Recurrent/Subsequent Stroke and Associated Outcomes in Geriatric Patients with OSA and Prior Stroke Events: A Retrospective Study Using the 2019 National Inpatient Sample
Abstract
1. Introduction
2. Methods
2.1. Data Source
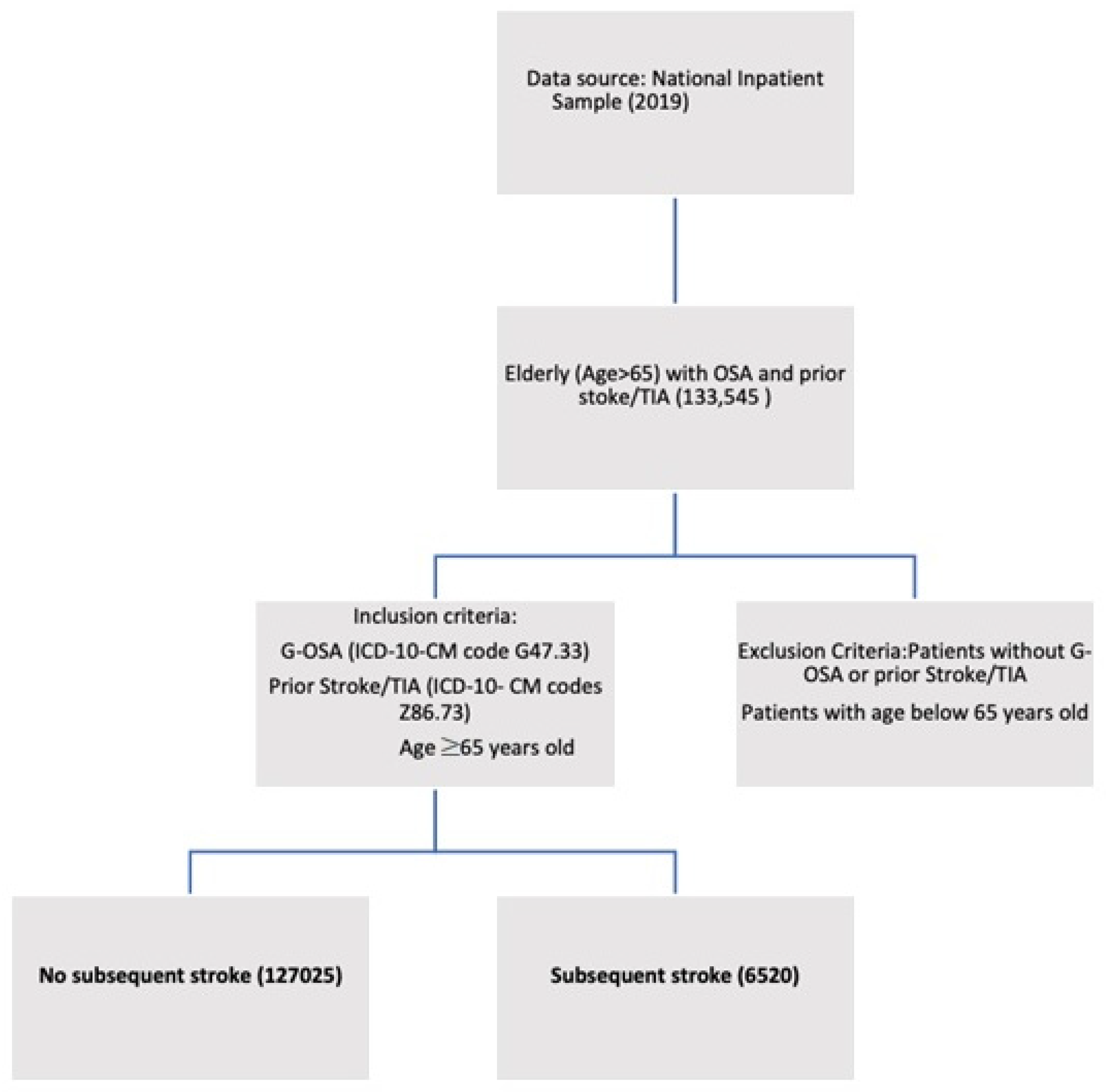
2.2. Study Population
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hale, E.; Gottlieb, E.; Usseglio, J.; Shechter, A. Post-stroke sleep disturbance and recurrent cardiovascular and cerebrovascular events: A systematic review and meta-analysis. Sleep Med. 2023, 104, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.; Adra, N.; Chuprevich, S.; Attarian, H. Screening for OSA in stroke patients: The role of a sleep educator. Sleep Med. 2022, 100, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.L.; Durkalski, V.; Durmer, J.S.; Broderick, J.P.; Zahuranec, D.B.; Levine, D.A.; Anderson, C.S.; Bravata, D.M.; Yaggi, H.K.; Morgenstern, L.B.; et al. Sleep for Stroke Management and Recovery Trial (Sleep SMART): Rationale and methods. Int. J. Stroke 2020, 15, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, D.; Loffler, K.A.; Wang, X.; McEvoy, R.D.; Woodman, R.J.; Luo, Y.; Lorenzi-Filho, G.; Barbe, F.; Tripathi, M.; et al. Sleep duration and risk of cardiovascular events: The SAVE study. Int. J. Stroke 2020, 15, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality; Rockville, M.D. HCUP Databases. Healthcare Cost and Utilization Project (HCUP). 2021. Available online: www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 22 July 2021).
- Agency for Healthcare Research and Quality; Rockville, M.D. Publishing with HCUP Data. Healthcare Cost and Utilization Project (HCUP). 2022. Available online: www.hcup-us.ahrq.gov/db/publishing.jsp (accessed on 16 May 2022).
- Xia, X.; Yue, W.; Chao, B.; Li, M.; Cao, L.; Wang, L.; Shen, Y.; Li, X. Prevalence and risk factors of stroke in the elderly in Northern China: Data from the National Stroke Screening Survey. J Neurol. 2019, 266, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Jamee Shahwan, A.; Abed, Y.; Desormais, I.; Magne, J.; Preux, P.M.; Aboyans, V.; Lacroix, P. Epidemiology of coronary artery disease and stroke and associated risk factors in Gaza community—Palestine. PLoS ONE 2019, 14, e0211131. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Leino, A.; Westeren-Punnonen, S.; Töyräs, J.; Myllymaa, S.; Leppänen, T.; Ylä-Herttuala, S.; Muraja-Murro, A.; Kantanen, A.-M.; Autere, J.; Jäkälä, P.; et al. Acute stroke and TIA patients have specific polygraphic features of obstructive sleep apnea. Sleep Breath. 2020, 24, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Sico, J.J.; Yaggi, H.K.; Ofner, S.; Concato, J.; Austin, C.; Ferguson, J.; Qin, L.; Tobias, L.; Taylor, S.; Vaz Fragoso, C.A.; et al. Development, validation, and assessment of an ischemic stroke or transient ischemic attack-specific prediction tool for obstructive sleep apnea. J. Stroke Cerebrovasc. Dis. 2017, 26, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Alloubani, A.; Saleh, A.; Abdelhafiz, I. Hypertension and diabetes mellitus as a predictive risk factors for stroke. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, C.; Sandberg, O.; Gustafson, Y.; Bucht, G.; Carlberg, B.; Stenlund, H.; Franklin, K.A. Obstructive sleep apnea is a risk factor for death in patients with stroke: A 10-year follow-up. Arch. Intern. Med. 2008, 168, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Mondragon, E.; Jimenez, A.; Palladino-Davis, A.G.; Davis, D.; Escamilla-Cejudo, J.A. Hispanic health in the USA: A scoping review of the literature. Public Health Rev. 2016, 37, 31. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.M.; Bryant, A.S. Racial and Ethnic Disparities in Health and Health Care. Obstet. Gynecol. Clin. 2017, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Farag, M.; Zizi, F.; Pandi-Perumal, S.R.; Chung, A.; Truong, A.; Tello, D.; McFarlane, S.I. Obstructive sleep apnea and stroke. Sleep Med. Dis. Int. J. 2018, 2, 120–125. [Google Scholar] [CrossRef]
- Ramos, A.R.; Seixas, A.; Dib, S.I. Obstructive sleep apnea and stroke: Links to health disparities. Sleep Health 2015, 1, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, P.; Cocuzza, S.; Maniaci, A.; Ferlito, S.; Rasà, D.; Anzivino, R.; Vicini, C.; Iannella, G.; La Mantia, I. The effect of adenotonsillectomy on children’s behavior and cognitive performance with obstructive sleep apnea syndrome: State of the art. Children 2021, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, S.; Maniaci, A.; Di Luca, M.; La Mantia, I.; Grillo, C.; Spinato, G.; Motta, G.; Testa, D.; Ferlito, S. Long-term results of nasal surgery: Comparison of mini-invasive turbinoplasty. J. Biol. Regul. Homeost. Agents 2020, 34, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
| Variables | No Subsequent Stroke (n = 127,025) | Subsequent Stroke (n = 6520) | |||
|---|---|---|---|---|---|
| Percentage % | Percentage % | p-Value | |||
| Age (years) at admission | Median [IQR] | 75 (70–81) | 75 (70–81) | <0.001 | |
| Sex | Male | 57.40% | 61.40% | <0.001 | |
| Female | 42.60% | 38.60% | |||
| Race | White | 81.20% | 82.00% | <0.001 | |
| Black | 11.50% | 10.70% | |||
| Hispanic | 4.40% | 3.70% | |||
| Asian/Pacific Islanders | 1.10% | 1.50% | |||
| Native Americans | 0.50% | 0.50% | |||
| Others | 1.30% | 1.60% | |||
| Median household income national quartile for patient ZIP Code | 0–25th | 25.90% | 26.50% | <0.001 | |
| 26–50th | 27.00% | 25.10% | |||
| 51–75th | 26.30% | 25.40% | |||
| 76–100th | 20.80% | 23.00% | |||
| Primary expected payer | Medicare | 90.10% | 89.00% | 0.003 | |
| Medicaid | 0.60% | 0.80% | |||
| Private including HMO | 6.80% | 7.70% | |||
| Self-pay | 0.40% | 0.20% | |||
| No charges | 0.00% | 0.00% | |||
| Others | 2.10% | 2.20% | |||
| Type of admission | Non-elective | 81.30% | 95.30% | <0.001 | |
| Elective | 18.70% | 4.70% | |||
| Bed size of the hospital | Small | 21.00% | 16.50% | <0.001 | |
| Medium | 28.10% | 29.80% | |||
| Large | 50.90% | 53.70% | |||
| Location/teaching status of the hospital | Rural | 8.40% | 5.80% | <0.001 | |
| Urban non-teaching | 17.80% | 16.70% | |||
| Urban teaching | 73.80% | 77.50% | |||
| Region of hospital | North-East | 14.20% | 13.40% | <0.001 | |
| Mid-West | 33.80% | 30.70% | |||
| South | 36.10% | 36.00% | |||
| West | 15.90% | 19.90% | |||
| Comorbidities | |||||
| Acquired immune deficiency syndrome | 0.20% | 0.20% | 0.817 | ||
| Alcohol abuse | 1.60% | 2.80% | <0.001 | ||
| Arthropathies | 5.90% | 3.80% | <0.001 | ||
| Leukemia | 0.80% | 0.70% | 0.288 | ||
| Lymphoma | 1.00% | 0.60% | 0.001 | ||
| Metastatic cancer | 1.80% | 2.10% | 0.109 | ||
| Solid tumor without metastasis, in situ | 0.00% | 0.20% | <0.001 | ||
| Solid tumor without metastasis, malignant | 3.20% | 2.90% | 0.266 | ||
| Dementia | 12.50% | 12.50% | 0.93 | ||
| Depression | 20.70% | 17.70% | <0.001 | ||
| Hypertension, complicated | 50.40% | 48.10% | <0.001 | ||
| Hypertension, uncomplicated | 29.70% | 42.70% | <0.001 | ||
| Diabetes with chronic complications | 40.20% | 34.20% | <0.001 | ||
| Diabetes without chronic complications | 12.80% | 17.90% | <0.001 | ||
| Hyperlipidemia | 71.70% | 76.50% | <0.001 | ||
| Obesity | 35.30% | 29.90% | <0.001 | ||
| Peripheral vascular disease | 13.20% | 13.00% | 0.637 | ||
| Prior MI | 17.60% | 14.10% | <0.001 | ||
| Prior PCI | 1.40% | 0.80% | <0.001 | ||
| Prior CABG | 17.00% | 14.90% | <0.001 | ||
| Tobacco Use Disorder | 7.40% | 8.20% | 0.019 | ||
| Chronic pulmonary disease | 42.40% | 27.70% | <0.001 | ||
| Hypothyroidism | 22.30% | 20.50% | <0.001 | ||
| Other thyroid disorders | 1.40% | 2.40% | <0.001 | ||
| Cancer | 6.60% | 6.20% | 0.174 | ||
| In-hospital outcomes | |||||
| All-cause mortality | 1.60% | 5.10% | <0.001 | ||
| All-cause mortality by gender | Male | Female | |||
| No stroke | 1.80% | 1.30% | <0.001 | ||
| Stroke | 5.20% | 4.80% | 0.407 | ||
| All-cause mortality by race | White | Black | Hispanic | ||
| No stroke | 1.60% | 1.50% | 1.60% | 0.088 | |
| Stroke | 4.90% | 4.40% | 10.60% | <0.001 | |
| Disposition of patient | Routine | 46.20% | 32.30% | <0.001 | |
| Transfers to Short term hospital | 1.90% | 2.80% | |||
| Transfer–other: Includes Skilled Nursing Facility (SNF), Intermediate Care Facility (ICF), Another type of facility | 25.50% | 42.20% | |||
| Home Health Care (HHC) | 24.40% | 17.30% | |||
| Against Medical Advice (AMA) | 0.40% | 0.30% | |||
| Length of stay (days) | Median [IQR] | 4 | 3 | <0.001 | |
| Total charges (USD) | Median [IQR] | $39,035 | $42,865 | <0.001 | |
| Variables | Adjusted Odds Ratio | 95% CI | p Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Female | 1.11 | 0.98 | 1.25 | 0.096 |
| Non-elective vs. Elective admission | 6.51 | 4.83 | 8.76 | <0.001 |
| Location/teaching status of the hospital | ||||
| Urban non-teaching vs. Rural | 1.48 | 1.09 | 2.03 | 0.008 |
| Urban teaching vs. Rural | 1.68 | 1.27 | 2.23 | |
| Comorbidities | ||||
| Hypertension complicated | 2.17 | 1.78 | 2.64 | <0.001 |
| Hypertension Uncomplicated | 3.18 | 2.58 | 3.92 | <0.001 |
| Diabetes with chronic complications | 1.28 | 1.08 | 1.51 | 0.004 |
| Hyperlipidemia | 1.24 | 1.08 | 1.43 | 0.002 |
| Obesity | 0.86 | 0.76 | 0.98 | 0.025 |
| Prior myocardial infarction | 0.8 | 0.67 | 0.96 | 0.016 |
| Chronic pulmonary disease | 0.54 | 0.47 | 0.62 | <0.001 |
| Other thyroid disorders | 1.69 | 1.14 | 2.49 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, R.; Singh, S.; Mellacheruvu, S.P.; Mohammed, A.S.; Soni, R.; Perera, A.; Makarla, V.A.; Santhosh, S.; Siddiqui, M.A.; Mohammed, B.K.; et al. Recurrent/Subsequent Stroke and Associated Outcomes in Geriatric Patients with OSA and Prior Stroke Events: A Retrospective Study Using the 2019 National Inpatient Sample. J. Pers. Med. 2023, 13, 782. https://doi.org/10.3390/jpm13050782
Desai R, Singh S, Mellacheruvu SP, Mohammed AS, Soni R, Perera A, Makarla VA, Santhosh S, Siddiqui MA, Mohammed BK, et al. Recurrent/Subsequent Stroke and Associated Outcomes in Geriatric Patients with OSA and Prior Stroke Events: A Retrospective Study Using the 2019 National Inpatient Sample. Journal of Personalized Medicine. 2023; 13(5):782. https://doi.org/10.3390/jpm13050782
Chicago/Turabian StyleDesai, Rupak, Sandeep Singh, Sai Priyanka Mellacheruvu, Adil Sarvar Mohammed, Roshni Soni, Ayodya Perera, Venkata Akhil Makarla, Sarayu Santhosh, Muneeb Ali Siddiqui, Bilal Khan Mohammed, and et al. 2023. "Recurrent/Subsequent Stroke and Associated Outcomes in Geriatric Patients with OSA and Prior Stroke Events: A Retrospective Study Using the 2019 National Inpatient Sample" Journal of Personalized Medicine 13, no. 5: 782. https://doi.org/10.3390/jpm13050782
APA StyleDesai, R., Singh, S., Mellacheruvu, S. P., Mohammed, A. S., Soni, R., Perera, A., Makarla, V. A., Santhosh, S., Siddiqui, M. A., Mohammed, B. K., Mohammed, Z. U. R., Gandhi, Z., Vyas, A., Jain, A., Sachdeva, R., & Kumar, G. (2023). Recurrent/Subsequent Stroke and Associated Outcomes in Geriatric Patients with OSA and Prior Stroke Events: A Retrospective Study Using the 2019 National Inpatient Sample. Journal of Personalized Medicine, 13(5), 782. https://doi.org/10.3390/jpm13050782






