic, communication and research purposes. Knee 2011, 18, 417–423. [Google Scholar] [CrossRef]
- Thompson, L.R.; Boudreau, R.; Hannon, M.J.; Newman, A.B.; Chu, C.R.; Jansen, M.; Nevitt, M.C.; Kwoh, C.K. The knee pain map: Reliability of a method to identify knee pain location and pattern. Arthritis Care Res. 2009, 61, 725–731. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Qiu, L.; Huang, X.; He, C.; Zhang, J.; Gong, Q. Structural and Functional Abnormalities in Knee Osteoarthritis Pain Revealed With Multimodal Magnetic Resonance Imaging. Front. Hum. Neurosci. 2021, 15, 783355. [Google Scholar] [CrossRef]
- Pujol, J.; Martínez-Vilavella, G.; Llorente-Onaindia, J.; Harrison, B.J.; López-Solà, M.; López-Ruiz, M.; Blanco-Hinojo, L.; Benito, P.; Deus, J.; Monfort, J. Brain imaging of pain sensitization in patients with knee osteoarthritis. Pain 2017, 158, 1831–1838. [Google Scholar] [CrossRef]
- Monfort, J.; Pujol, J.; López-Ruiz, M.; Llorente-Onaindia, J.; Martínez-Vilavella, G.; Macià, D.; Montañes, F.; Benito, P.; Deus, J. Functional magnetic resonance imaging evaluation of pain central sensitization phenomena in subjects with knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, A60. [Google Scholar] [CrossRef]
- Suokas, A.K.; Walsh, D.A.; McWilliams, D.F.; Condon, L.; Moreton, B.; Wylde, V.; Arendt-Nielsen, L.; Zhang, W. Quantitative sensory testing in painful osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2012, 20, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Alavinia, M.; Singh, M.; Kumbhare, D. Pressure Pain Threshold in patients with chronic pain: A Systematic Review and Meta-analysis. Am. J. Phys. Med. Rehabil. 2021, 100, 656–674. [Google Scholar] [CrossRef]
- Sylwander, C.; Larsson, I.; Haglund, E.; Bergman, S.; Andersson, M.L.E. Pressure pain thresholds in individuals with knee pain: A cross-sectional study. BMC Musculoskelet. Disord. 2021, 22, 516. [Google Scholar] [CrossRef] [PubMed]
- Jaber, K.; McAuliffe, M.; Pedler, A.; Sterling, M.; O’Leary, S. Further exploring the relationship between pressure pain thresholds and function in knee osteoarthritis. Musculoskelet. Sci. Pract. 2022, 59, 102542. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Nir, R.-R.; Sprecher, E.; Yarnitsky, D. Psychological Factors and Conditioned Pain Modulation: A Meta-Analysis. Clin. J. Pain 2016, 32, 541–554. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Wodehouse, T. Conditioned pain modulation—A comprehensive review. Neurophysiol. Clin. 2021, 51, 197–208. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Wodehouse, T.; Langford, R.M.; Arendt-Nielsen, L.; Kidd, B.L. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012, 64, 2907–2916. [Google Scholar] [CrossRef]
- Vriezekolk, J.E.; Peters, Y.A.S.; Steegers, M.A.H.; Blaney Davidson, E.N.; van den Ende, C.H.M. Pain descriptors and determinants of pain sensitivity in knee osteoarthritis: A community-based cross-sectional study. Rheumatol. Adv. Pract. 2022, 6, rkac016. [Google Scholar] [CrossRef]
- van Berkel, A.C.; Ringelenberg, R.; Bindels, P.J.E.; Bierma-Zeinstra, S.M.A.; Schiphof, D. Nocturnal pain, is the pain different compared with pain during the day? An exploratory cross-sectional study in patients with hip and knee osteoarthritis. Fam. Pract. 2022, 40, 75–82. [Google Scholar] [CrossRef]
- Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Okubo, N.; Takahashi, I.; Nakaji, S.; Ishibashi, Y. Nocturnal Knee Pain Increases With the Severity of Knee Osteoarthritis, Disturbing Patient Sleep Quality. Arthritis Care Res. 2014, 66, 1027–1032. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Peat, G.; Bierma-Zeinstra, M.A.; Arden, N.K.; Bresnihan, B.; Herrero-Beaumont, G.; Kirschner, S.; Leeb, B.F.; Lohmander, L.S.; et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 483–489. [Google Scholar] [CrossRef]
- Guermazi, A.; Niu, J.; Hayashi, D.; Roemer, F.W.; Englund, M.; Neogi, T.; Aliabadi, P.; McLennan, C.E.; Felson, D.T. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: Population based observational study (Framingham Osteoarthritis Study). BMJ Br. Med. J. 2012, 345, e5339. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Gale, D.R.; Chaisson, C.E.; Skinner, K.; Kazis, L.; Gale, M.E.; Felson, D.T. Periarticular lesions detected on magnetic resonance imaging: Prevalence in knees with and without symptoms. Arthritis Rheum. 2003, 48, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Kim, D.H.; Song, Y.W.; Guermazi, A.; Crema, M.D.; Hunter, D.J.; Seo, Y.I.; Kim, H.A. The prevalence of periarticular lesions detected on magnetic resonance imaging in middle-aged and elderly persons: A cross-sectional study. BMC Musculoskelet. Disord. 2016, 17, 186. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. PAIN 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Krebs, E.E.; Gravely, A.; Nugent, S.; Jensen, A.C.; DeRonne, B.; Goldsmith, E.S.; Kroenke, K.; Bair, M.J.; Noorbaloochi, S. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA 2018, 319, 872–882. [Google Scholar] [CrossRef]
- Grøvle, L.; Hasvik, E.; Haugen, A.J. Impact of rescue medication in placebo-controlled trials of pharmacotherapy for neuropathic pain and low back pain. Pain 2022, 163, e417–e425. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Driban, J.B.; Henrotin, Y.; Hunter, D.J.; Jiang, G.L.; Skou, S.T.; Wang, S.; Schnitzer, T. OARSI Clinical Trials Recommendations: Design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, 747–760. [Google Scholar] [CrossRef]
- Goubert, D.; Danneels, L.; Cagnie, B.; Van Oosterwijck, J.; Kolba, K.; Noyez, H.; Meeus, M. Effect of Pain Induction or Pain Reduction on Conditioned Pain Modulation in Adults: A Systematic Review. Pain Pract. 2015, 15, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, M.C.; Marchi, E.; Machado, E.G.; de Azevedo e Souza Munhoz, M. Detection of Possible Problematic Polypharmacy in Patients with Osteoarthritis and Associated Factors. Osteoarthr. Cartil. 2022, 30, S195–S196. [Google Scholar] [CrossRef]
- Englund, M.; Turkiewicz, A. Pos1354 Regression to the Mean for Pain Outcomes in Clinical Trials for Knee Osteoarthritis. Ann. Rheum. Dis. 2023, 82, 1028–1029. [Google Scholar] [CrossRef]
- Pocock, S.J.; Bakris, G.; Bhatt, D.L.; Brar, S.; Fahy, M.; Gersh, B.J. Regression to the Mean in SYMPLICITY HTN-3: Implications for Design and Reporting of Future Trials. J. Am. Coll. Cardiol. 2016, 68, 2016–2025. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H.; Lamb, S.E.; Lee, H. Disentangling contextual effects from musculoskeletal treatments. Osteoarthr. Cartil. 2021, 29, 297–299. [Google Scholar] [CrossRef]
- Kun, Z.; Jean, W.; Natasya, A.; Xi, C.; Toby, S.; Michael, D.; Weiya, Z. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: Meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2016, 75, 1964–1970. [Google Scholar] [CrossRef]
- Bannuru, R.R.; McAlindon, T.E.; Sullivan, M.C.; Wong, J.B.; Kent, D.M.; Schmid, C.H. Effectiveness and Implications of Alternative Placebo Treatments. Ann. Intern. Med. 2015, 163, 365–372. [Google Scholar] [CrossRef]
- Erpelding, N.; Evans, K.; Lanier, R.K.; Elder, H.; Katz, N.P. Placebo Response Reduction and Accurate Pain Reporting Training Reduces Placebo Responses in a Clinical Trial on Chronic Low Back Pain: Results From a Comparison to the Literature. Clin. J. Pain 2020, 36, 950–954. [Google Scholar] [CrossRef]
- Branders, S.; Pereira, A.; Bernard, G.; Ernst, M.; Dananberg, J.; Albert, A. Leveraging historical data to optimize the number of covariates and their explained variance in the analysis of randomized clinical trials. Stat. Methods Med. Res. 2022, 31, 240–252. [Google Scholar] [CrossRef]
| Self-Reporting Outcome Measures | Method | Advantages | Disadvantages |
|---|---|---|---|
| Numerical rating scale | Pain level is indicated by a number between 2 set numbers, e.g., between 0 and 10 [60] | Feasible [60] Can be administered verbally [60,61] and quickly [61] High test–retest reliability [61] Correlates well to the visual analogue scale in patients with chronic pain conditions [61] A small change in score is considered clinically important [61] | Less distinct discernment of pain intensity [60] Inconsistencies in administration can lead to over and underestimation and inaccuracy [62] |
| Visual analogue scale | Pain level is marked on a line between two endpoints (no pain and worse ever pain) [60] | Sensitive to treatment effects [60,63] Can be administered quickly [61] Good test–retest reliability [61] Has been shown to be sensitive when detecting changes in chronic inflammatory and degenerative joint pain [61] | Unclear cut-off for clinical significance [60] Time consuming [60] Difficult to understand [60] Cannot be administered verbally [61] |
| McGill Pain Questionnaire | Consists of a pain rating index where each word has a numerical score, depending on sensory, affective, and evaluative implications of pain, number of words chosen to describe pain and pain intensity (between 1 and 5) [60] | Very extensive [60] Good test–retest reliability within 1 day of testing but poor test–retest reliability within 7 days [61] Able to detect mild pain [61] Differentiates between sensorial and emotional components of pain and has been validated in patients with KOA [6] | Time consuming [61] Difficult to administer and understand [61] |
| Likert scale | A total of 5 to 7 options between two-end points, e.g., completely better and worse [64] | Practical [64] Easy to understand [64] | Restrictive [64] |
| Intermittent and constant pain score (ICOAP) | An 11-question questionnaire, each scored between 0 and 4 [65] | Easy to administer [61] Excellent validity for hip and KOA with good test–retest reliability [61] Able to detect changes in osteoarthritis pain intensity in response to pharmacologic therapies [61] | Atukorala et al. (2016) found that ICOAP does not prognosticate pain flares associated with KOA pain flares [65] Does not assess osteoarthritis associated disability [61] |
| Western Ontario and McMaster Universities (WOMAC) Osteoarthritis index | A 24-question questionnaire about pain, stiffness, and function, scored between 0 and 100, with 100 being the best joint health [66,67] | Good reliability and internal consistency [67,68] Can be self-administered | WOMAC subscale on stiffness is often vague [67] |
| Knee injury and osteoarthritis outcome score | A 42-item questionnaire about the short and long term impacts of participants’ knee injuries with questions about pain, activities of daily living, function and quality of life, scored between 0 and 100, with 100 being asymptomatic [69] | Self-administered, quick administration, assesses long term outcomes [69] Good test–retest reliability [70,71] | High number of items to be answered, making the assessment time consuming for participants [72] |
| Lequesne index | An 11-item questionnaire about pain, maximum walking distance, and activities of daily living, with scores ranging from 0 to 8, with a total maximum score of 24, with higher scores indicating worse joint health [68] | Average to good reliability and internal consistency [68] | Needs to be administered by an interviewer [68] Poorer internal consistency and reliability as compared to WOMAC [68] |
| European health-related quality of life measures (EuroQol) | The 5 questions about pain, mobility, activities of daily living and anxiety [73] | Quick administration and simple to use [73,74] Measures quality of life [73,74] Good reliability and internal consistency [75] | Large ceiling effect [75] |
| Short Form-36 health survey (SF-36) | A 2-item scale evaluating pain intensity between 6 points ranging from none to very severe [61] | Easy to administer, can be completed quickly with good test–retest reliability [61] Able to detect improvements in pain intensities [61] | Not specific to a disease [61] Hard to distinguish between different intensities of pain, making treatment effect difficult to assess [61] |
| Neuropathic Pain Scale | A 10-item questionnaire based on 8 qualities of neuropathic pain, such as sharp, hot, cold, sensitive, cold, and itchy [76,77] | Able to differentiate between neuropathic and non-neuropathic pain [76] | Does not include all pain descriptors that patients with neuropathic pain experience [77] Mainly used for monitoring neuropathic pain [78] |
| Central sensitisation inventory | Questionnaire split into 2 parts. First part involves a 25-item questionnaire with a total score ranging from 0 to 100. Second part involves questions regarding physician diagnosed disorders, such as depression and anxiety [79]. | Good test–retest reliability [79], with good sensitivity and specificity [80] | Cut-off scores vary depending on patient sample [80] |
| Characteristic | Relevance |
|---|---|
| Pain location |
|
| Type |
|
| Aggravating vs. relieving factors |
|
| Onset |
|
| Severity |
|
| Guideline | Criteria for Clinical Diagnosis of KOA |
|---|---|
| American College of Rheumatology (ACR) [96] |
|
| National Institute for Health and Care Excellence (NICE) [15] |
|
| European Alliance of Associations for Rheumatology (EULAR) [97] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirumaran, A.J.; Deveza, L.A.; Atukorala, I.; Hunter, D.J. Assessment of Pain in Osteoarthritis of the Knee. J. Pers. Med. 2023, 13, 1139. https://doi.org/10.3390/jpm13071139
Thirumaran AJ, Deveza LA, Atukorala I, Hunter DJ. Assessment of Pain in Osteoarthritis of the Knee. Journal of Personalized Medicine. 2023; 13(7):1139. https://doi.org/10.3390/jpm13071139
Chicago/Turabian StyleThirumaran, Aricia Jieqi, Leticia Alle Deveza, Inoshi Atukorala, and David J. Hunter. 2023. "Assessment of Pain in Osteoarthritis of the Knee" Journal of Personalized Medicine 13, no. 7: 1139. https://doi.org/10.3390/jpm13071139
APA StyleThirumaran, A. J., Deveza, L. A., Atukorala, I., & Hunter, D. J. (2023). Assessment of Pain in Osteoarthritis of the Knee. Journal of Personalized Medicine, 13(7), 1139. https://doi.org/10.3390/jpm13071139






