1. Introduction
Inflammatory bowel disease (IBD) consists of Crohn’s disease (CD) and ulcerative colitis (UC), both of which are characterized by chronic inflammation of the gastrointestinal tract [
1,
2]. CD can affect the entire gastrointestinal tract, whereas UC is restricted to the colon. Abdominal pain is one of the major symptoms of IBD, especially for those with coexisting irritable bowel syndrome (IBS) [
3,
4]. Chronic abdominal pain could persist even when IBD is in clinical remission, which can be debilitating and significantly affect patients’ quality of life [
5,
6]. However, patients’ self-reported symptoms may not correlate with disease activity and inflammation [
7,
8]. Patients with overlapping IBD-IBS are associated with increased psychiatric diagnoses and lower quality of life [
9]. It was postulated that IBD and IBS share many different mechanisms, including increased mucosal permeability [
10], increased production of bio-mediators [
11], abnormal enteric nerves [
12], psychological stress [
13,
14], and gut microbiota dysbiosis [
4,
15,
16].
Acute pain in IBD could be due to active inflammation, fistulas, abscesses, strictures, adhesions, bowel obstruction, and dysmotility [
17]. These are treated by addressing the underlying cause. Management of chronic abdominal pain in the absence of active inflammation and IBD-related complications can be challenging [
18]. Causes of chronic abdominal pain in the absence of inflammation can include surgical adhesions, fibrostenotic bowel, small intestinal bacterial overgrowth (SIBO), post-surgical complications, or disorders of gut-brain interaction, or any other etiologies like those in the general population (cholecystitis, pancreatitis, etc.). A meta-analysis in 2020 reported that the overall prevalence of IBS in IBD patients was 32.5%; specifically, the prevalence of IBS in patients with CD and UC in remission was estimated to be 36.6% and 28.7%, respectively [
19].
Even though against recommendations, opioids are commonly prescribed to control chronic abdominal pain in patients with IBD in the real-world clinical setting, which may result in an increased risk of developing opioid addiction in this population [
20]. Current guidelines do not support the use of opioids except in acute IBD-related admission. Opioids are well known to cause various gastrointestinal side effects, such as delaying gastrointestinal transit, constipation, nausea, vomiting, bloating, and gastroesophageal reflux disease [
21,
22]. In addition, opioids are associated with increased healthcare utilization, mortality, and decreased quality of life in patients with IBD [
23,
24,
25]. Patients with coexisting CD and functional gastrointestinal disorders are especially prone to chronic opioid use [
26]. Previously, it has been reported that patients with overlapping IBD-IBS were associated with increased opioid use and adverse outcomes in a cross-sectional study [
27]. Nevertheless, large-scale database studies addressing this topic are currently lacking. It is essential for clinicians to be aware of the potential risks of opioid misuse in this particular population and refrain from prescribing opioids for the management of functional abdominal pain in IBD patients.
The aim of the study was to assess the risks of opioid prescription use and related complications in patients with overlapping IBD-IBS using a large clinical database. Simultaneously, we aim to raise awareness among clinicians regarding the potential over-prescription of opioids in patients with concurrent IBD and IBS, particularly in light of the ongoing opioid epidemic in the US.
2. Materials and Methods
2.1. Data Source
TriNetX is a global federated health research network that provided access to aggregated de-identified electronic healthcare record (EHR) data across 93 large healthcare organizations (HCOs) with over 120 million patients. The majority of HCOs are from the US and Europe. This database provides real-time longitudinal patient information, which allows for customizable cohort selection. It contains information including diagnoses, procedures, medications, and laboratory values from both inpatient and outpatient settings. In this study, patients were enrolled from the US Collaborative Network of the TriNetX platform. The US collaborative Network contained 56 HCOs with over 92 million patients by May 2023. Review and approval by Metrohealth Medical Center Institutional Review Board (IRB) was exempted since TriNetX is a de-identified database without the involvement of any identifiable patients’ personal information.
2.2. Cohort Definitions
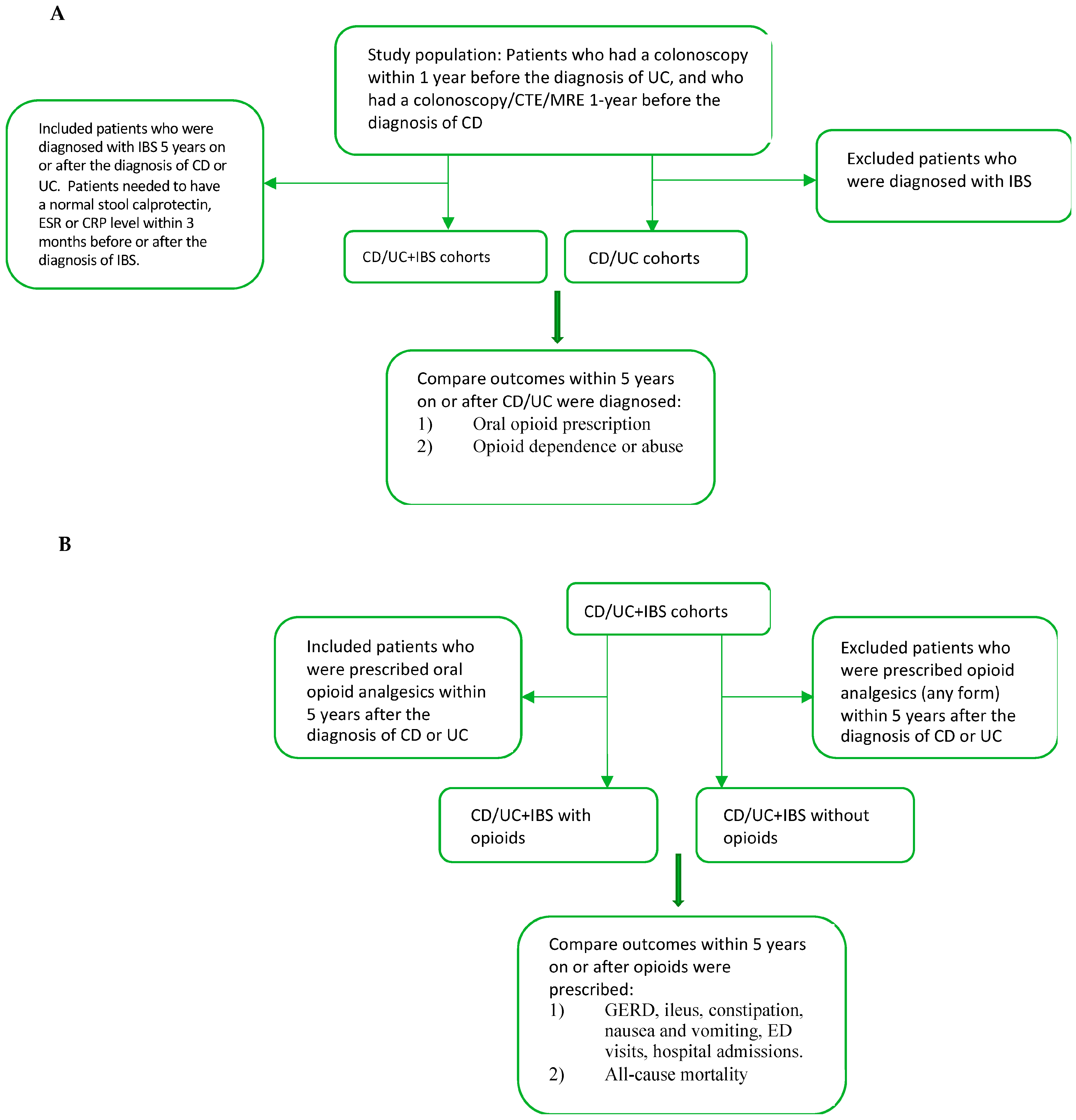
A cohort study was performed using the TriNetX database. We identified four separate study populations: concomitant CD and IBS (CD+IBS), concomitant UC and IBS (UC+IBS), CD+IBS with opioids, and UC+IBS with opioids. There were four control groups: CD, UC, CD+IBS without opioids, and UC+IBS without opioids (
Figure 1).
2.2.1. CD/UC+IBS Cohorts
We identified all patients who had a colonoscopy (UMLS:SNOMED:73761001 or UMLS:CPT:1022231) within 1 year before the diagnosis of UC (UMLS:ICD10CM:K51). We identified patients who had completed colonoscopy, or computed tomography of abdomen and pelvis, or Magnetic Resonance abdomen and pelvis, or Computed Tomography/Magnetic Resonance Enterography (CTE or MRE) within 1 year before the diagnosis of CD (UMLS:ICD10CM:K50). Patients needed to be subsequently diagnosed with IBS (UMLS:ICD10CM:K58) within 5 years of the diagnosis of CD or UC. A normal stool calprotectin (UMLS:LNC:38445-3, between 0.00 and 200.00 ug/g), erythrocyte sedimentation rate (ESR, TNX:9066, between 0.00 and 20.00 mm/h) or C-reactive protein (CRP, TNX:9063, between 0.00 and 10.00 mg/L) level within 3 months of the diagnosis of IBS was required to ensure that IBD was in remission when IBS was diagnosed.
2.2.2. CD/UC Cohorts
Two control groups consisted of patients with CD and UC without IBS. We identified all patients who had a colonoscopy within 1 year before the diagnosis of UC. We identified all patients who had a colonoscopy, or Computed Tomography of the abdomen and pelvis, or Magnetic Resonance abdomen and pelvis, or CTE/MRE within 1 year before the diagnosis of CD. Patients with a diagnosis of IBS were excluded from the cohorts.
2.2.3. CD/UC+IBS with Opioids Cohorts
A subgroup analysis was performed by selecting patients (CD+IBS and UC+IBS) who were prescribed oral opioids (NLM:VA:CN101, oral product). Patients needed to be prescribed oral opioids within 5 years after the diagnosis of CD or UC.
2.2.4. CD/UC+IBS without Opioids Cohorts
Two control groups consisted of patients (CD+IBS and UC+IBS) who were not prescribed any forms of opioids (including topical, injection, oral, and intranasal) within 5 years after the diagnosis of CD or UC.
2.3. Outcome Measures
We compared the risks of 5-year incident prescription of oral opioids (NLM:VA:CN101), oral oxycodone (NLM:RXNORM:7804), oral hydromorphone (NLM:RXNORM:3423), oral morphine (NLM:RXNORM:7052), oral hydrocodone (NLM:RXNORM:5489), oral tramadol (NLM:RXNORM:10689), opioid dependence (UMLS:ICD10CM:F11.2), and opioid abuse (UMLS:ICD10CM:F11.2) in CD (UC)+IBS and CD (UC) cohorts. Opioid use disorder included both opioid dependence and opioid abuse. The index event was defined as the onset of CD or UC in each cohort. We excluded patients with outcomes prior to the diagnosis of CD or UC.
We compared the 5-year incidence of new onset gastroesophageal reflux disease (GERD), (UMLS:ICD10CM:K21), ileus (UMLS:ICD10CM:K56.0 or K56.7), constipation (UMLS:ICD10CM:K59.0), nausea and vomiting (UMLS:ICD10CM:R11) in CD/UC+IBS with and without opioids. We also compared the 5-year incidence of emergency department (ED) visits and inpatient admissions in both cohorts. The index event was defined as the prescription of oral opioids. Patients with outcomes before the time window were excluded.
2.4. Statistical Analysis
Chi-square tests and analysis of variance (ANOVA) were used to compare categorical and continuous parameters. We used the TriNetX built-in algorithm to perform the propensity score matching for baseline characteristics, which was based on 1:1 nearest neighbor matching with a caliper of 0.1 SD. We accounted for covariates, including age at the index event, gender, race, and ethnicity. We also accounted for covariates that could contribute to opioid use, including chronic pain syndrome, osteoarthritis, rheumatoid arthritis, low back pain, mental disorders, inflammatory polyarthropathies, radiculopathy, cervicalgia, and thoracic pain. We obtained the number of patients with individual outcomes and the risks of the outcomes in the cohorts. Risk is defined as the fraction of patients with the outcomes within the 5-year window of the index event. Odds ratios (OR) with a 95% confidence interval were obtained to compare the cohorts. p < 0.05 was deemed statistically significant. Kaplan–Meier survival analyses and log-rank tests were performed to compare the survival probability in CD/UC+IBS with and without opioids cohorts. The hazard ratio (HR) for the risk of death with a 95% confidence interval was obtained by the Cox regression model.
4. Discussion
Our study reveals that IBS is an independent risk factor for receiving oral opioids and developing opioid use disorder in patients with IBD. We also found that opioids increased the risks of developing several gastrointestinal complications and resulting in a higher mortality rate and healthcare utilization in patients with concomitant IBD and IBS. This is one of the first studies to determine the risks of opioid prescription and related gastrointestinal complications in concomitant IBD and IBS using a large database.
Our study shows that IBS brings a 1.57 and 1.80-fold increase in the risks of receiving oral opioid analgesics in patients with concomitant CD or UC, respectively. A retrospective study with a sample size of 931 patients showed that chronic prescription opioid use was more common in patients with concomitant CD and functional gastrointestinal disorders (FGID) than in patients with CD alone (44 vs. 18%,
p < 0.001) [
26]. IBS is also a strong risk factor for inpatient narcotic use for IBD patients who were admitted to the hospital [
28]. A cross-sectional study showed that patients with concurrent CD-IBS and UC-IBS were associated with higher risks of opioid use (16.5 vs. 10.5%; 9.3 vs. 4.7%) [
27]. Our results show a consistent trend with the data reported in the literature: 24.6% of patients with CD and IBS, and 20.2% of patients with UC and IBS were prescribed oral opioid analgesics at least once (
p < 0.001). Our data also showed a consistent trend with the literature that patients with CD were more likely to suffer from pain than UC [
29]. However, our study did not differentiate whether oral opioids were prescribed for short-term use or for chronic use and whether they were prescribed in the inpatient setting or outpatient setting, which may have overestimated the overall risks of opioid prescription.
IBS is a strong risk factor for patients to develop opioid use disorder. We observed a 2.30-fold and 1.92-fold increase in opioid abuse in patients with concomitant CD+IBS and UC+IBS, respectively. It has been reported that IBD is an independent risk factor for a patient to become a heavy opioid user [
20]. Moreover, a study has shown that FGID was associated with a 4.5-fold increase in opioid dependence and a 5-fold increase in opioid misuse in patients with UC [
30]. Another study reported that opioid misuse in patients with CD is strongly associated with a concurrent diagnosis of FGID [
26]. However, the previous studies did not differentiate IBS from other FGIDs. Overall, our results showed a consistent trend with the literature that IBS appears to be a risk factor for opioid-related disorders, and this is one of the first studies to separate IBS from other FGIDs.
Opioid analgesics are associated with increased risks of developing various gastrointestinal complications in patients with overlapping IBD and IBS, including nausea, vomiting, constipation, GERD, and ileus. The association was the strongest with ileus. Opioids are known to cause various gastrointestinal symptoms, including constipation, nausea, vomiting, and motility issues such as esophageal dysmotility and ileus [
31,
32,
33]. Opioids can also cause narcotic bowel syndrome, characterized by the development or worsening of abdominal pain with continuous or escalating doses of narcotics [
34]. However, this is the first study to investigate the gastrointestinal side effects of opioids in patients with concurrent IBD and IBS. It is reasonable to postulate that opioids may be contributing to the worsening gastrointestinal symptoms and side effects.
Patients with concurrent IBD and IBS who were prescribed oral opioids appear to be high utilizers of the healthcare system. They were more likely to have ED visits and be admitted to the hospital, though we could not determine the chief complaints leading to the specific admission or ED visit. It was previously reported that IBS symptoms affected more than 60% of patients with IBD during a 6-year follow-up period and they tend to be high utilizers of the healthcare system [
35]. This population warrants more frequent outpatient monitoring by gastroenterologists and their primary care physicians to avoid the overutilization of the healthcare system.
Furthermore, our study shows that opioid analgesics are associated with a higher all-cause mortality rate in patients with concomitant IBD and IBS. Previous studies have shown that opioid analgesics are associated with a higher mortality rate in patients with IBD [
20,
36]. We demonstrated consistent results for patients with overlapping IBD and IBS.
We would like to emphasize that the use of opioid prescription medications is associated with worse clinical outcomes and higher healthcare utilization. Even though prescribing opioids may be an easy decision for clinicians to make to satisfy patients’ immediate needs and avoid lengthy discussions, it may easily feed into a vicious cycle and even contribute to worsening mortality in the long term. Clinicians should prioritize the principle of "do no harm" and avoid prescribing opioids when managing functional abdominal pain in patients with IBD. Future research areas should be focused on better symptom control for IBD patients with overlapping IBS features to avoid opioid analgesic use. Current guideline recommends a low FODMAP diet and psychological therapies in managing functional GI symptoms in IBD patients [
37]. A low FODMAP diet has been shown to reduce IBS-like symptoms and improve quality of life in IBD patients [
38,
39]. Clinicians should offer such guideline-compliant therapy options to patients instead of opioids.
There are several limitations to the current study. The data obtained were aggregated, and it was not possible to access patients’ data on an individual level. Certain confounding factors may not have been accounted for. The diagnoses are based on billing codes and could not be verified. For example, it is difficult to tell if IBS and IBD were diagnosed by gastroenterologists or not and whether the diagnoses were accurate or not. ESR and CRP can be nonspecific, thus a normal value can still be associated with active IBD; whereas elevated fecal calprotectin is more specific for assessing disease activity.
In conclusion, this is one of the first studies to evaluate the risks of opioid prescription and related complications in patients with concomitant IBD and IBS. We found that IBS is an independent risk factor for developing opioid addiction in patients with IBD and that patients who were prescribed opioids are more likely to have gastrointestinal side effects and higher mortality than those with IBD alone. Clinicians should be aware of the risks of opioid-related complications in this population and refrain from prescribing opioids in managing IBS symptoms in IBD.