Exploring the Association of Burning Mouth Syndrome with Depressive and Anxiety Disorders in Middle-Aged and Older Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Extraction
2.2. Protocol and Registration
2.3. Quality Assessment within and across Studies and Overall Quality Assessment
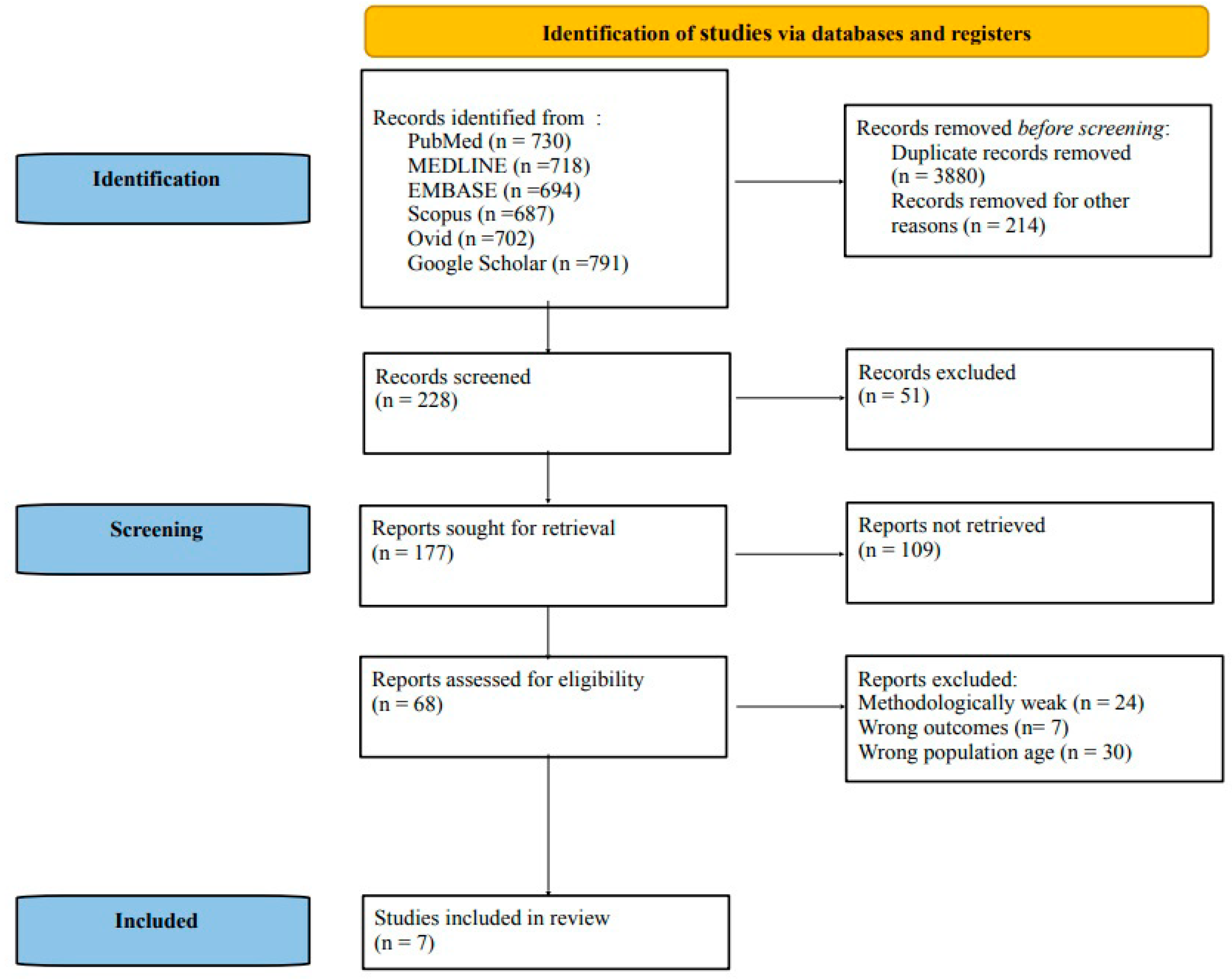
3. Results
3.1. Depressive and Anxiety Disorders and Their Distribution across Different Studies
3.2. Assessment Tools of Depressive Disorders and Their Distribution across Different Studies
3.3. Assessment Tools of Anxiety Disorders and Their Distribution across Different Studies
3.4. Summary of Findings on the Association of Burning Mouth Syndrome with Depressive and Anxiety Disorders in Middle-Aged and Older Adults
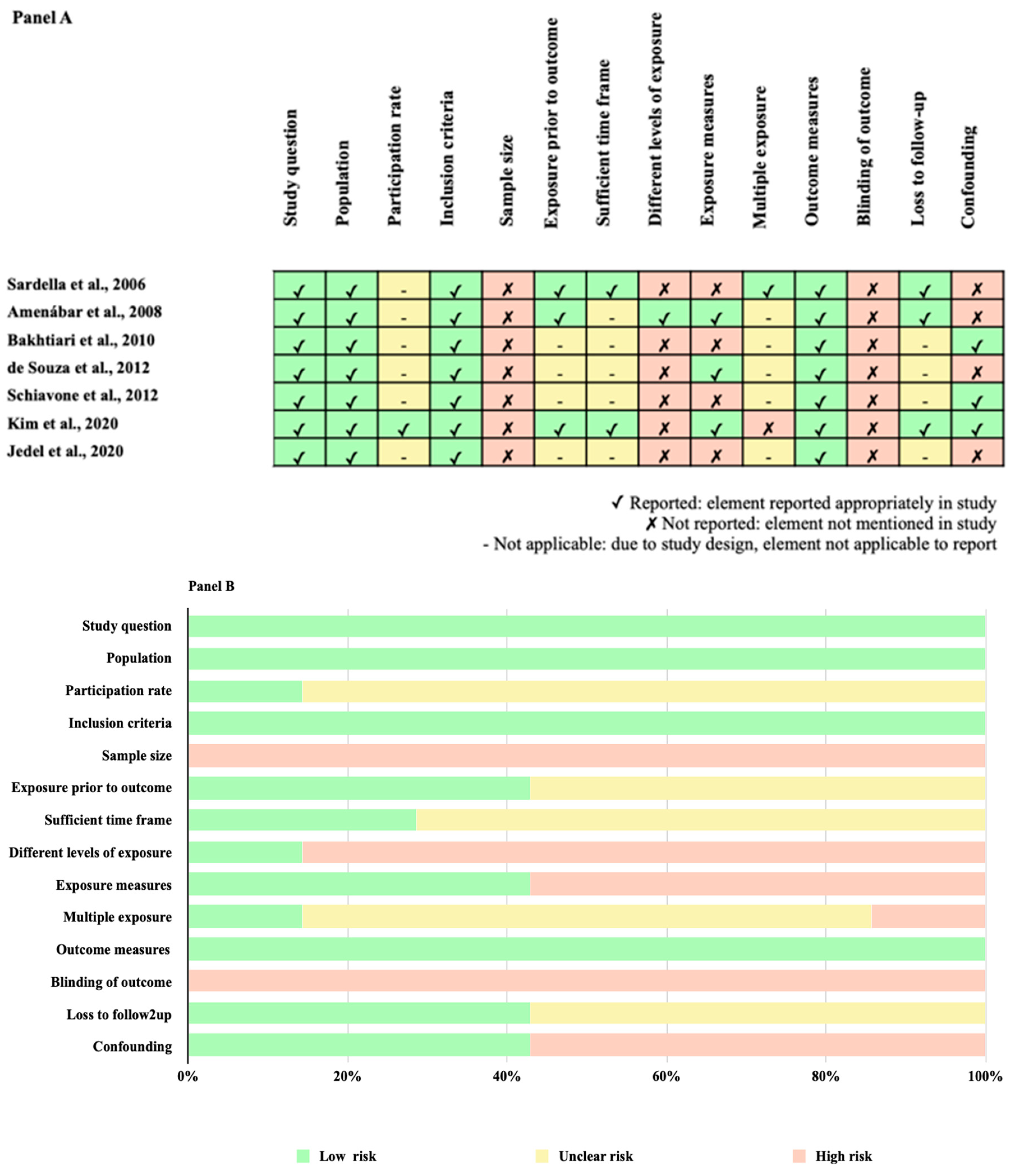
3.5. Methodological Quality Assessment within Studies and Overall Quality Assessment across Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.Y.; Kim, Y.S.; Ko, I.; Kim, D.K. Association Between Burning Mouth Syndrome and the Development of Depression, Anxiety, Dementia, and Parkinson Disease. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 561–569. [Google Scholar] [CrossRef]
- Scala, A.; Checchi, L.; Montevecchi, M.; Marini, I.; Giamberardino, M.A. Update on burning mouth syndrome: Overview and patient management. Crit. Rev. Oral Biol. Med. 2003, 14, 275–291. [Google Scholar] [CrossRef]
- Barker, K.; Savage, N. Burning mouth syndrome: An update on recent findings. Aust. Dent. J. 2005, 50, 220–223. [Google Scholar] [CrossRef] [PubMed]
- International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [CrossRef] [PubMed]
- Lamey, P.J. Burning mouth syndrome. Dermatol. Clin. 1996, 14, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Gurvits, G.E.; Tan, A. Burning mouth syndrome. World J. Gastroenterol. 2013, 19, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Coon, E.A.; Laughlin, R.S. Burning mouth syndrome in Parkinson’s disease: Dopamine as cure or cause? J. Headache Pain. 2012, 13, 255–257. [Google Scholar] [CrossRef]
- Forssell, H.; Teerijoki-Oksa, T.; Puukka, P.; Estlander, A.M. Symptom severity in burning mouth syndrome associates with psychological factors. J. Oral Rehabil. 2020, 47, 713–719. [Google Scholar] [CrossRef]
- Davies, S.J.; Underhill, H.C.; Abdel-Karim, A.; Christmas, D.M.; Bolea-Alamanac, B.M.; Potokar, J.; Herrod, J.; Prime, S.S. Individual oral symptoms in burning mouth syndrome may be associated differentially with depression and anxiety. Acta Odontol. Scand. 2016, 74, 155–160. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, C.; Yu, H.; Kim, D.K. Relationship of Depression, Anxiety, and Bipolar Disease with Burning Mouth Syndrome: A Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 3391. [Google Scholar] [CrossRef]
- Galli, F.; Lodi, G.; Sardella, A.; Vegni, E. Role of psychological factors in burning mouth syndrome: A systematic review and meta-analysis. Cephalalgia 2017, 37, 265–277. [Google Scholar] [CrossRef]
- Orliaguet, M.; Misery, L. Neuropathic and Psychogenic Components of Burning Mouth Syndrome: A Systematic Review. Biomolecules 2021, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, W.; Yan, J.; Noma, N.; Young, A.; Yan, Z. Worldwide prevalence estimates of burning mouth syndrome: A systematic review and meta-analysis. Oral Dis. 2022, 28, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies; NIH Library: Bethesda, MD, USA, 2022. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 2 April 2023).
- Sardella, A.; Lodi, G.; Demarosi, F.; Uglietti, D.; Carrassi, A. Causative or precipitating aspects of burning mouth syndrome: A case-control study. J. Oral Pathol. Med. 2006, 35, 466–471. [Google Scholar] [CrossRef]
- Amenábar, J.M.; Pawlowski, J.; Hilgert, J.B.; Hugo, F.N.; Bandeira, D.; Lhüller, F.; Lopes de Souza, M.A. Anxiety and salivary cortisol levels in patients with burning mouth syndrome: Case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, S.; Khalighi, H.R.; Azimi, S.; Alavi, K.; Ayoobi Valoogerdi, H.; Namazi, Z. Correlation between Burning Mouth Syndrome and Anxiety in the Elderly Inmates of Sanitaria in Tehran. J. Dent. Res. Dent. Clin. Dent. Prospect. 2010, 4, 37–41. [Google Scholar]
- de Souza, F.T.; Teixeira, A.L.; Amaral, T.M.; dos Santos, T.P.; Abreu, M.H.; Silva, T.A.; Kummer, A. Psychiatric disorders in burning mouth syndrome. J. Psychosom Res. 2012, 72, 142–146. [Google Scholar] [CrossRef]
- Schiavone, V.; Adamo, D.; Ventrella, G.; Morlino, M.; De Notaris, E.B.; Ravel, M.G.; Kusmann, F.; Piantadosi, M.; Pollio, A.; Fortuna, G.; et al. Anxiety, depression, and pain in burning mouth syndrome: First chicken or egg? Headache 2012, 52, 1019–1025. [Google Scholar] [CrossRef]
- Jedel, E.; Elfström, M.L.; Hägglin, C. Differences in personality, perceived stress and physical activity in women with burning mouth syndrome compared to controls. Scand. J. Pain 2020, 21, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Iida, T.; Komiyama, O.; Masuda, M.; Uchida, T.; Nishimura, H.; Okubo, M.; Shimosaka, M.; Narita, N.; Niwa, H.; et al. Characteristics of middle-aged and older patients with temporomandibular disorders and burning mouth syndrome. J. Oral Sci. 2015, 57, 355–360. [Google Scholar] [CrossRef]
- Dibello, V.; Panza, F.; Mori, G.; Ballini, A.; Di Cosola, M.; Lozupone, M.; Dibello, A.; Santarcangelo, F.; Vertucci, V.; Dioguardi, M.; et al. Temporomandibular Disorders as a Risk Factor for Suicidal Behavior: A Systematic Review. J. Pers. Med. 2022, 12, 1782. [Google Scholar] [CrossRef]
- Christopher, M. A broader view of trauma: A biopsychosocial-evolutionary view of the role of the traumatic stress response in the emergence of pathology and/or growth. Clin. Psychol. Rev. 2004, 24, 75–98. [Google Scholar] [CrossRef]
- Law, L.F.; Sluka, K.A. How does physical activity modulate pain? Pain 2017, 158, 369–370. [Google Scholar] [CrossRef]
- Petruzzi, M.; De Benedittis, M.; Pastore, L.; Serpico, R. Vulvostomatodynia. Maturitas 2007, 58, 102–106. [Google Scholar] [CrossRef]
- Tokura, T.; Kimura, H.; Ito, M.; Nagashima, W.; Sato, N.; Kimura, Y.; Arao, M.; Aleksic, B.; Yoshida, K.; Kurita, K.; et al. Temperament and character profiles of patients with burning mouth syndrome. J. Psychosom. Res. 2015, 78, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; Albert, U.; Gandolfo, S.; Vitalucci, A.; Bogetto, F. Personality disorders in patients with burning mouth syndrome. J. Pers. Disord. 2005, 19, 84–93. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Lucero-Berdugo, M.; Castillo-Felipe, C.; Zamora Lavella, C.; Ferrandez-Pujante, A.; Pons-Fuster, A. Assessment of self-reported sleep disturbance and psychological status in patients with burning mouth syndrome. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1285–1290. [Google Scholar] [CrossRef]
- Santoro, V.; Caputo, G.; Peluso, F. Clinical and therapeutic experience in twenty eight patients with burning mouth syndrome. Minerva Stomatol. 2005, 54, 489–496. [Google Scholar]
- Hamon, B.; Orliaguet, M.; Misery, L.; Boisramé, S. Burning mouth syndrome and pelvodynia: A literature review. Medicine 2023, 102, e32648. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int. J. Dermatol. 2003, 42, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Ford, B.; Louis, E.D.; Greene, P.; Fahn, S. Oral and genital pain syndromes in Parkinson’s disease. Mov. Disord. 1996, 11, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Antoun Reyad, A.; Mishriky, R.; Girgis, E. Pharmacological and nonpharmacological management of burning mouth syn-drome: A systematic review. Dent. Med. Probl. 2020, 57, 295–304. [Google Scholar] [CrossRef] [PubMed]

| Authors, Year [Reference] | Outcome(s) | Outcome(s) Assessment Tool(s) and Pain Assessment Tools | Design (Follow-Up) | N | Age | Sex | Setting(s) | Country | Quality Assessment | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Sardella et al., 2006 [16] | Depressive disorders Anxiety disorders | Hospital Anxiety and Depression Scale VAS | Longitudinal Case-control (3 years) | 67/54 | 62.3 years (4.6)/56.8 years (5) | 15% M, 85% F/10% M, 90% F | Hospital (outpatients) | Europe (Italy) | High | This prospective case-control study showed significant differences between BMS and control subjects regarding anxiety and depressive disorders. |
| Amenábar et al., 2008 [17] | Anxiety disorders | Beck Anxiety Inventory | Longitudinal Case-control (N/A) | 30/30 | 61.6 years (10.7)/63.2 years (9.7) | 20% M, 80% F/N/A | Hospital (outpatients) | South America (Brazil) | High | BMS was positively associated with higher anxiety and elevated salivary cortisol levels. |
| Bakhtiari et al., 2010 [18] | Anxiety disorders | Cattell Anxiety Scale VAS | Cross-sectional Case-control | 50/50 | 60 years+ 70.3 years (9.9)/70.9 years (9.8) | 12% M, 88% F/22% M, 78% F | Hospital (outpatients) | Asia (Iran) | Moderate | This cross-sectional study suggested that both state and trait anxiety were related to the presence of BMS. |
| de Souza et al., 2012 [19] | Depressive disorders Anxiety disorders | The Mini-International Neuropsychiatric Interview-Plus (MINI-Plus) Hamilton Rating Scale for Depression Beck Depression Inventory State-Trait Anxiety Inventory VAS | Cross-sectional Case-control | 30/31 | 63.8 years (11.8)/63.8 years (11.8) | 3.3% M, 97.7% F/3.2% M, 97.8% F | Hospital (outpatients) | South America (Brazil) | Moderate | The findings of this cross-sectional study confirmed clinical observations on the fact that subjects with BMS may have a particular psychiatric and/or psychological profile. |
| Schiavone et al., 2012 [20] | Depressive disorders Anxiety disorders | Hamilton Rating Scale for Depression State-Trait Anxiety Inventory Form Y 1–2 Symptom Checklist-90-Revised VAS | Cross-sectional Case-control | 53/51 | 55.26 years (11.50)/54.02 years (13.28) | 30.2% M, 69.8 F/33.3% M, 66.7% F | Hospital (outpatients) | Europe (Italy) | Moderate | This cross-sectional study highlighted that in BMS there were psychiatric symptoms (anxiety and depression) with a possible association with pain. |
| Kim et al., 2020 [1] | Depressive disorders Anxiety disorders | Korean Standard Classification of Diseases, Sixth Revision | Retrospective Case-control (10 years) | 695/362 | 45 years+ | 38.6% M, 61.4% F/38.1% M 61.9% F | Hospital (inpatients and outpatients) | Asia (South Korea) | High | Findings of this observational study suggested that BMS was associated with increased incidence of depression and anxiety but not of dementia or Parkinson’s disease. |
| Jedel et al., 2020 [21] | Anxiety disorders | Swedish universities Scales of Personality VAS | Cross-sectional Case-control | 56/56 | 67.8 years (8.9)/67.8 years (8.9) | (100% F/N/A) | Hospital (outpatients) | Europe (Sweden) | Low | SSP subscales Somatic Trait Anxiety, Psychic Trait Anxiety, Stress Susceptibility, and Verbal Trait Aggression differed between women with BMS and controls, and the personality factor scores for neuroticism and aggressiveness were higher. |
| Psychiatric Disorders | Evidence Base | Strength of Association | Strength of Evidence (GRADE) | Comments |
|---|---|---|---|---|
| Depressive disorders [1,16,19,20] | Four studies n = 1.343 | BMS vs. depression (45–64 years): HR = 1.45, 95% CI = 1.03–2.04; BMS vs. depression (64 years+): HR = 1.68, 95% CI = 1.16–2.43 [1] BMS vs. depression: OR = 3.833, 95% CI = 1.528–9.572 [16] BMS vs. current major depressive disorder (BMS group vs. control group): p = 0.004 (Chi-square test); BMS vs. past major depressive disorder (BMS group vs. control group): p = 0.006 (Chi-square test) [19] BMS vs. depression (SCL-90-R) (BMS group vs. control group): p =< 0.001 (ANOVA); BMS vs. depression (HAM-D) (BMS group vs. control group): p =< 0.001 (ANOVA) [20] | ⊕⊕ Low | Low association of BMS with depressive disorders, with estimates partly provided; a few studies included but with a large sample size. |
| Anxiety disorders [1,16,17,18,19,20,21] | Seven studies n = 1.615 | BMS vs. anxiety (45–64 years): HR 1.72, 95% CI 1.30–2.29; BMS vs. anxiety (64 years+): HR 2.13, 95% CI 1.57–2.91 [1] BMS vs. anxiety (HAD Scale): OR 4.256, 95% CI 1.780–10.148 [16] BMS vs. anxiety (BMS group vs. control group): p = 0.001 (Fisher exact test) [17] BMS vs. anxiety: r = 0.431, p < 0.001 [18] BMS vs. generalized anxiety disorder (BMS group vs. control group): p = 0.012 (Chi-square test) [19] BMS vs. anxiety (SCL-90-R), (BMS group vs. control group): p = 0.002 (ANOVA); BMS vs. anxiety (STAI Y1), (BMS group vs. control group): p = 0.026 (ANOVA); BMS vs. anxiety (STAI Y2), (BMS group vs. control group): p = 0.046 (ANOVA) [20] BMS vs. somatic trait anxiety (BMS group vs. control group): (p < 0.001) (Wilcoxon sign rank test) [21] | ⊕⊕⊕ Moderate | Moderate association of BMS with anxiety disorders, with estimates partly provided; multiple studies included, with also a large sample size. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibello, V.; Ballini, A.; Lozupone, M.; Custodero, C.; Cantore, S.; Sardone, R.; Dibello, A.; Santarcangelo, F.; Barulli Kofler, B.; Petruzzi, M.; et al. Exploring the Association of Burning Mouth Syndrome with Depressive and Anxiety Disorders in Middle-Aged and Older Adults: A Systematic Review. J. Pers. Med. 2023, 13, 1014. https://doi.org/10.3390/jpm13061014
Dibello V, Ballini A, Lozupone M, Custodero C, Cantore S, Sardone R, Dibello A, Santarcangelo F, Barulli Kofler B, Petruzzi M, et al. Exploring the Association of Burning Mouth Syndrome with Depressive and Anxiety Disorders in Middle-Aged and Older Adults: A Systematic Review. Journal of Personalized Medicine. 2023; 13(6):1014. https://doi.org/10.3390/jpm13061014
Chicago/Turabian StyleDibello, Vittorio, Andrea Ballini, Madia Lozupone, Carlo Custodero, Stefania Cantore, Rodolfo Sardone, Antonio Dibello, Filippo Santarcangelo, Bianca Barulli Kofler, Massimo Petruzzi, and et al. 2023. "Exploring the Association of Burning Mouth Syndrome with Depressive and Anxiety Disorders in Middle-Aged and Older Adults: A Systematic Review" Journal of Personalized Medicine 13, no. 6: 1014. https://doi.org/10.3390/jpm13061014
APA StyleDibello, V., Ballini, A., Lozupone, M., Custodero, C., Cantore, S., Sardone, R., Dibello, A., Santarcangelo, F., Barulli Kofler, B., Petruzzi, M., Daniele, A., Solfrizzi, V., & Panza, F. (2023). Exploring the Association of Burning Mouth Syndrome with Depressive and Anxiety Disorders in Middle-Aged and Older Adults: A Systematic Review. Journal of Personalized Medicine, 13(6), 1014. https://doi.org/10.3390/jpm13061014