Randomized Clinical Trials and Observational Tribulations: Providing Clinical Evidence for Personalized Surgical Pain Management Care Models
Abstract
1. Introduction
2. How to Manage Skill in Surgical Trials
3. Major Errors in Clinical Trials in Surgery
4. Differences between Drug Trials and Surgical Trials
5. Why Are Patients Reluctant to Participate in Surgical Trials?
6. Why Do Surgeons Not Like to Do Clinical Trials?
7. Should We Even Perform Randomized Clinical Trials in Surgery?
8. Opportunities and Limitations of Observational Studies Compared to Clinical Trials
9. Providing Evidence on Surgical Methods for Which There Are No RCTs
10. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moher, D.; Schulz, K.F.; Altman, D.G. The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials. Ann. Intern. Med. 2001, 134, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W. Re-evaluating “Success” as It Pertains to Surgical Trials. J. Minim. Invasive Gynecol. 2021, 28, 496–501. [Google Scholar] [CrossRef]
- Bogduk, N.; Fraifeld, E.M. Proof or consequences: Who shall pay for the evidence in pain medicine? Pain Med. 2010, 11, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Joeris, A.; Knoll, C.; Kalampoki, V.; Blumenthal, A.; Gaskell, G. Patient-reported outcome measurements in clinical routine of trauma, spine and craniomaxillofacial surgeons: Between expectations and reality: A survey among 1212 surgeons. BMJ Open 2018, 8, e020629. [Google Scholar] [CrossRef] [PubMed]
- Hansson-Hedblom, A.; Jonsson, E.; Fritzell, P.; Hagg, O.; Borgstrom, F. The Association Between Patient Reported Outcomes of Spinal Surgery and Societal Costs: A Register Based Study. Spine 2019, 44, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Maldaner, N.; Joswig, H.; Corniola, M.V.; Bellut, D.; Prömmel, P.; Regli, L.; Weyerbrock, A.; Schaller, K.; Gautschi, O.P. Objective functional assessment using the “Timed Up and Go” test in patients with lumbar spinal stenosis. Neurosurg. Focus 2019, 46, E4. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Chalmers, I.; Hayes, R.J.; Altman, D.G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef]
- Henriksen, K.; Kaplan, H. Hindsight bias, outcome knowledge and adaptive learning. Qual. Saf. Health Care 2003, 12 (Suppl. 2), ii46–ii50. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Karanicolas, P.J.; Thoma, A.; Simunovic, M.; Bhandari, M.; Devereaux, P.J.; Anvari, M.; Adili, A.; Guyatt, G. Randomized Controlled Trials of Surgical Interventions. Ann. Surg. 2010, 251, 409–416. [Google Scholar] [CrossRef]
- Lilford, R.; Braunholtz, D.; Harris, J.; Gill, T. Trials in surgery. Br. J. Surg. 2004, 91, 6–16. [Google Scholar] [CrossRef]
- Doerr-Harim, C.; Bruckner, T.; Diener, M.K.; Knebel, P. Insights into surgical trials: Methodological challenges and solutions. Langenbeck’s Arch. Surg. 2014, 399, 273–278. [Google Scholar] [CrossRef]
- Vonlanthen, R.; Lodge, P.; Barkun, J.S.; Farges, O.; Rogiers, X.; Soreide, K.; Kehlet, H.; Reynolds, J.V.; Käser, S.A.; Naredi, P.; et al. Toward a Consensus on Centralization in Surgery. Ann. Surg. 2018, 268, 712–724. [Google Scholar] [CrossRef] [PubMed]
- McLeod, R.S. Issues in surgical randomized controlled trials. World J. Surg. 1999, 23, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.; Batzer, F.R. Is a placebo-controlled surgical trial an oxymoron? J. Minim. Invasive Gynecol. 2007, 14, 278–283. [Google Scholar] [CrossRef]
- Schain, W.S. Barriers to clinical trials: Part II: Knowledge and attitudes of potential participants. Cancer 1994, 74 (Suppl. 9), 2666–2671. [Google Scholar]
- Foster, J.M.; Sawyer, S.M.; Smith, L.; Reddel, H.K.; Usherwood, T. Barriers and facilitators to patient recruitment to a cluster randomized controlled trial in primary care: Lessons for future trials. BMC Med. Res. Methodol. 2015, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.D.; Lytle, B.L.; Biswas, M.S.; Coombs, L. Willingness to participate in cardiac trials. Am. J. Geriatr. Cardiol. 2004, 13, 11–15. [Google Scholar] [CrossRef]
- Barcot, O.; Boric, M.; Dosenovic, S.; Cavar, M.; Kadic, A.J.; Pericic, T.P.; Vukicevic, I.; Vuka, I.; Puljak, L. Adequacy of risk of bias assessment in surgical vs non-surgical trials in Cochrane reviews: A methodological study. BMC Med. Res. Methodol. 2020, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. 2022. Available online: https://www.equator-network.org/reporting-guidelines/strobe/ (accessed on 10 June 2023).
- Phillips, F.M.; Cheng, I.; Rampersaud, Y.R.; Akbarnia, B.A.; Pimenta, L.; Rodgers, W.B.; Uribe, J.S.; Khanna, N.; Smith, W.D.; Youseff, J.A.; et al. Breaking Through the “Glass Ceiling” of Minimally Invasive Spine Surgery. Spine 2016, 41 (Suppl. 8), S39–S43. [Google Scholar] [CrossRef]
- Solheim, O. Randomized controlled trials in surgery and the glass ceiling effect. Acta Neurochir. 2019, 161, 623–625. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; León, J.F.R.; Dowling, Á.; Garcia, M.R.; Rugeles, J.G.; Ramirez, C.; Garcia, A.; Valerio, J.; de Carvalho, P.S.T.; Rodríguez, L.M.D.; et al. Breaking through the glass ceiling effect of high-grade clinical evidence creation in orthopaedics & trauma. Rev. Colomb. Ortop. Traumatol. 2022, 36, 215–228. [Google Scholar] [CrossRef]
- Lunceford, J.K.; Davidian, M. Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Stat. Med. 2004, 23, 2937–2960, Correction in Stat. Med. 2017, 36, 2320. [Google Scholar] [CrossRef]
- Austin, P.C.; Mamdani, M.M. A comparison of propensity score methods: A case-study estimating the effectiveness of post-AMI statin use. Stat. Med. 2006, 25, 2084–2106. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Spertus, J.A.; Krumholz, H.M. Data-Driven Individualized Surgical Decision-making: Beyond “Better on Average” Clinical Trial Results. JAMA Surg. 2022, 157, 93–94. [Google Scholar] [CrossRef]
- Dwan, K.; Altman, D.G.; Cresswell, L.; Blundell, M.; Gamble, C.L.; Williamson, P.R. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database Syst. Rev. 2011, 2011, Mr000031. [Google Scholar] [CrossRef]
- Quoix, E. Methodology for the assessment of new targeted treatments in clinical trials. Future perspectives. Rev. Pneumol. Clin. 2004, 60 Pt 2, 3s72–3s78. [Google Scholar]
- Kazaryan, I.; Sevikyan, A. Patients in need of medicine information. Int. J. Risk Saf. Med. 2015, 27 (Suppl. 1), S21–S22. [Google Scholar] [CrossRef]
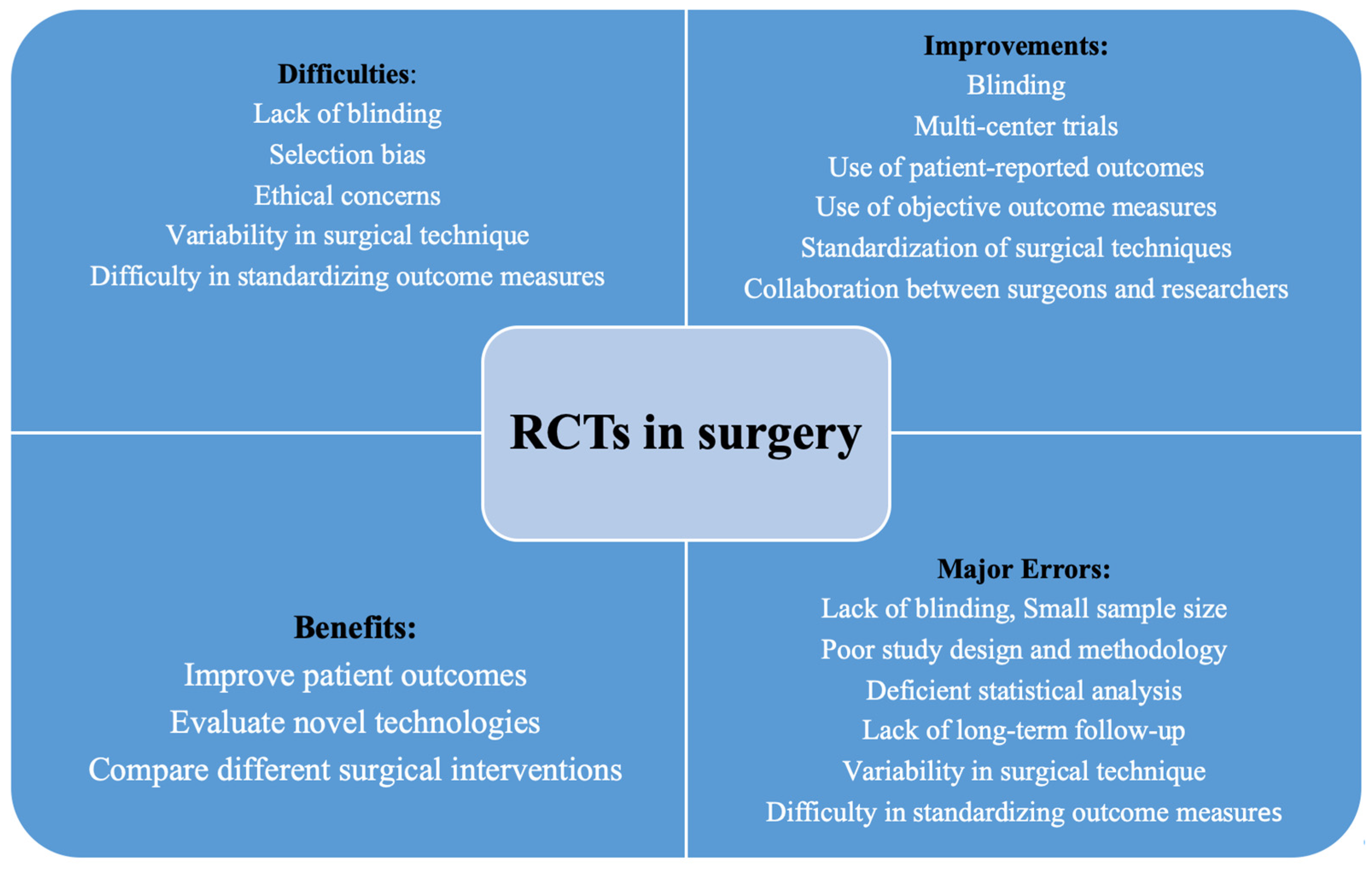

| Problem | Description | |
|---|---|---|
| Managing surgical skills | Surgeon training and standardization | Educating surgeons and standardizing surgical methods can assist in minimizing surgical skill variability and improve the efficacy of surgical trials. |
| Surgeon selection | Choosing experienced and highly qualified surgeons to join investigations may reduce variability of surgical results. | |
| Centralization of procedure | Centralizing treatments to a few competent surgeons can guarantee that surgical interventions are conducted consistently and skillfully. | |
| Monitoring of surgical performance | Surgical-skill-related problems can be found and resolved by regular monitoring of surgical performance, such as by employing surgical checklists. | |
| Assessment of surgical skill | Integrating objective measurements of surgical ability, such as time-motion assessment or surgical evaluation tools, can guarantee that surgical operations are continuously and effectively performed. | |
| Errors | Selection bias | A clinical trial’s sample may be biased if patients who are candidates for surgery are not eligible to participate. |
| Blinding | In many surgical trials, it can be challenging to blind the patient and surgeon to the intervention, which can result in observer bias. | |
| Variability in surgical technique | Surgical procedures and approaches can differ substantially amongst surgeons, which can make controlling this source of variation in a clinical trial challenging. | |
| Difficulty in standardizing outcome measures | Surgical results can be hard to measure and are susceptible to observer bias, making it difficult to develop accurate and valid measurements in surgical trials. | |
| Small sample size | Several surgical trials have limited sample numbers, which makes detecting substantial differences in outcomes across groups problematic. | |
| Poor study design | The reliability and accuracy of trial outcomes can be affected by poorly designed studies, such as those with insufficient randomization or an absence of a control group. | |
| Lack of long-term follow-up | Numerous surgical trials lack proper long-term follow-up, which makes it impossible to evaluate the long-term advantages and disadvantages of a surgical intervention. |
| Barrier | Description |
|---|---|
| Lack of understanding | Most patients seem to be unaware of the benefits and risks of joining RCTs. |
| Fear of being a “guinea pig” | There are patients who might be hesitant to participate in RCTs because they believe that they will be given unproven or experimental healthcare. |
| Concerns about receiving a placebo | Patients might be hesitant to provide informed permission for surgical trials because they are concerned about being assigned to a control group and not receiving the complete surgical treatment. |
| Logistical issues | Individuals who may find it challenging to engage in RCTs would conclusively require more time and transportation. |
| Health insurance | Some patients may not have enough health insurance or may be concerned about the costs of participating in RCTs. |
| Trust in the medical community | Whether they have doubts about the medical profession or fear of being taken advantage of, patients can be hesitant to take part in clinical research. |
| Lack of standardization | In clinical trials, surgical techniques must be standardized in order to account for variability and deliver accurate results. Some surgeons may argue that standardization restricts their ability to perform surgery in the manner in which they believe is best for their patients. |
| Time constraints | For busy surgeons, clinical studies can be time-consuming and may require extra follow-up and documentation. |
| Financial considerations | Clinical trials may not be as economically advantageous as ordinary surgical interventions are, and surgeons may be unwilling to take part if they believe that it will have a detrimental influence on their profession or their practice. |
| Perception of research | Clinical trials may not be viewed by many surgeons as a useful and important part of surgical practice, but rather as a merely academic exercise. |
| Ethical considerations | It can be challenging to obtain patients’ informed consent for surgical trials, and there can be ethical issues in restraining a patient from a potentially helpful surgical intervention. |
| Factors | Description | |
|---|---|---|
| Difficulties | Selection bias | Patients who are surgical candidates may not be eligible for a clinical study, resulting in a biased sample. |
| Blinding | It is challenging to blind the patient and surgeon to the intervention in many surgical studies, which causes bias on the part of the observers. | |
| Ethical concerns | Patients’ informed consents may be hard to obtain, and there may be ethical concerns regarding rejecting a patient from potentially helpful surgical intervention. | |
| Variability in surgical technique | Surgical procedures and strategies can differ substantially amongst surgeons, and this source of variation is difficult to manage in a clinical study. | |
| Difficulty in standardizing outcome measures | Surgical consequences can be hard to measure and are susceptible to observer bias, making it difficult to develop precise and reliable outcome data in surgical trials. | |
| Improving factors | Standardization of surgical techniques | The development and promotion of standardized surgical procedures and practices can help with decreasing variability and improving the level of surgical trials. |
| Use of patient-reported outcomes | A more complete picture of the advantages and disadvantages of a surgical intervention can be obtained by incorporating patient-reported outcomes into surgical trials. | |
| Blinding | To lessen observer bias, efforts should be taken to obliterate knowledge of the intervention from the patients’ and surgeons’ perspectives. | |
| Use of objective outcome measures | Using objective data in surgical trials, such as imaging findings or the outcomes of biopsies, can lessen observer bias and improve the accuracy of outcome measurements. | |
| Multi-center trials | Conducting multi-center tests can improve the outcome generalization and overcome the issue of limited sample numbers that might emerge in single-center trials. | |
| Collaboration between surgeons and researchers | Collaborations between surgeons and scientists can aid in the design and conduct of high-quality surgical trials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abraham, I.; Lewandrowski, K.-U.; Elfar, J.C.; Li, Z.-M.; Fiorelli, R.K.A.; Pereira, M.G.; Lorio, M.P.; Burkhardt, B.W.; Oertel, J.M.; Winkler, P.A.; et al. Randomized Clinical Trials and Observational Tribulations: Providing Clinical Evidence for Personalized Surgical Pain Management Care Models. J. Pers. Med. 2023, 13, 1044. https://doi.org/10.3390/jpm13071044
Abraham I, Lewandrowski K-U, Elfar JC, Li Z-M, Fiorelli RKA, Pereira MG, Lorio MP, Burkhardt BW, Oertel JM, Winkler PA, et al. Randomized Clinical Trials and Observational Tribulations: Providing Clinical Evidence for Personalized Surgical Pain Management Care Models. Journal of Personalized Medicine. 2023; 13(7):1044. https://doi.org/10.3390/jpm13071044
Chicago/Turabian StyleAbraham, Ivo, Kai-Uwe Lewandrowski, John C. Elfar, Zong-Ming Li, Rossano Kepler Alvim Fiorelli, Mauricio G. Pereira, Morgan P. Lorio, Benedikt W. Burkhardt, Joachim M. Oertel, Peter A. Winkler, and et al. 2023. "Randomized Clinical Trials and Observational Tribulations: Providing Clinical Evidence for Personalized Surgical Pain Management Care Models" Journal of Personalized Medicine 13, no. 7: 1044. https://doi.org/10.3390/jpm13071044
APA StyleAbraham, I., Lewandrowski, K.-U., Elfar, J. C., Li, Z.-M., Fiorelli, R. K. A., Pereira, M. G., Lorio, M. P., Burkhardt, B. W., Oertel, J. M., Winkler, P. A., Yang, H., León, J. F. R., Telfeian, A. E., Dowling, Á., Vargas, R. A. A., Ramina, R., Asefi, M., de Carvalho, P. S. T., Defino, H., ... on behalf of Teams/Organizations/Institutions. (2023). Randomized Clinical Trials and Observational Tribulations: Providing Clinical Evidence for Personalized Surgical Pain Management Care Models. Journal of Personalized Medicine, 13(7), 1044. https://doi.org/10.3390/jpm13071044












