Acute Coronary Syndrome: Disparities of Pathophysiology and Mortality with and without Peripheral Artery Disease
Abstract
1. Introduction
2. Anatomy of Coronary Arteries: Normal and Pathological Aspects
3. Anatomy of Lower Extremity Arteries: Normal Aspects
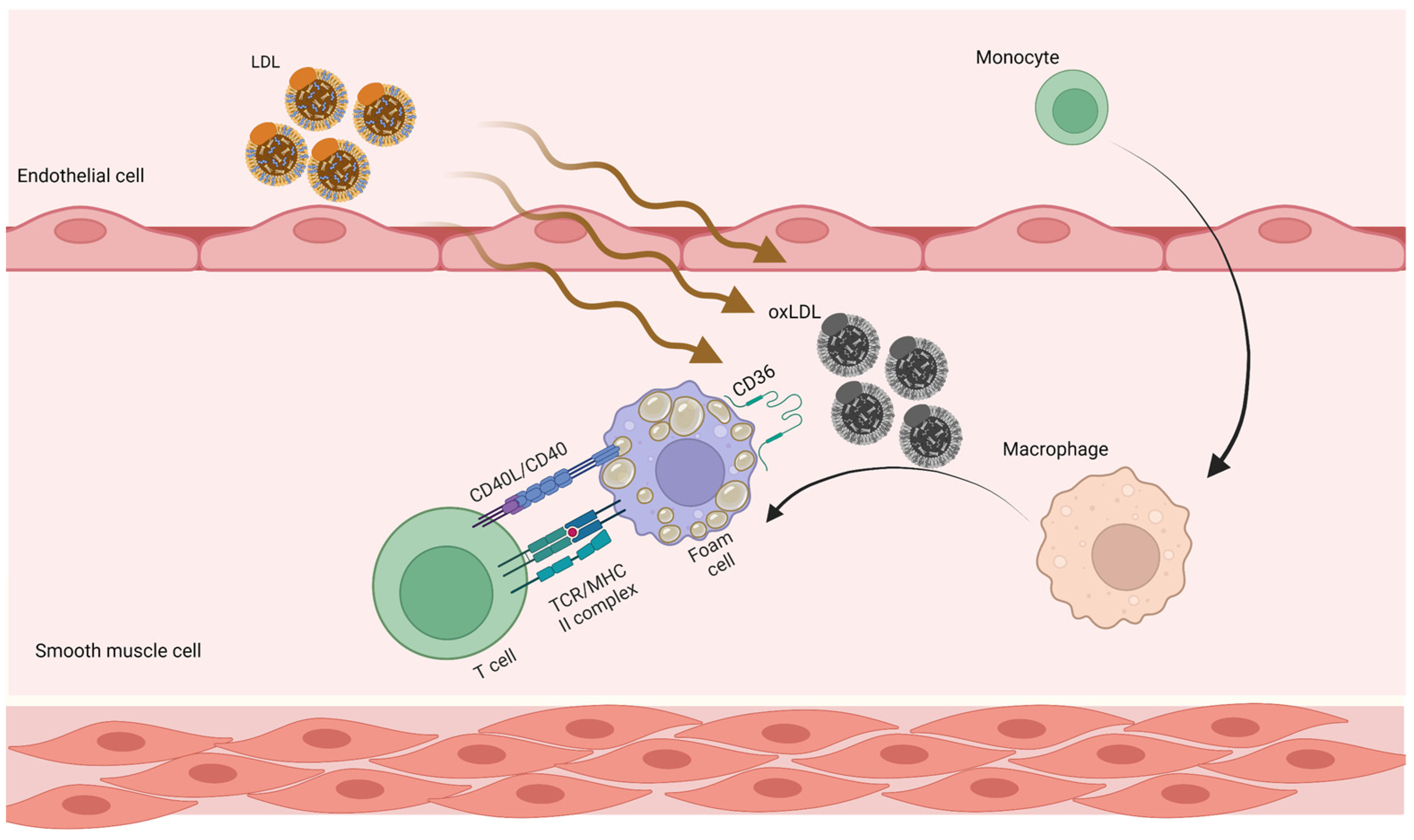
4. Atherosclerosis
4.1. Pathogenesis of Atherosclerosis
4.2. Immune Response in Atherosclerosis
4.3. Atherosclerosis and Autoreactive CD4+ T cells
4.4. Future Atherosclerosis Prevention
5. Diagnostic Approach to Coronary Artery Disease
6. Diagnostic Approach to Peripheral Artery Disease
7. Pathology of Coronary Artery Disease and Acute Coronary Events
8. Pathology of Peripheral Artery Disease
9. Pathological Comparison of Arteries of Patients with Coronary Artery Disease and Peripheral Artery Disease
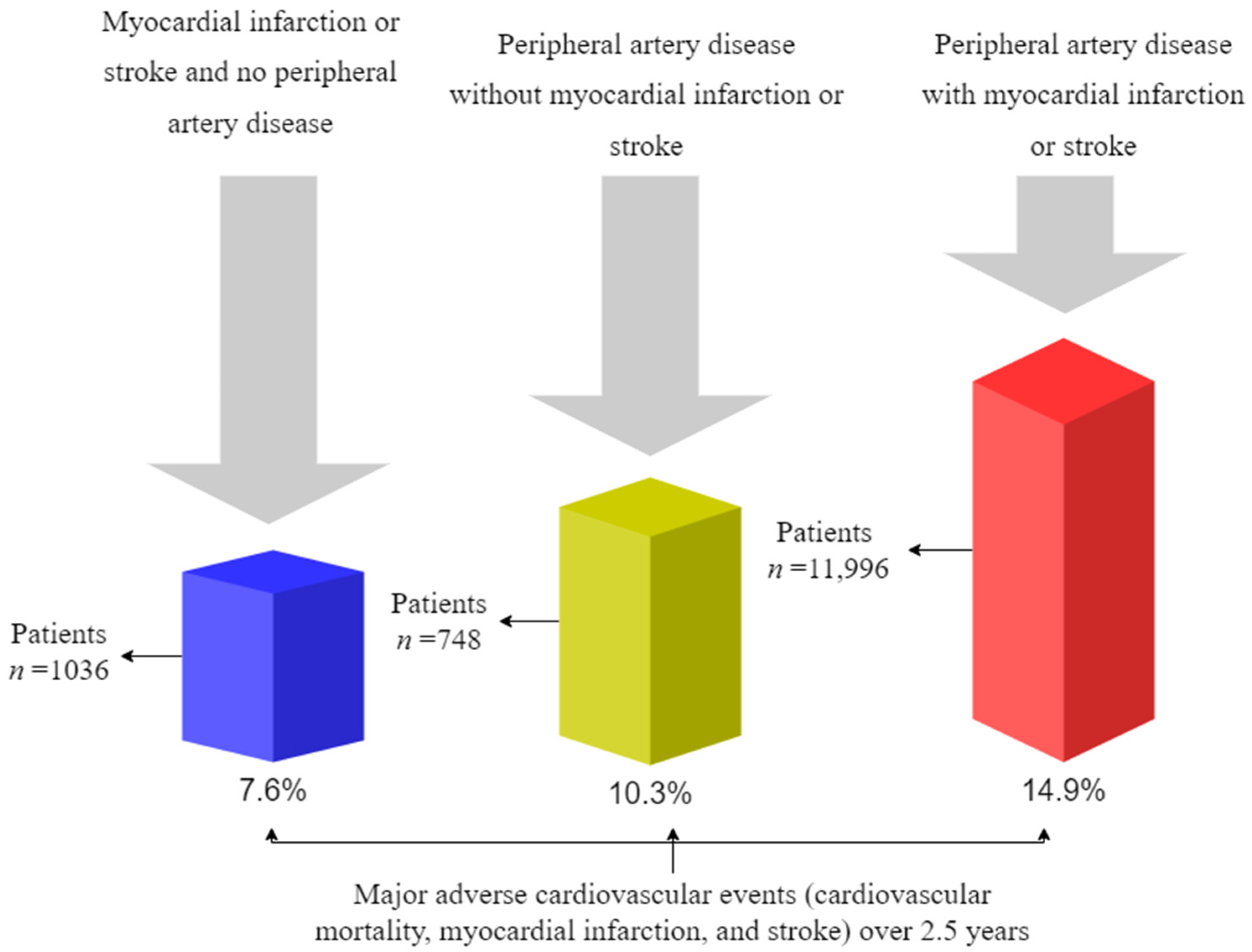
10. Mortality and Cardiovascular Outcomes
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, F.J.; Sechtem, U.; Banning, A.P.; Bonaros, N.; Bueno, H.; Bugiardini, R.; Chieffo, A.; Crea, F.; Czerny, M.; Delgado, V.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary SyndromesThe Task Force for the Diagnosis and Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Verner, V.A.; Mel’Nik, M.V.; Knjazeva, S.A. Cardio-Ankle Vascular Index (CAVI) in Diagnostics, Risk and Severity Evaluation of Magistral Vessels Lesion in Patients with Cardio-Vascular Diseases and Type 2 Diabetes. Ter. Arkhiv 2021, 93, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Thiruvoipati, T.; Kielhorn, C.E.; Armstrong, E.J. Peripheral Artery Disease in Patients with Diabetes: Epidemiology, Mechanisms, and Outcomes. World J. Diabetes 2015, 6, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Foussard, N.; Dari, L.; Ducasse, E.; Rigalleau, V.; Mohammedi, K.; Caradu, C. Lower-Limb Peripheral Arterial Disease and Amputations in People with Diabetes: Risk Factors, Prognostic Value and Management. Presse Med. 2023, 52, 104164. [Google Scholar] [CrossRef]
- Chun, D.I.; Kim, S.; Kim, J.; Yang, H.J.; Kim, J.H.; Cho, J.H.; Yi, Y.; Kim, W.J.; Won, S.H. Epidemiology and Burden of Diabetic Foot Ulcer and Peripheral Arterial Disease in Korea. J. Clin. Med. 2019, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Aday, A.W.; Everett, B.M. Dyslipidemia Profiles in Patients with Peripheral Artery Disease. Curr. Cardiol. Rep. 2019, 21, 42. [Google Scholar] [CrossRef]
- Saleh, A.; Makhamreh, H.; Qoussoos, T.; Alawwa, I.; Alsmady, M.; Salah, Z.A.; Shakhatreh, A.; Alhazaymeh, L.; Jabber, M. Prevalence of Previously Unrecognized Peripheral Arterial Disease in Patients Undergoing Coronary Angiography. Medicine 2018, 97, 11519. [Google Scholar] [CrossRef]
- Moussa, I.D.; Jaff, M.R.; Mehran, R.; Gray, W.; Dangas, G.; Lazic, Z.; Moses, J.W. Prevalence and Prediction of Previously Unrecognized Peripheral Arterial Disease in Patients with Coronary Artery Disease: The Peripheral Arterial Disease in Interventional Patients Study. Catheter. Cardiovasc. Interv. 2009, 73, 719–724. [Google Scholar] [CrossRef]
- Kumar, A.; Bano, S.; Bhurgri, U.; Kumar, J.; Ali, A.; Dembra, S.; Kumar, L.; Shahid, S.; Khalid, D.; Rizwan, A.; et al. Peripheral Artery Disease as a Predictor of Coronary Artery Disease in Patients Undergoing Coronary Angiography. Cureus 2021, 13, 15094. [Google Scholar] [CrossRef] [PubMed]
- Aursulesei Onofrei, V.; Ceasovschih, A.; Marcu, D.T.M.; Adam, C.A.; Mitu, O.; Mitu, F. Mortality Risk Assessment in Peripheral Arterial Disease—The Burden of Cardiovascular Risk Factors over the Years: A Single Center’s Experience. Diagnostics 2022, 12, 2499. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral Arterial Disease Detection, Awareness, and Treatment in Primary Care. J. Am. Med. Assoc. 2001, 286, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): Executive Summary a Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines f. J. Am. Coll. Cardiol. 2006, 47, 1239–1312. [Google Scholar] [CrossRef] [PubMed]
- Loukas, M.; Sharma, A.; Blaak, C.; Sorenson, E.; Mian, A. The Clinical Anatomy of the Coronary Arteries. J. Cardiovasc. Transl. Res. 2013, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kosar, P.; Ergun, E.; Öztürk, C.; Kosar, U. Anatomic Variations and Anomalies of the Coronary Arteries: 64-Slice CT Angiographic Appearance. Diagn. Interv. Radiol. 2009, 15, 275–283. [Google Scholar] [CrossRef]
- Alexander, R.W.; Griffith, G.C. Anomalies of the Coronary Arteries and Their Clinical Significance. Circulation 1956, 14, 800–805. [Google Scholar] [CrossRef]
- Rabin, D.N.; Rabin, S.; Mintzer, R.A. A Pictorial Review of Coronary Artery Anatomy on Spiral CT. Chest 2000, 118, 488–491. [Google Scholar] [CrossRef]
- Woodhouse, C.; Janowitz, W.; Viamonte, M. Coronary Arteries: Retrospective Cardiac Gating Technique to Reduce Cardiac Motion Artifact at Spiral CT. Radiology 1997, 204, 566–569. [Google Scholar] [CrossRef]
- Von Lüdinghausen, M. The Clinical Anatomy of Coronary Arteries. In Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2003; Volume 167, ISBN 978-3-540-43689-8. [Google Scholar]
- De Cecco, C.N.; Bastarrika, G.; Arraiza, M.; Maurizi Enrici, M.; Pueyo, J.; Muscogiuri, G.; Fina, P.; Anselmi, A.; Di Girolamo, M.; David, V. Dual Source CT: State of the Art in the Depiction of Coronary Arteries Anatomy, Anatomical Variants and Myocardial Segments. Minerva Cardioangiol. 2012, 60, 133–146. [Google Scholar]
- Young, P.M.; Gerber, T.C.; Williamson, E.E.; Julsrud, P.R.; Herfkens, R.J. Cardiac Imaging: Part 2, Normal, Variant, and Anomalous Configurations of the Coronary Vasculature. Am. J. Roentgenol. 2012, 197, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Gracia, P.; Ormiston, J.; Webster, M.; Beier, S.; Young, A.; Ellis, C.; Wang, C.; Smedby, Ö.; Cowan, B. A Computational Atlas of Normal Coronary Artery Anatomy. EuroIntervention 2016, 12, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, A.; Saini, A.; Aggarwal, A.; Gupta, T.; Saikia, U.N.; Rohit, M.K.; Sahni, D. Variant Origin and Course of Left Circumflex Coronary Artery. Surg. Radiol. Anat. SRA 2017, 39, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Qazi, E.; Wilting, J.; Patel, N.R.; Alenezi, A.O.; Kennedy, S.A.; Tan, K.T.; Jaberi, A.; Mafeld, S. Arteries of the Lower Limb-Embryology, Variations, and Clinical Significance. Can. Assoc. Radiol. J. 2022, 73, 259–270. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Y.; Wang, Y.; Li, X. Patterns of Disease Distribution of Lower Extremity Peripheral Arterial Disease. Angiology 2015, 66, 211–218. [Google Scholar] [CrossRef]
- Xie, D.; Na, J.; Zhang, M.; Dong, S.; Xiao, X. CT Angiography of the Lower Extremity and Coronary Arteries Using 256-Section CT: A Preliminary Study. Clin. Radiol. 2015, 70, 1281–1288. [Google Scholar] [CrossRef]
- Kropman, R.H.J.; Kiela, G.; Moll, F.L.; De Vries, J.P.P.M. Variations in Anatomy of the Popliteal Artery and Its Side Branches. Vasc. Endovasc. Surg. 2011, 45, 536–540. [Google Scholar] [CrossRef]
- Shah, S.; Fischman, A.; Marin, M.; Won, J. Spontaneous Tibioperoneal Trunk and Anterior Tibial Artery Pseudoaneurysms. Vasc. Med. 2012, 17, 164–167. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Atherosclerosis and Inflammation. New Therapeutic Approaches. Med. Clin. 2020, 155, 256–262. [Google Scholar] [CrossRef]
- Zárate, A.; Manuel-Apolinar, L.; Basurto, L.; De la Chesnaye, E.; Saldívar, I. Cholesterol and Atherosclerosis. Historical Considerations and Treatment. Arch. Cardiol. Mex. 2016, 86, 163–169. [Google Scholar] [CrossRef]
- Sarmah, D.; Datta, A.; Raut, S.; Sarkar, A.; Shah, B.; Bohra, M.; Singh, U.; Jagtap, P.; Baidya, F.; Kalia, K.; et al. The Role of Inflammasomes in Atherosclerosis and Stroke Pathogenesis. Curr. Pharm. Des. 2020, 26, 4234–4245. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, L.; Chen, Y.; Xie, Q.; Yan, Z.; Liu, Y.; Kang, J.; Li, S. Risk Factors for Ischemic Stroke: Differences between Cerebral Small Vessel and Large Artery Atherosclerosis Aetiologies. Folia Neuropathol. 2021, 59, 378–385. [Google Scholar] [CrossRef]
- Razavi, A.C.; Agatston, A.S.; Shaw, L.J.; De Cecco, C.N.; van Assen, M.; Sperling, L.S.; Bittencourt, M.S.; Daubert, M.A.; Nasir, K.; Blumenthal, R.S.; et al. Evolving Role of Calcium Density in Coronary Artery Calcium Scoring and Atherosclerotic Cardiovascular Disease Risk. Cardiovasc. Imaging 2022, 15, 1648–1662. [Google Scholar] [CrossRef]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front. Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Weitgasser, R.; Ratzinger, M.; Hemetsberger, M.; Siostrzonek, P. LDL-Cholesterol and Cardiovascular Events: The Lower the Better? Wien. Med. Wochenschr. 2018, 168, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Kroon, A.A.; Stalenhoef, A.F.H. LDL-Cholesterol Lowering and Atherosclerosis—Clinical Benefit and Possible Mechanisms: An Update. Neth. J. Med. 1997, 51, 16–27. [Google Scholar] [CrossRef]
- Gencer, B.; Marston, N.A.; Im, K.A.; Cannon, C.P.; Sever, P.; Keech, A.; Braunwald, E.; Giugliano, R.P.; Sabatine, M.S. Efficacy and Safety of Lowering LDL Cholesterol in Older Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet 2020, 396, 1637–1643. [Google Scholar] [CrossRef]
- Stoekenbroek, R.M.; Lambert, G.; Cariou, B.; Hovingh, G.K. Inhibiting PCSK9—Biology beyond LDL Control. Nat. Rev. Endocrinol. 2018, 15, 52–62. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The Evolving Future of PCSK9 Inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Reduction in C-Reactive Protein and LDL Cholesterol and Cardiovascular Event Rates after Initiation of Rosuvastatin: A Prospective Study of the JUPITER Trial. Lancet 2009, 373, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Closing the Loop on Inflammation and Atherothrombosis: Why Perform the CIRT and CANTOS Trials? Trans. Am. Clin. Climatol. Assoc. 2013, 124, 174–190. [Google Scholar] [PubMed]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of Interleukin-1beta Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef]
- Merhi-Soussi, F.; Kwak, B.R.; Magne, D.; Chadjichristos, C.; Berti, M.; Pelli, G.; James, R.W.; MacH, F.; Gabay, C. Interleukin-1 Plays a Major Role in Vascular Inflammation and Atherosclerosis in Male Apolipoprotein E-Knockout Mice. Cardiovasc. Res. 2005, 66, 583–593. [Google Scholar] [CrossRef]
- Isoda, K.; Sawada, S.; Ishigami, N.; Matsuki, T.; Miyazaki, K.; Kusuhara, M.; Iwakura, Y.; Ohsuzu, F. Lack of Interleukin-1 Receptor Antagonist Modulates Plaque Composition in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1068–1073. [Google Scholar] [CrossRef]
- Kimura, T.; Tse, K.; Sette, A.; Ley, K. Vaccination to Modulate Atherosclerosis. Autoimmunity 2015, 48, 152–160. [Google Scholar] [CrossRef]
- Kimura, T.; Kobiyama, K.; Winkels, H.; Tse, K.; Miller, J.; Vassallo, M.; Wolf, D.; Ryden, C.; Orecchioni, M.; Dileepan, T.; et al. Regulatory CD4+ T Cells Recognize Major Histocompatibility Complex Class II Molecule–Restricted Peptide Epitopes of Apolipoprotein B. Circulation 2018, 138, 1130–1143. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef]
- Sekiya, M.; Osuga, J.; Nagashima, S.; Ohshiro, T.; Igarashi, M.; Okazaki, H.; Takahashi, M.; Tazoe, F.; Wada, T.; Ohta, K.; et al. Ablation of Neutral Cholesterol Ester Hydrolase 1 Accelerates Atherosclerosis. Cell Metab. 2009, 10, 219–228. [Google Scholar] [CrossRef]
- Sukhorukov, V.N.; Khotina, V.A.; Chegodaev, Y.S.; Ivanova, E.; Sobenin, I.A.; Orekhov, A.N. Lipid Metabolism in Macrophages: Focus on Atherosclerosis. Biomedicines 2020, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Fu, Y.; Zhang, D.; Yin, K.; Tang, C. Foam Cells in Atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated Cholesterol Handling in Atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Cagnina, A.; Chabot, O.; Davin, L.; Lempereur, M.; Maréchal, P.; Oury, C.; Lancellotti, P. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 302–309. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque Hemorrhage and Progression of Coronary Atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef]
- Ma, J.; Luo, J.; Sun, Y.; Zhao, Z. Cytokines Associated with Immune Response in Atherosclerosis. Am. J. Transl. Res. 2022, 14, 6424–6444. [Google Scholar] [PubMed]
- Tsioufis, P.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. The Impact of Cytokines in Coronary Atherosclerotic Plaque: Current Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 15937. [Google Scholar] [CrossRef]
- Creager, M.A.; Belkin, M.; Bluth, E.I.; Casey, D.E.; Chaturvedi, S.; Dake, M.D.; Fleg, J.L.; Hirsch, A.T.; Jaff, M.R.; Kern, J.A.; et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS Key Data Elements and Definitions for Peripheral Atherosclerotic Vascular Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease). Circulation 2012, 125, 395–467. [Google Scholar] [CrossRef]
- Lundberg, A.M.; Hansson, G.K. Innate Immune Signals in Atherosclerosis. Clin. Immunol. 2010, 134, 5–24. [Google Scholar] [CrossRef]
- Marchini, T.; Mitre, L.S.; Wolf, D. Inflammatory Cell Recruitment in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 207. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Salomon, B.L.; Potteaux, S.; Robertson, A.K.L.; Gourdy, P.; Zoll, J.; Merval, R.; Esposito, B.; Cohen, J.L.; Fisson, S.; et al. Natural Regulatory T Cells Control the Development of Atherosclerosis in Mice. Nat. Med. 2006, 12, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Saleheen, D.; Haycock, P.C.; Zhao, W.; Rasheed, A.; Taleb, A.; Imran, A.; Abbas, S.; Majeed, F.; Akhtar, S.; Qamar, N.; et al. Apolipoprotein(a) Isoform Size, Lipoprotein(a) Concentration, and Coronary Artery Disease: A Mendelian Randomisation Analysis. Lancet Diabetes Endocrinol. 2017, 5, 524–533. [Google Scholar] [CrossRef]
- Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; Nelson, C.P.; et al. Interleukin-6 Receptor Pathways in Coronary Heart Disease: A Collaborative Meta-Analysis of 82 Studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Holmes, M.V.; Kuchenbaecker, K.B.; Engmann, J.E.L.; Shah, T.; Sofat, R.; Guo, Y.; Chung, C.; Peasey, A.; Pfister, R.; et al. The Interleukin-6 Receptor as a Target for Prevention of Coronary Heart Disease: A Mendelian Randomisation Analysis. Lancet 2012, 379, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Zhang, Y.; Ho, Y.L.; Link, N.; Sun, J.; Huang, J.; Cai, T.A.; Damrauer, S.; Ahuja, Y.; Honerlaw, J.; et al. Association of Interleukin 6 Receptor Variant with Cardiovascular Disease Effects of Interleukin 6 Receptor Blocking Therapy: A Phenome-Wide Association Study. JAMA Cardiol. 2018, 3, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kishimoto, T. Interplay between Interleukin-6 Signaling and the Vascular Endothelium in Cytokine Storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef]
- Shuvalova, Y.A.; Kaminnaya, V.; Kaminnyi, A.I. Contribution of Interleukin-6 System Genes Polymorphisms to the Development of Coronary Atherosclerosis. Gene 2023, 861, 147253. [Google Scholar] [CrossRef]
- Ortega-Rivera, O.A.; Shin, M.D.; Moreno-Gonzalez, M.A.; Pokorski, J.K.; Steinmetz, N.F. A Single-Dose Qβ VLP Vaccine against S100A9 Protein Reduces Atherosclerosis in a Preclinical Model. Adv. Ther. 2022, 5, 2200092. [Google Scholar] [CrossRef]
- Ahamad, S.; Bhat, S.A. Recent Update on the Development of PCSK9 Inhibitors for Hypercholesterolemia Treatment. J. Med. Chem. 2022, 65, 15513–15539. [Google Scholar] [CrossRef]
- Ramonfaur, D.; Hinojosa-González, D.E.; Paredes-Vázquez, J.G. Killip-Kimball Classification in Octogenarians with Acute Coronary Syndrome: An 11-Year Experience. Arch. Cardiol. Mex. 2022, 92, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Killip, T.; Kimball, J.T. Treatment of Myocardial Infarction in a Coronary Care Unit. A Two Year Experience with 250 Patients. Am. J. Cardiol. 1967, 20, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zeymer, U.; Bueno, H.; Granger, C.B.; Hochman, J.; Huber, K.; Lettino, M.; Price, S.; Schiele, F.; Tubaro, M.; Vranckx, P.; et al. Acute Cardiovascular Care Association Position Statement for the Diagnosis and Treatment of Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur. Heart J. 2020, 9, 183–197. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute Coronary Syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment ElevationThe Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Neumann, J.; Twerenbold, R.; Ojeda, F.; Sörensen, N.A.; Chapman, A.R.; Shah, A.S.V.; Anand, A.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; et al. Application of High-Sensitivity Troponin in Suspected Myocardial Infarction. N. Engl. J. Med. 2019, 380, 2529–2540. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; Dimaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E18–E114. [Google Scholar] [CrossRef]
- Saraste, A.; Knuuti, J. ESC 2019 Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes: Recommendations for Cardiovascular Imaging. Herz 2020, 45, 409–420. [Google Scholar] [CrossRef]
- Matetzky, S.; Natanzon, S.S.; Shlomo, N.; Atar, S.; Pollak, A.; Yosefy, C.; Zahger, D.; Fefer, P.; Iakobishvili, Z.; Mazin, I.; et al. Peripheral Arterial Disease in Patients With Acute Coronary Syndrome: Results From a Large Real-World Registry. Heart Lung Circ. 2022, 31, 1093–1101. [Google Scholar] [CrossRef]
- Bonacchi, M.; Parise, O.; Matteucci, F.; Tetta, C.; Moula, A.I.; Micali, L.R.; Dokollari, A.; Martino, M.D.; Sani, G.; Grasso, A.; et al. Is Peripheral Artery Disease an Independent Predictor of Isolated Coronary Artery Bypass Outcome? Heart Lung Circ. 2020, 29, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Attar, R.; Wu, A.; Wojdyla, D.; Jensen, S.E.; Andell, P.; Mahaffey, K.W.; Roe, M.T.; James, S.K.; Wallentin, L.; Vemulapalli, S.; et al. Outcomes After Acute Coronary Syndrome in Patients With Diabetes Mellitus and Peripheral Artery Disease (from the TRACER, TRILOGY-ACS, APPRAISE-2, and PLATO Clinical Trials). Am. J. Cardiol. 2022, 178, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Shishehbor, M.H.; Takahashi, E.A.; Aronow, H.D.; Brewster, L.P.; Bunte, M.C.; Kim, E.S.H.; Lindner, J.R.; Rich, K. Perfusion Assessment in Critical Limb Ischemia: Principles for Understanding and the Development of Evidence and Evaluation of Devices: A Scientific Statement from the American Heart Association. Circulation 2019, 140, E657–E672. [Google Scholar] [CrossRef] [PubMed]
- Cassar, K. Intermittent Claudication. BMJ 2006, 333, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of Classification Systems in Peripheral Artery Disease. Semin. Interv. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Kieback, A.G.; Gähwiler, R.; Thalhammer, C. PAD Screening: Why? Whom? When? How?—A Systematic Review. Vasa 2021, 50, 85–91. [Google Scholar] [CrossRef]
- Marco, M.; Valentina, I.; Daniele, M.; Valerio, D.R.; Andrea, P.; Roberto, G.; Laura, G.; Luigi, U. Peripheral Arterial Disease in Persons with Diabetic Foot Ulceration: A Current Comprehensive Overview. Curr. Diabetes Rev. 2021, 17, 474–485. [Google Scholar] [CrossRef]
- Park, S.C.; Choi, C.Y.; Ha, Y.I.; Yang, H.E. Utility of Toe-Brachial Index for Diagnosis of Peripheral Artery Disease. Arch. Plast. Surg. 2012, 39, 227–231. [Google Scholar] [CrossRef]
- Mills, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk Stratification Based on Wound, Ischemia, and Foot Infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef]
- Obara, H.; Matsubara, K.; Kitagawa, Y. Acute Limb Ischemia. Ann. Vasc. Dis. 2018, 11, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Yusrizal, T.; Dewi, I.P.; Wardhani, L.F.K.; Lefi, A. Massive Thrombosis in Elderly with Acute Limb Ischemia: Lessons Learned from Difficult Case. Vis. J. Emerg. Med. 2021, 25, 101173. [Google Scholar] [CrossRef]
- Roush, W.P.; Peters, A.; Vogel, T.R.; Balasundaram, N.; Bath, J. Balloon-Assisted Endovascular Thrombectomy for Tibial Thromboembolism. Ann. Vasc. Surg. 2022, 79, 440.e1–440.e5. [Google Scholar] [CrossRef]
- King, E.G.; Farber, A. What Is the Best Treatment for Acute Limb Ischemia? Adv. Surg. 2022, 56, 287–304. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.M.; Univers, J. Acute Limb Ischemia. Surg. Clin. N. Am. 2018, 98, 1081–1096. [Google Scholar] [CrossRef] [PubMed]
- Buls, N.; de Brucker, Y.; Aerden, D.; Devos, H.; Van Gompel, G.; Boonen, P.T.; Nieboer, K.; Leiner, T.; de Mey, J. Improving the Diagnosis of Peripheral Arterial Disease in Below-the-Knee Arteries by Adding Time-Resolved CT Scan Series to Conventional Run-off CT Angiography. First Experience with a 256-Slice CT Scanner. Eur. J. Radiol. 2019, 110, 136–141. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Campbell, J.E. Overview of Peripheral Arterial Disease of the Lower Extremity. In Noninvasive Vascular Diagnosis: A Practical Textbook for Clinicians; AbuRahma, A.F., Perler, B.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 449–488. ISBN 978-3-030-60626-8. [Google Scholar]
- Metaxas, V.I.; Dimitroukas, C.P.; Efthymiou, F.O.; Zampakis, P.E.; Panayiotakis, G.S.; Kalogeropoulou, C.P. Patient Dose in CT Angiography Examinations: An Institutional Survey. Radiat. Phys. Chem. 2022, 195, 110083. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar] [CrossRef]
- Buckler, A.J.; Gotto, A.M.; Rajeev, A.; Nicolaou, A.; Sakamoto, A.; Pierre, S.S.; Phillips, M.; Virmani, R.; Villines, T.C. Atherosclerosis Risk Classification with Computed Tomography Angiography: A Radiologic-Pathologic Validation Study. Atherosclerosis 2023, 366, 42–48. [Google Scholar] [CrossRef]
- van Zandvoort, L.J.C.; Otsuka, K.; Villiger, M.; Neleman, T.; Dijkstra, J.; Zijlstra, F.; van Mieghem, N.M.; Bouma, B.E.; Daemen, J. Polarimetric Signatures of Coronary Thrombus in Patients with Acute Coronary Syndrome. Circ. J. 2021, 85, 1806–1813. [Google Scholar] [CrossRef]
- Falk, E.; Shah, P.K.; Fuster, V. Coronary Plaque Disruption. Circulation 1995, 92, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Medaković, P.; Jukić, M.; Biloglav, Z. Vulnerable Plaque Characteristics at Coronary Computed Tomography Angiography. Cardiol. Croat. 2023, 18, 7–21. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Kaufman, J.A.; Conte, M.S. Clinical Practice. Acute Limb Ischemia. N. Engl. J. Med. 2012, 366, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.P.J.; Banerjee, A.; Fairhead, J.F.; Hands, L.; Silver, L.E.; Rothwell, P.M. Population-Based Study of Incidence, Risk Factors, Outcome, and Prognosis of Ischemic Peripheral Arterial Events: Implications for Prevention. Circulation 2015, 132, 1805–1815. [Google Scholar] [CrossRef]
- Narula, N.; Olin, J.W.; Narula, N. Pathologic Disparities between Peripheral Artery Disease and Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1982–1989. [Google Scholar] [CrossRef]
- Kuyama, N.; Kaikita, K.; Ishii, M.; Mitsuse, T.; Nakanishi, N.; Fujisue, K.; Otsuka, Y.; Hanatani, S.; Sueta, D.; Takashio, S.; et al. Increased Thrombogenicity Is Associated with Revascularization Outcomes in Patients with Chronic Limb-Threatening Ischemia. J. Vasc. Surg. 2022, 76, 513–522.e3. [Google Scholar] [CrossRef]
- Torii, S.; Mustapha, J.A.; Narula, J.; Mori, H.; Saab, F.; Jinnouchi, H.; Yahagi, K.; Sakamoto, A.; Romero, M.E.; Narula, N.; et al. Histopathologic Characterization of Peripheral Arteries in Subjects With Abundant Risk Factors: Correlating Imaging With Pathology. JACC Cardiovasc. Imaging 2019, 12, 1501–1513. [Google Scholar] [CrossRef]
- Soor, G.S.; Vukin, I.; Leong, S.W.; Oreopoulos, G.; Butany, J. Peripheral Vascular Disease: Who Gets It and Why? A Histomorphological Analysis of 261 Arterial Segments from 58 Cases. Pathology 2008, 40, 385–391. [Google Scholar] [CrossRef]
- Narula, J.; Ibáñez, B.; Fuster, V. From Heart to Head, Thrombi to Emboli, and Inferences to Extrapolation. J. Am. Coll. Cardiol. 2019, 73, 1000–1003. [Google Scholar] [CrossRef]
- Ho, C.Y.; Shanahan, C.M. Medial Arterial Calcification: An Overlooked Player in Peripheral Arterial Disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.F.; Zhang, K.L.; Shen, X.B.; Lin, W.C.; Hu, B.; Cai, H.P.; Huang, G. Retrospective Study Postoperative Adverse Cardiac Events in Acute Myocardial Infarction with High Thrombus Load and Best Time for Stent Implantation. World J. Clin. Cases 2022, 10, 2106–2114. [Google Scholar] [CrossRef]
- Yoshida, K.; Yang, T.; Yamamoto, Y.; Kurosaki, Y.; Funaki, T.; Kikuchi, T.; Ishii, A.; Kataoka, H.; Miyamoto, S. Expansive Carotid Artery Remodeling: Possible Marker of Vulnerable Plaque. J. Neurosurg. 2019, 133, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Hibi, K. Intravascular Ultrasound in Vulnerable Plaque and Acute Coronary Syndrome. Interv. Cardiol. Clin. 2023, 12, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Schantl, A.E.; Ivarsson, M.E.; Leroux, J.C. Investigational Pharmacological Treatments for Vascular Calcification. Adv. Ther. 2019, 2, 1800094. [Google Scholar] [CrossRef]
- Anand, S.S.; Caron, F.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Aboyans, V.; Abola, M.T.; Branch, K.R.H.; Keltai, K.; Bhatt, D.L.; et al. Major Adverse Limb Events and Mortality in Patients With Peripheral Artery Disease: The COMPASS Trial. J. Am. Coll. Cardiol. 2018, 71, 2306–2315. [Google Scholar] [CrossRef]
- Nakamura, S.; Ishibashi-Ueda, H.; Niizuma, S.; Yoshihara, F.; Horio, T.; Kawano, Y. Coronary Calcification in Patients with Chronic Kidney Disease and Coronary Artery Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1892–1900. [Google Scholar] [CrossRef]
- Rerkasem, A.; Nopparatkailas, R.; Nantakool, S.; Rerkasem, R.; Chansakaow, C.; Apichartpiyakul, P.; Phrommintikul, A.; Rerkasem, K. The Ability of Clinical Decision Rules to Detect Peripheral Arterial Disease: A Narrative Review. Int. J. Low. Extrem. Wounds 2022, 15347346221104590. [Google Scholar] [CrossRef]
- Sakamoto, A.; Virmani, R.; Finn, A.V.; Gupta, A. Calcified Nodule as the Cause of Acute Coronary Syndrome: Connecting Bench Observations to the Bedside. Cardiology 2018, 139, 101–104. [Google Scholar] [CrossRef]
- Eligini, S.; Gianazza, E.; Mallia, A.; Ghilardi, S.; Banfi, C. Macrophage Phenotyping in Atherosclerosis by Proteomics. Int. J. Mol. Sci. 2023, 24, 2613. [Google Scholar] [CrossRef]
- Kitada, R.; Otsuka, K.; Fukuda, D. Role of Plaque Imaging for Identification of Vulnerable Patients beyond the Stage of Myocardial Ischemia. Front. Cardiovasc. Med. 2023, 10, 1095806. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, G.; Fowkes, F.G.R.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; et al. Ankle Brachial Index Combined With Framingham Risk Score to Predict Cardiovascular Events and Mortality: A Meta-Analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Grenon, S.M.; Hiramoto, J.; Smolderen, K.G.; Vittinghoff, E.; Whooley, M.A.; Cohen, B.E. Association between Depression and Peripheral Artery Disease: Insights from the Heart and Soul Study. J. Am. Heart Assoc. 2012, 1, e002667. [Google Scholar] [CrossRef]
- Wickström, J.E.; Laivuori, M.; Aro, E.; Sund, R.T.; Hautero, O.; Venermo, M.; Jalkanen, J.; Hakovirta, H. Toe Pressure and Toe Brachial Index Are Predictive of Cardiovascular Mortality, Overall Mortality, and Amputation Free Survival in Patients with Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Sacks, C.A.; Avorn, J.; Kesselheim, A.S. The Failure of Solanezumab—How the FDA Saved Taxpayers Billions. N. Engl. J. Med. 2017, 376, 1706–1708. [Google Scholar] [CrossRef]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Dullaart, R.P.F. PCSK9 Inhibition to Reduce Cardiovascular Events. N. Engl. J. Med. 2017, 376, 1790–1791. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S. A PCSK9 Missense Variant Associated with a Reduced Risk of Early-Onset Myocardial Infarction. N. Engl. J. Med. 2008, 358, 2299–2300. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, J.C.; Conti, C.R. Intensive Lipid Lowering with Atorvastatin in Patients with Stable Coronary Disease. ACC Cardiosource Rev. J. 2006, 15, 97–99. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Fowkes, F.G.R.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; Mahaffey, K.W.; Norgren, L.; Jones, W.S.; et al. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N. Engl. J. Med. 2017, 376, 32–40. [Google Scholar] [CrossRef]
- Bundó Vidiella, M.; Pérez Pérez, C.; Montero Alia, J.J.; Cobos Solórzano, M.D.; Aubà Llambrich, J.; Cabezas Peña, C. Peripheral Artery Disease of the Lower Limbs and Morbidity/Mortality in Type 2 Diabetics. Atención Primaria 2006, 38, 139–144. [Google Scholar] [CrossRef]
- Mueller, T.; Hinterreiter, F.; Poelz, W.; Haltmayer, M.; Dieplinger, B. Mortality Rates at 10 Years Are Higher in Diabetic than in Non-Diabetic Patients with Chronic Lower Extremity Peripheral Arterial Disease. Vasc. Med. 2016, 21, 445–452. [Google Scholar] [CrossRef]
- Schuyler Jones, W.; Patel, M.R.; Dai, D.; Vemulapalli, S.; Subherwal, S.; Stafford, J.; Peterson, E.D. High Mortality Risks after Major Lower Extremity Amputation in Medicare Patients with Peripheral Artery Disease. Am. Heart J. 2013, 165, 809–815.e1. [Google Scholar] [CrossRef]
- Voci, D.; Fedeli, U.; Valerio, L.; Schievano, E.; Righini, M.; Kucher, N.; Spirk, D.; Barco, S. Mortality Rate Related to Peripheral Arterial Disease: A Retrospective Analysis of Epidemiological Data (Years 2008–2019). Nutr. Metab. Cardiovasc. Dis. 2023, 33, 516–522. [Google Scholar] [CrossRef]
| Stage I | Asymptomatic |
| Stage IIa | Intermittent claudication after more than 200 m of walking |
| Stage IIb | Intermittent claudication after less than 200 m of walking |
| Stage III | Rest pain. Rest pain appears especially during the night when the legs are raised up on to the bed, which diminishes the gravitational effect present by day |
| Stage IV | Ischaemic ulcers or gangrene (which may be dry or humid) [86] |
| Stage 0 | Asymptomatic |
| Stage 1 | Mild claudication |
| Stage 2 | Moderate claudication—the distance that delineates mild, moderate, and severe claudication is not specified in the Rutherford classification, as it is in the Fontaine classification. |
| Stage 3 | Severe claudication |
| Stage 4 | Rest pain |
| Stage 5 | Ischaemic ulceration not exceeding ulcers of the digits of the foot |
| Stage 6 | Severe ischaemic ulcers or frank gangrene [86] |
| Study Name | Number of Patients | Trial Type | End Point |
|---|---|---|---|
| FOURIER trial | 27,564 | Prospective, randomized, double-blind, placebo-controlled trial | Evolocumab significantly reduced the risk of the primary composite end point of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization [127]. |
| High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease 2 | 186,338 patients with identified PAD who underwent major LE amputation | Retrospective study | Mortality rate 13.5% at 30 days, 48.3% at 1 year, and 70.9% at 3 years. Age per 5-year increase (hazard ratio [HR] 1.29, 95% CI 1.29–1.29), history of heart failure (HR 1.71, 95% CI 1.71–1.72), renal disease (HR 1.84. 95% CI 1.83–1.85), cancer (HR 1.71, 95% CI 1.70–1.72), and chronic obstructive pulmonary disease (HR 1.33, 95% CI, 1.32–1.33) were all independently associated with death after major LE amputation [140]. |
| LIPAD study | 331 | Prospective study | Mortality rates at 10 years were 29% in non-diabetic PAD patients versus 14% in age- and sex-matched non-diabetic controls (risk ratio (RR), 2.31; 95% confidence interval (CI), 1.54–3.47; p < 0.001), and 58% in diabetic PAD patients versus 19% in age- and sex-matched diabetic controls (RR, 4.06; 95% CI, 2.67–6.18; p < 0.001) [139]. |
| Peripheral Artery Disease of the Lower Limbs and Morbidity/Mortality in Type 2 Diabetics | 269 type 2 diabetics, of which 63 had peripheral artery disease | Retrospective study | 39 patients had died, of whom 19 had PAD in 1996 (30.1%) and 20 did not (9.7%) (p = 0.001).16 died in the group with an ABI of <0.9 (30.2%) and 21 (10.1%) in the group with normal ABI values (p = 0.001). Seven (13.2%) patients died due to a cardiovascular cause with a pathological ABI, and eight (3.9%) with a normal value (p = 0.009) [138]. |
| EUCLID study | 13,885 | Prospective study, multicenter, randomized, double-blind | A total of 1263 out of 13,885 (9.1%) patients died (median follow-up: 30 months). There were 706 patients (55.9%) with a cardiovascular cause of death and 522 (41.3%) with a noncardiovascular cause of death [137]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherasie, F.-A.; Popescu, M.-R.; Bartos, D. Acute Coronary Syndrome: Disparities of Pathophysiology and Mortality with and without Peripheral Artery Disease. J. Pers. Med. 2023, 13, 944. https://doi.org/10.3390/jpm13060944
Gherasie F-A, Popescu M-R, Bartos D. Acute Coronary Syndrome: Disparities of Pathophysiology and Mortality with and without Peripheral Artery Disease. Journal of Personalized Medicine. 2023; 13(6):944. https://doi.org/10.3390/jpm13060944
Chicago/Turabian StyleGherasie, Flavius-Alexandru, Mihaela-Roxana Popescu, and Daniela Bartos. 2023. "Acute Coronary Syndrome: Disparities of Pathophysiology and Mortality with and without Peripheral Artery Disease" Journal of Personalized Medicine 13, no. 6: 944. https://doi.org/10.3390/jpm13060944
APA StyleGherasie, F.-A., Popescu, M.-R., & Bartos, D. (2023). Acute Coronary Syndrome: Disparities of Pathophysiology and Mortality with and without Peripheral Artery Disease. Journal of Personalized Medicine, 13(6), 944. https://doi.org/10.3390/jpm13060944






