Long-Term Outcomes after Transcatheter Mitral Valve-in-Valve or Valve-in-Ring Procedures
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Inclusion and Exclusion Criteria
2.2. Data Collection, Ethic Statement, and Study Endpoints
2.3. Patients, Study Groups, and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Echocardiographic Baselines
3.3. Procedural Data
3.4. Postoperative Course and Hospital Outcomes
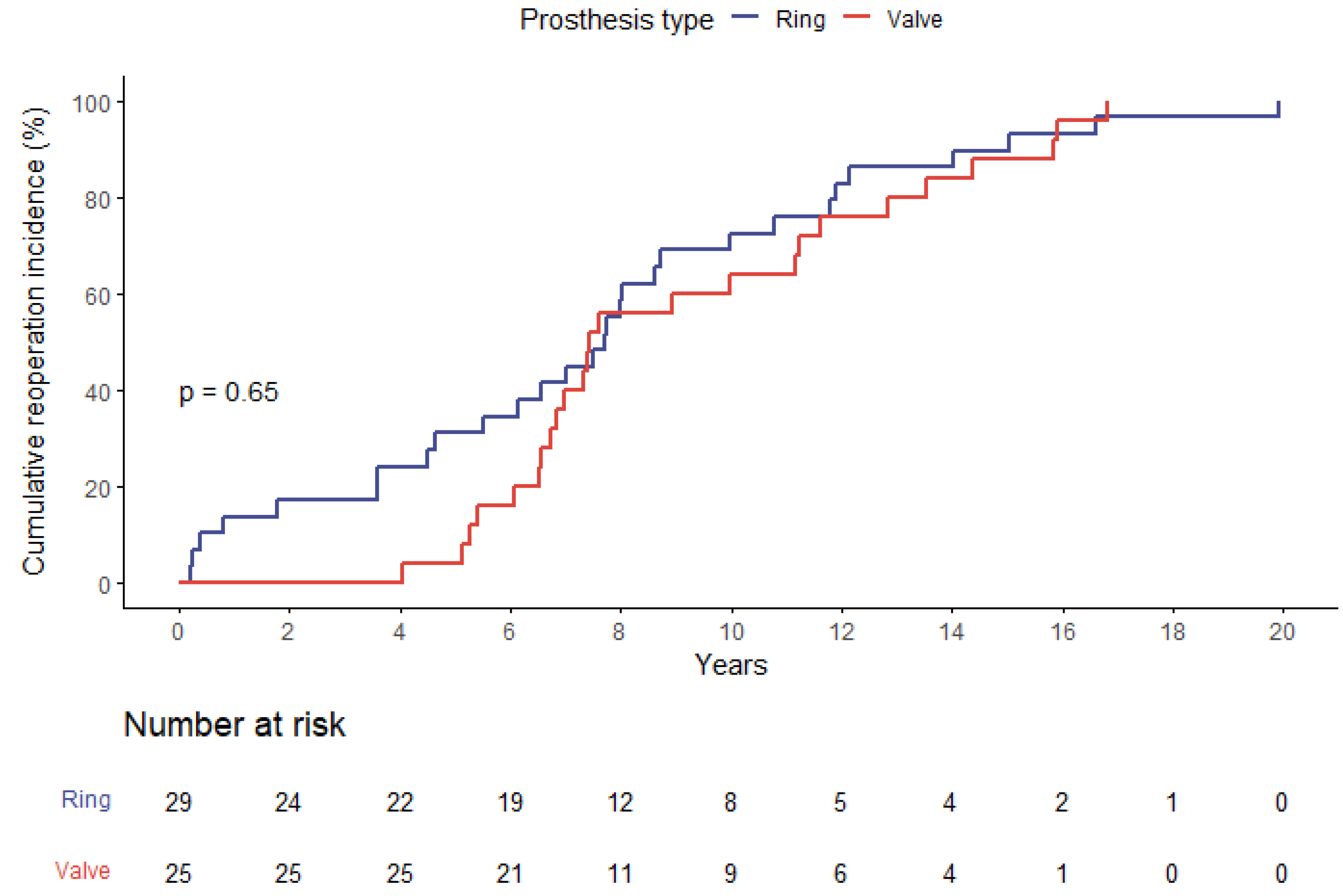
3.5. Follow-Up Data
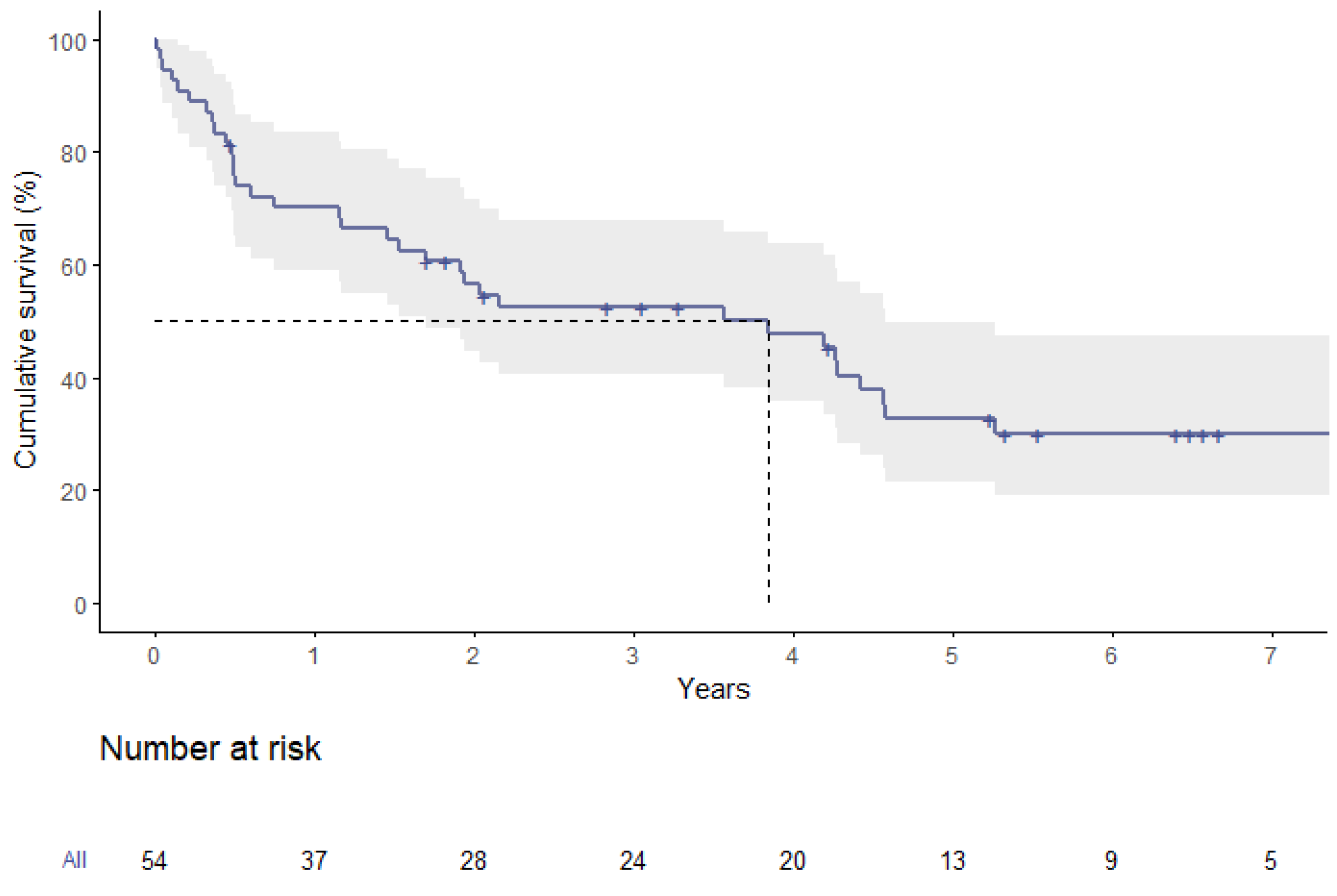
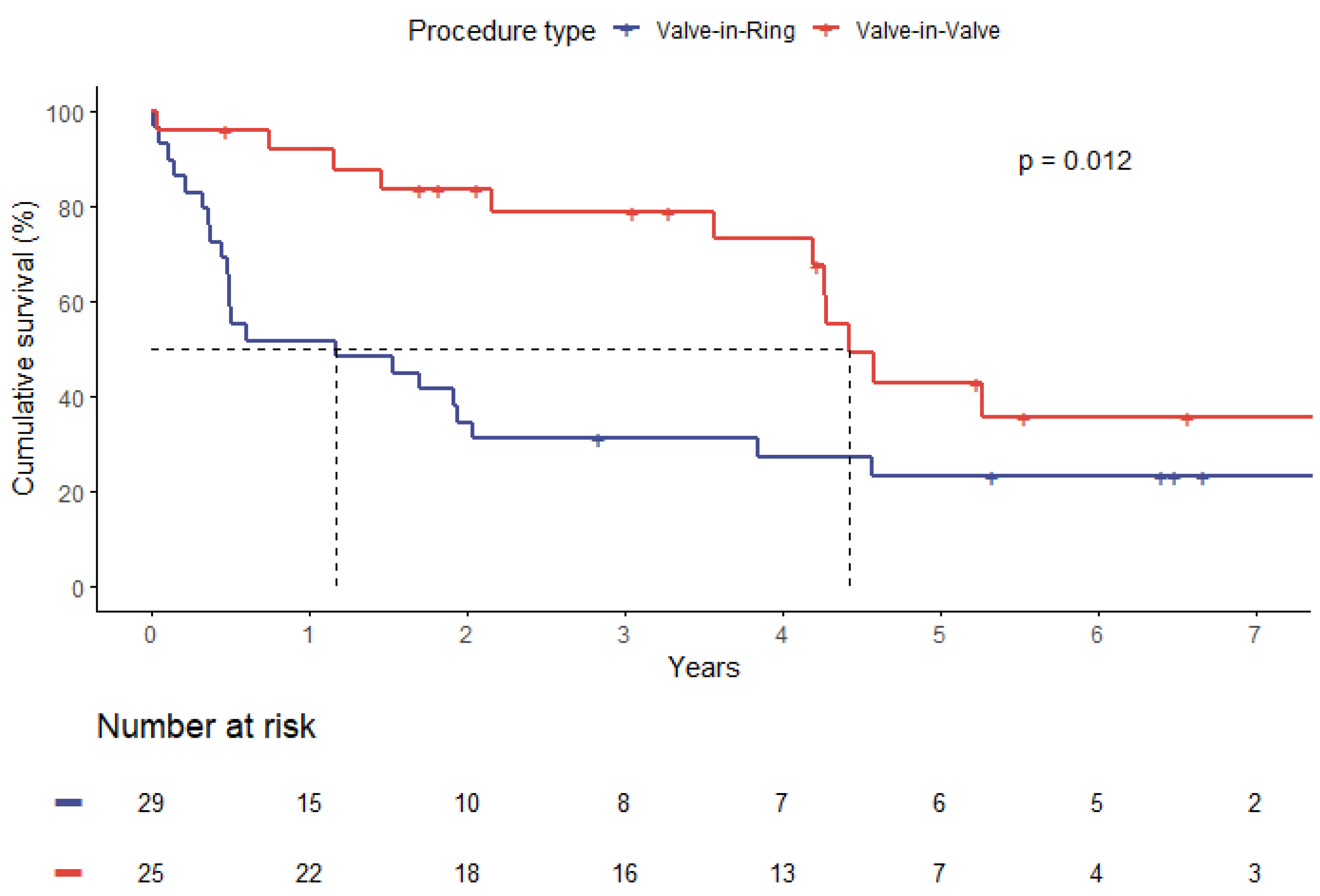
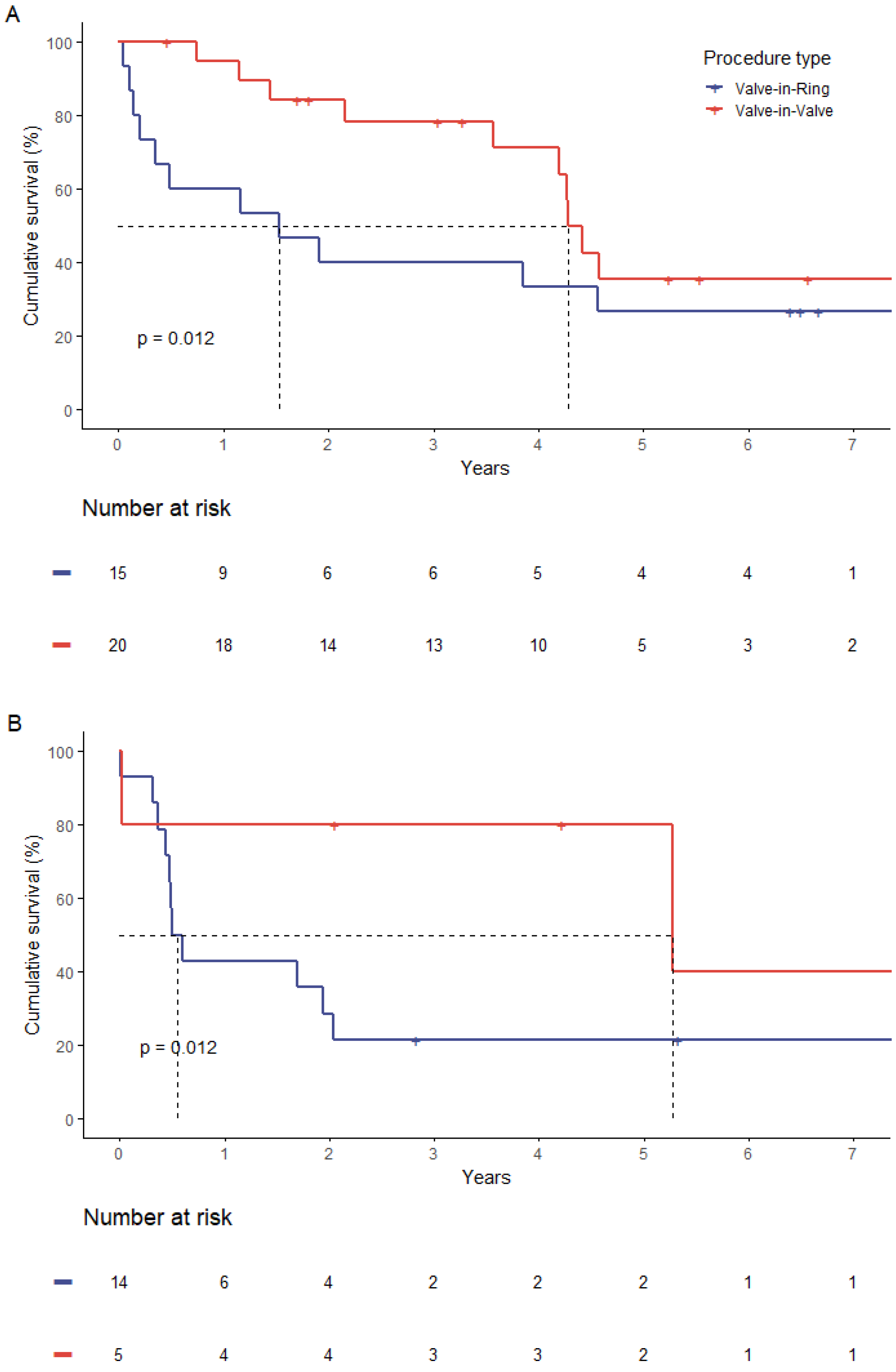
3.6. Survival Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; de Bonis, M.; de Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Kempfert, J.; van Linden, A.; Linke, A.; Borger, M.A.; Rastan, A.; Mukherjee, C.; Ender, J.; Schuler, G.; Mohr, F.W.; Walther, T. Transapical off-pump valve-in-valve implantation in patients with degenerated aortic xenografts. Ann. Thorac. Surg. 2010, 89, 1934–1941. [Google Scholar] [CrossRef]
- Walther, T.; Falk, V.; Dewey, T.; Kempfert, J.; Emrich, F.; Pfannmüller, B.; Bröske, P.; Borger, M.A.; Schuler, G.; Mack, M.; et al. Valve-in-a-valve concept for transcatheter minimally invasive repeat xenograft implantation. J. Am. Coll. Cardiol. 2007, 50, 56–60. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Wilbring, M.; Alexiou, K.; Tugtekin, S.M.; Arzt, S.; Ibrahim, K.; Matschke, K.; Kappert, U. Pushing the limits-further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J. Thorac. Cardiovasc. Surg. 2014, 147, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Wilbring, M.; Alexiou, K.; Tugtekin, S.M.; Sill, B.; Hammer, P.; Schmidt, T.; Simonis, G.; Matschke, K.; Kappert, U. Transapical transcatheter valve-in-valve implantation for deteriorated mitral valve bioprostheses. Ann. Thorac. Surg. 2013, 95, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Webb, J.G.; Wong, D.R.; Ye, J.; Masson, J.-B.; Carere, R.G.; Lichtenstein, S.V. Transapical transcatheter mitral valve-in-valve implantation in a human. Ann. Thorac. Surg. 2009, 87, e18–e20. [Google Scholar] [CrossRef] [PubMed]
- Speiser, U.; Pohling, D.; Tugtekin, S.-M.; Charitos, E.; Matschke, K.; Wilbring, M. Redo surgery for noninfective isolated mitral valve disease: Initial outcome and further follow-up compared to primary surgery. J. Card. Surg. 2022, 37, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Schofer, N.; Eschenbach, L.; Fujita, B.; Sharma, R.; Ancona, M.; Yzeiraj, E.; et al. Transcatheter Mitral Valve Replacement for Degenerated Bioprosthetic Valves and Failed Annuloplasty Rings. J. Am. Coll. Cardiol. 2017, 70, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J.; et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; Whisenant, B.; Ribeiro, H.B.; Webb, J.G.; Kornowski, R.; Guerrero, M.; Wijeysundera, H.; Søndergaard, L.; de Backer, O.; Villablanca, P.; et al. Transcatheter Mitral Valve Replacement after Surgical Repair or Replacement: Comprehensive Midterm Evaluation of Valve-in-Valve and Valve-in-Ring Implantation from the VIVID Registry. Circulation 2021, 143, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; de Bonis, M.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.-H.; Leon, M.B.; Piazza, N.; et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 2: Endpoint definitions: A consensus document from the Mitral Valve Academic Research Consortium. Eur. Heart J. 2015, 36, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.T.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2—Isolated valve surgery. Ann. Thorac. Surg. 2009, 88, S23–S42. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744; discussion 744–745. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Guerrero, M.; Vemulapalli, S.; Xiang, Q.; Wang, D.D.; Eleid, M.; Cabalka, A.K.; Sandhu, G.; Salinger, M.; Russell, H.; Greenbaum, A.; et al. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data from the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ. Cardiovasc. Interv. 2020, 13, e008425. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Y.; Cheng, S.; Zhang, S.; Wu, K.; Wang, W.; Zhou, Y. Transcatheter mitral valve implantation for degenerated mitral bioprostheses or failed surgical annuloplasty rings: A systematic review and meta-analysis. J. Card. Surg. 2018, 33, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Lee, A.P.W.; Jin, C.-N.; Wong, R.H.L.; Chan, H.H.M.; Ng, C.S.H.; Wan, I.Y.P.; Underwood, M.J. The choice of mitral annuloplastic ring-beyond “surgeon’s preference”. Ann. Cardiothorac. Surg. 2015, 4, 261–265. [Google Scholar] [CrossRef] [PubMed]

| Valve-in-Valve (n = 25) | Valve-in-Ring (n = 29) | p-Value | |
|---|---|---|---|
| Age (years) | 77.4 ± 6.3 | 75.8 ± 6.7 | 0.35 |
| Gender male | 13 (52.0%) | 17 (58.6%) | 0.83 |
| BMI (kg/m2) | 25.4 ± 4.5 | 28.1 ± 5.5 | 0.08 |
| Arterial hypertension | 24 (96.0%) | 27 (93.1%) | 1.00 |
| Diabetes mellitus | 9 (36.0%) | 11 (37.9%) | 1.00 |
| Dyslipidemia | 16 (64.0%) | 20 (69.0%) | 0.92 |
| Coronary artery disease | 15 (60.0%) | 19 (65.5%) | 0.89 |
| Chronic obstructive lung disease | 6 (24.0%) | 4 (13.8%) | 0.49 |
| Pulmonary arterial hypertension | |||
| None | 4 (16.0%) | 6 (20.7%) | |
| Moderate | 7 (28.0%) | 16 (55.2%) | 0.06 |
| Severe | 14 (56.0%) | 7 (24.1%) | |
| Chronic kidney disease | 21 (84.0%) | 29 (100%) | 0.04 |
| Preoperative dialysis | 1 (4.0%) | 1 (3.4%) | 1.00 |
| GFR (ml/min) | 53.0 ± 21.2 | 44.5 ± 19.0 | 0.12 |
| Peripheral arterial disease | 6 (24.0%) | 4 (13.8%) | 0.49 |
| History of stroke | 0.54 | ||
| None | 21 (84.0%) | 27 (93.1%) | |
| TIA | 2 (8.0%) | 1 (3.4%) | |
| Stroke | 2 (8.0%) | 1 (3.4%) | |
| Atrial fibrillation | 20 (80.0%) | 24 (82.8%) | 1.00 |
| Preoperative pacemaker | 5 (20.0%) | 20 (69.0%) | <0.001 |
| NYHA Class | 0.04 | ||
| I | 3 (12.0%) | 0 (0%) | |
| II | 3 (12.0%) | 0 (0%) | |
| III | 15 (60.0%) | 21 (72.4%) | |
| IV | 4 (16.0%) | 8 (27.6%) | |
| EuroSCORE II (%) | 11.5 ± 7.5 | 16.9 ± 9.0 | <0.01 |
| STS-PROM score (%) | 5.9 ± 3.7 | 8.7 ± 4.3 | <0.01 |
| Years since index operation | 9.3 ± 3.8 | 7.7 ± 5.0 | 0.200 |
| Initial operation for endocarditis | 7 (28.0%) | 0 (0%) | <0.01 |
| Initial operation indication | <0.001 | ||
| Structural mitral disease | 21 (84.0%) | 8 (27.6%) | |
| Functional mitral disease | 4 (16.0%) | 21 (72.4%) | |
| History of coronary bypass | 6 (24.0%) | 7 (24.1%) | 1.000 |
| Size of initial prosthesis (mm) | 29.7 ± 1.1 | 28.4 ± 1.5 | <0.001 |
| Valve-in-Valve (n = 25) | Valve-in-Ring (n = 29) | p-Value | |
|---|---|---|---|
| Ejection fraction (%) | 53.6 ± 12.4 | 39.3 ± 15.1 | <0.001 |
| LVEDD (mm) | 49.1 ± 6.5 | 58.6 ± 7.8 | <0.001 |
| Right ventricular function | 0.56 | ||
| Normal | 8 (32.0%) | 9 (31.0%) | |
| Mildly reduced | 6 (24.0%) | 9 (31.0%) | |
| Moderately reduced | 9 (36.0%) | 7 (24.1%) | |
| Severely reduced | 2 (8.0%) | 4 (13.8%) | |
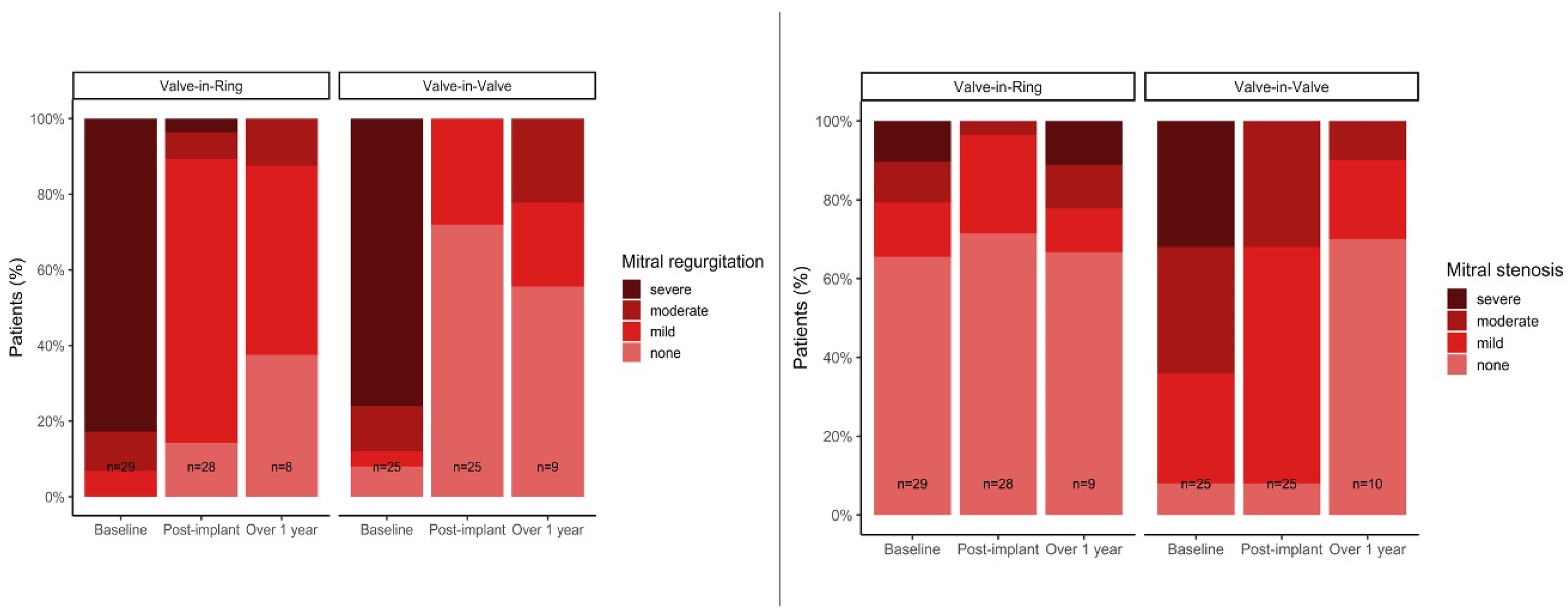
| Mitral regurgitation | 0.62 | ||
| None | 2 (8.0%) | 0 (0%) | |
| Mild | 1 (4.0%) | 2 (6.9%) | |
| Moderate | 3 (12.0%) | 3 (10.3%) | |
| Severe | 19 (76.0%) | 24 (82.8%) | |
| Mitral stenosis | <0.001 | ||
| None | 2 (8.0%) | 19 (65.5%) | |
| Mild | 7 (28.0%) | 4 (13.8%) | |
| Moderate | 8 (32.0%) | 3 (10.3%) | |
| Severe | 8 (32.0%) | 3 (10.3%) | |
| Mitral valve area (cm2) | 1.3 ± 0.6 | 2.4 ± 1.2 | 0.16 |
| Peak gradient (mmHg) | 27.4 ± 6.4 | 18.4 ± 6.6 | <0.001 |
| Mean gradient (mmHg) | 10.7 ± 3.5 | 6.3 ± 2.7 | <0.001 |
| Valve-in-Valve (n = 25) | Valve-in-Ring (n = 29) | p-Value | |
|---|---|---|---|
| Indication for redo procedure (according to the leading pathology) | 0.76 | ||
| Mitral regurgitation | 18 (72.0%) | 23 (79.3%) | |
| Mitral stenosis | 7 (28.0%) | 6 (20.7%) | |
| Access | 0.48 | ||
| Transapical | 22 (88.0%) | 22 (75.9%) | |
| Transfemoral | 3 (12.0%) | 6 (20.7%) | |
| Right anterolateral minithoracotomy (transatrial) | 0 (0%) | 1 (3.4%) | |
| Prosthesis model | 1 | ||
| 8 (32.0%) | 10 (34.5%) | |
| 17 (68.0%) | 19 (65.5%) | |
| Prosthesis labeled size (mm) | 27.9 ± 1.5 | 25.9 ± 1.7 | <0.001 |
| Surgery time (min) | 62.7 ± 34.7 | 73.9 ± 70.9 | 0.59 |
| Conversion | 0 (0%) | 2 (6.9%) | 0.49 |
| M-VARC procedural success a | 5 (20.0%) | 3 (10.3%) | 0.45 |
| Modified procedural success b | 18 (72.0%) | 20 (69.0%) | 1 |
| Mitral stenosis | <0.001 | ||
| None | 2 (8.0%) | 21 (72.4%) | |
| Mild | 15 (60.0%) | 7 (24.1%) | |
| Moderate | 8 (32.0%) | 1 (3.4%) | |
| Severe | 0 (0%) | 0 (0%) | |
| Mitral regurgitation | <0.001 | ||
| None | 18 (72.0%) | 5 (17.2%) | |
| Mild | 7 (28.0%) | 21 (72.4%) | |
| Moderate | 0 (0%) | 2 (6.9%) | |
| Severe | 0 (0%) | 1 (3.4%) | |
| Type of regurgitation | <0.001 | ||
| None | 21 (84.0%) | 5 (17.2%) | |
| Transvalvular | 1 (4.0%) | 4 (13.8%) | |
| Paravalvular | 3 (12.0%) | 19 (65.5%) | |
| Both | 0 (0%) | 1 (3.4%) | |
| LVOT obstruction | 1 (4.0%) | 2 (6.9%) | 1.00 |
| Valve-in-Valve (n = 25) | Valve-in-Ring (n = 29) | p-Value | |
|---|---|---|---|
| Pacemaker implantation | 0 (0%) | 1 (3.5%) | 1.00 |
| Acute kidney injury | 6 (24.0%) | 6 (20.7%) | 1.00 |
| New-onset dialysis | 1 (4.0%) | 5 (17.2%) | 0.20 |
| Wound-healing disorder | 0 (0%) | 0 (0%) | 1.00 |
| Sepsis | 2 (8.0%) | 3 (10.3%) | 1.00 |
| Stroke | 2 (8.0%) | 1 (3.4%) | 0.59 |
| TIA | 0 (0%) | 1 (3.4%) | 1.00 |
| CPR | 3 (12.0%) | 2 (6.9%) | 0.65 |
| Ventilation time | 1.00 | ||
| Under 12 h | 0 (0%) | 0 (0%) | |
| Under 24 h | 23 (92.0%) | 25 (86.2%) | |
| Over 24 h | 1 (4.0%) | 1 (3.4%) | |
| RBC transfusion (units) | 0.7 ± 2.0 | 2.3 ± 3.8 | 0.02 |
| ICU stay (days) | 3.8 ± 6.8 | 4.3 ± 6.3 | 0.96 |
| Hospital stay (days) | 9.9 ± 5.9 | 13.5 ± 8.0 | 0.13 |
| 30-day mortality | 1 (4.0%) | 2 (6.9%) | 1.00 |
| Overall mortality | |||
| Cardiac death | 5 (38.5%) | 12 (52.2%) | 0.66 |
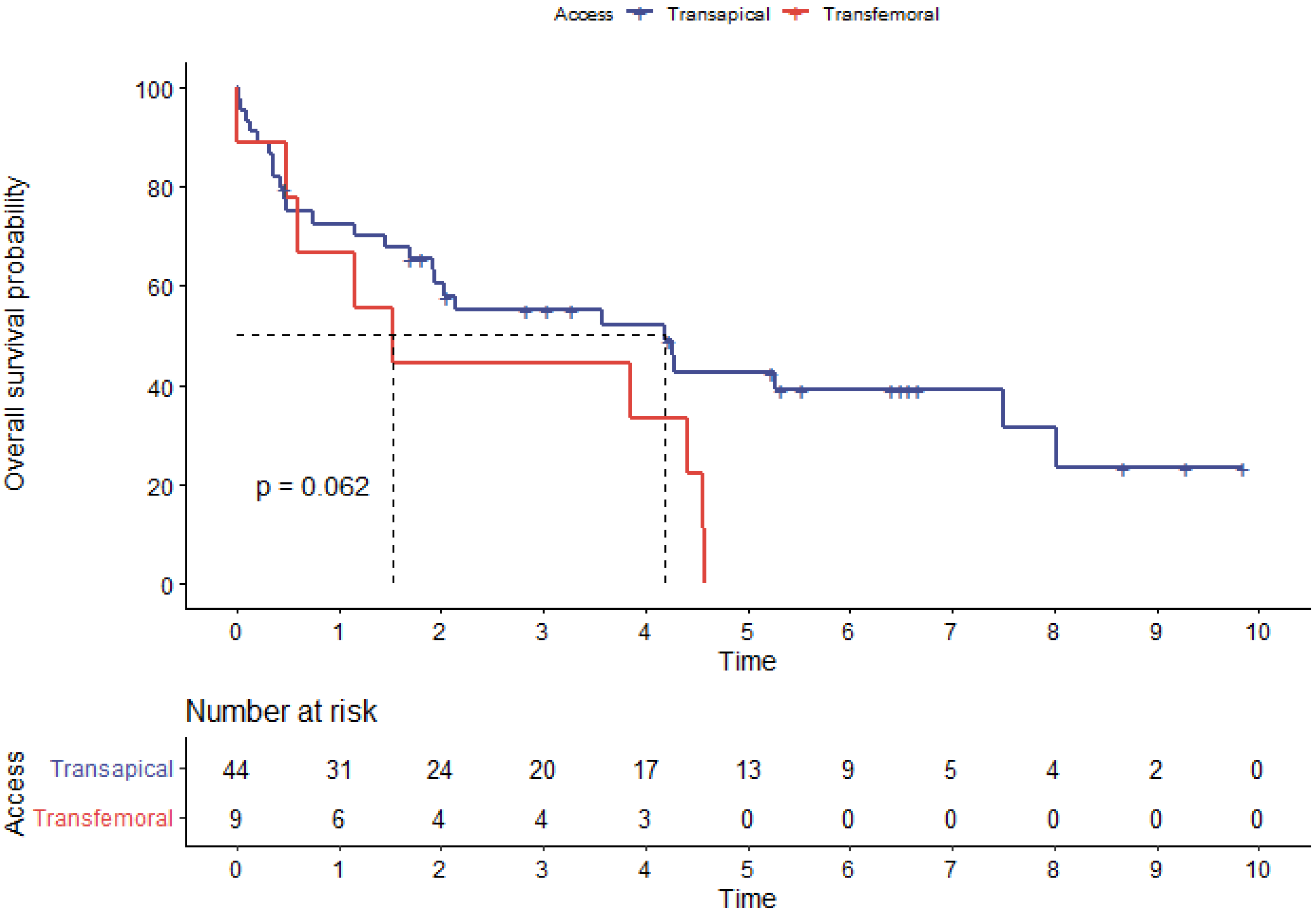
| Median survival time (years) | 4.2 | 1.2 | 0.01 |
| Hazard Ratio | 95% CI | p | |
|---|---|---|---|
| STS-PROM | 1.07 | 0.99–1.15 | 0.08 |
| EuroSCORE II | 1.04 | 1.00–1.08 | 0.06 |
| Age | 1.02 | 0.97–1.08 | 0.38 |
| Sex (female) | 2.14 | 1.07–4.27 | 0.03 |
| BMI | 1.03 | 0.97–1.09 | 0.39 |
| Diabetes on insulin | 1.68 | 0.59–4.77 | 0.33 |
| COPD | 1.25 | 0.51–3.07 | 0.62 |
| Pulmonary hypertension | 2.13 | 0.75–6.06 | 0.16 |
| Chronic kidney disease | 1.65 | 0.83–3.26 | 0.15 |
| GFR | 0.99 | 0.97–1.00 | 0.14 |
| Peripheral vascular disease | 0.76 | 0.29–1.96 | 0.57 |
| Atrial fibrillation | 1.44 | 0.34–6.03 | 0.62 |
| Preoperative pacemaker | 1.77 | 0.92–3.43 | 0.09 |
| Type of valve disease (functional) | 1.55 | 0.80–3.00 | 0.19 |
| Type of heart failure (HFrEF) | 1.70 | 0.84–3.41 | 0.14 |
| Baseline LVEF | 0.98 | 0.96–1.00 | 0.10 |
| History of stroke | 1.12 | 0.46–2.7 | 0.80 |
| Time since initial operation | 1.07 | 0.99–1.16 | 0.11 |
| Procedure duration | 1.02 | 1.01–1.03 | <0.001 |
| Procedure type (ViR) | 2.36 | 1.19–4.67 | 0.01 |
| Valve size | 0.93 | 0.77–1.10 | 0.41 |
| Conversion to sternotomy | 29.33 | 4.82–178.4 | <0.001 |
| Transfemoral access | 2.06 | 0.95–4.46 | 0.06 |
| Hazard Ratio | 95% CI | p | |
|---|---|---|---|
| Sex (female) | 2.49 | 1.19–5.20 | 0.02 |
| Conversion to sternotomy | 22.49 | 3.47–145.92 | 0.001 |
| Procedure type (ViR) | 2.06 | 1.01–4.21 | 0.05 |
| EuroSCORE II | 1.06 | 1.07–1.11 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilbring, M.; Petrov, A.; Arzt, S.; Eiselt, J.P.; Taghizadeh-Waghefi, A.; Matschke, K.; Kappert, U.; Alexiou, K. Long-Term Outcomes after Transcatheter Mitral Valve-in-Valve or Valve-in-Ring Procedures. J. Pers. Med. 2023, 13, 803. https://doi.org/10.3390/jpm13050803
Wilbring M, Petrov A, Arzt S, Eiselt JP, Taghizadeh-Waghefi A, Matschke K, Kappert U, Alexiou K. Long-Term Outcomes after Transcatheter Mitral Valve-in-Valve or Valve-in-Ring Procedures. Journal of Personalized Medicine. 2023; 13(5):803. https://doi.org/10.3390/jpm13050803
Chicago/Turabian StyleWilbring, Manuel, Asen Petrov, Sebastian Arzt, Julia Patricia Eiselt, Ali Taghizadeh-Waghefi, Klaus Matschke, Utz Kappert, and Konstantin Alexiou. 2023. "Long-Term Outcomes after Transcatheter Mitral Valve-in-Valve or Valve-in-Ring Procedures" Journal of Personalized Medicine 13, no. 5: 803. https://doi.org/10.3390/jpm13050803
APA StyleWilbring, M., Petrov, A., Arzt, S., Eiselt, J. P., Taghizadeh-Waghefi, A., Matschke, K., Kappert, U., & Alexiou, K. (2023). Long-Term Outcomes after Transcatheter Mitral Valve-in-Valve or Valve-in-Ring Procedures. Journal of Personalized Medicine, 13(5), 803. https://doi.org/10.3390/jpm13050803






