Abstract
Cerebral cavernous malformations (CCMs) arise when capillaries within the brain enlarge abnormally, causing the blood–brain barrier (BBB) to break down. The BBB serves as a sophisticated interface that controls molecular interactions between the bloodstream and the central nervous system. The neurovascular unit (NVU) is a complex structure made up of neurons, astrocytes, endothelial cells (ECs), pericytes, microglia, and basement membranes, which work together to maintain blood–brain barrier (BBB) permeability. Within the NVU, tight junctions (TJs) and adherens junctions (AJs) between endothelial cells play a critical role in regulating the permeability of the BBB. Disruptions to these junctions can compromise the BBB, potentially leading to a hemorrhagic stroke. Understanding the molecular signaling cascades that regulate BBB permeability through EC junctions is, therefore, essential. New research has demonstrated that steroids, including estrogens (ESTs), glucocorticoids (GCs), and metabolites/derivatives of progesterone (PRGs), have multifaceted effects on blood–brain barrier (BBB) permeability by regulating the expression of tight junctions (TJs) and adherens junctions (AJs). They also have anti-inflammatory effects on blood vessels. PRGs, in particular, have been found to play a significant role in maintaining BBB integrity. PRGs act through a combination of its classic and non-classic PRG receptors (nPR/mPR), which are part of a signaling network known as the CCM signaling complex (CSC). This network couples both nPR and mPR in the CmPn/CmP pathway in endothelial cells (ECs).
1. Introduction
Cerebral cavernous malformations (CCMs), among the most common brain vascular malformations, are characterized by abnormally dilated intracranial capillaries resulting in the destruction of the blood–brain barrier (BBB), leading to hemorrhagic stroke [1,2]. Three CCM genes, KRIT1 (CCM1) [3,4], MGC4607 (CCM2) [5,6], and PDCD10 (CCM3) [7], have been identified as causes of familial CCM (fCCM); at least one of these genes is disrupted in most human fCCM cases [1]. It has been defined that the three CCM proteins form the CCM signaling complex (CSC) that maintains the integrity of the blood–brain barrier (BBB) [8,9,10,11]. Although the majority of carriers of CCM gene mutations are asymptomatic, this autosomal dominant disorder with incomplete penetrance can lead to irreversible brain damage once symptoms manifest, typically in the form of focal hemorrhage. Despite ongoing research, the specific molecular mechanism that triggers the development of CCM pathology remains unclear.
The BBB, the blood vessel barrier in the brain, is a complex, dynamic, and selective interface between the bloodstream and central nervous system (CNS) [12,13,14]. The BBB is responsible for regulating the transfer of molecules between the bloodstream and the central nervous system (CNS) [15,16]. It acts as a barrier, blocking the entry of certain blood components, such as pathogens and drugs, into the cerebrospinal fluid (CSF) or CNS. However, it permits the selective passage of vital nutrients (glucose, amino acids, and lipid-soluble substances), hormones (neurosteroids), and signaling agents. It is formed by three structural features: tight junctions between non-fenestrated capillary endothelial cells, pericytes, basement membranes, and astrocyte endfeet (AE) [12,13,14]. The disruption of endothelial cell junctions within the blood–brain barrier (BBB) can lead to hemorrhagic stroke with high morbidity and mortality [17,18,19]. Likewise, any disruption to the four essential components of the BBB, namely, tight junctions between non-fenestrated capillary endothelial cells, pericytes, basement membranes, and astrocyte endfeet, can compromise the integrity of the BBB. Although there are structural and functional deviations between the peripheral blood vessels and the blood–brain barrier, and variations in their signaling pathways have been reported [13,20,21,22,23], there are also numerous molecular and cellular mechanisms shared between them, even though many questions remain unanswered [22,24,25]. Additionally, the two main lesion sites in CCM pathology have been identified in both the BBB and cutaneous peripheral blood vessels [1,26]. Therefore, in this review, we provide a brief description of the underlying mechanisms of vessel leakage between peripheral blood vessels and the BBB, with an emphasis on hemorrhages (equivalent to symptomatic CCMs for neurosurgery) related to the BBB, due to limited space.
It is crucial to comprehend the molecular mechanisms underlying the signaling pathways that regulate and maintain blood–brain barrier permeability in both physiological and pathological states. The elucidation of the molecular mechanisms involved in the disruption of the BBB can provide insights into the effects of various factors, including abusive drugs, toxins, and pathogens. This knowledge can also be instrumental in understanding numerous neurological pathologies, such as encephalitis, meningitis, and, in particular, hemorrhagic stroke [27,28]. The maintenance of blood–brain barrier (BBB) integrity primarily relies on two crucial endothelial cell junctions: adherens junctions (AJs) and tight junctions (TJs). These junctions are formed by different molecular components, but they are functionally and structurally interconnected [29]. This review exclusively examines the pathological leakage resulting from disturbed endothelial cell contacts in the disrupted BBB. While transport pathways across the BBB, such as receptor-mediated endocytosis, exosomal transport, and transcytosis of endothelial cells in the BBB [30,31,32,33], have immense potential for therapeutic use [34,35,36,37], they could not be covered in this review due to space constraints.
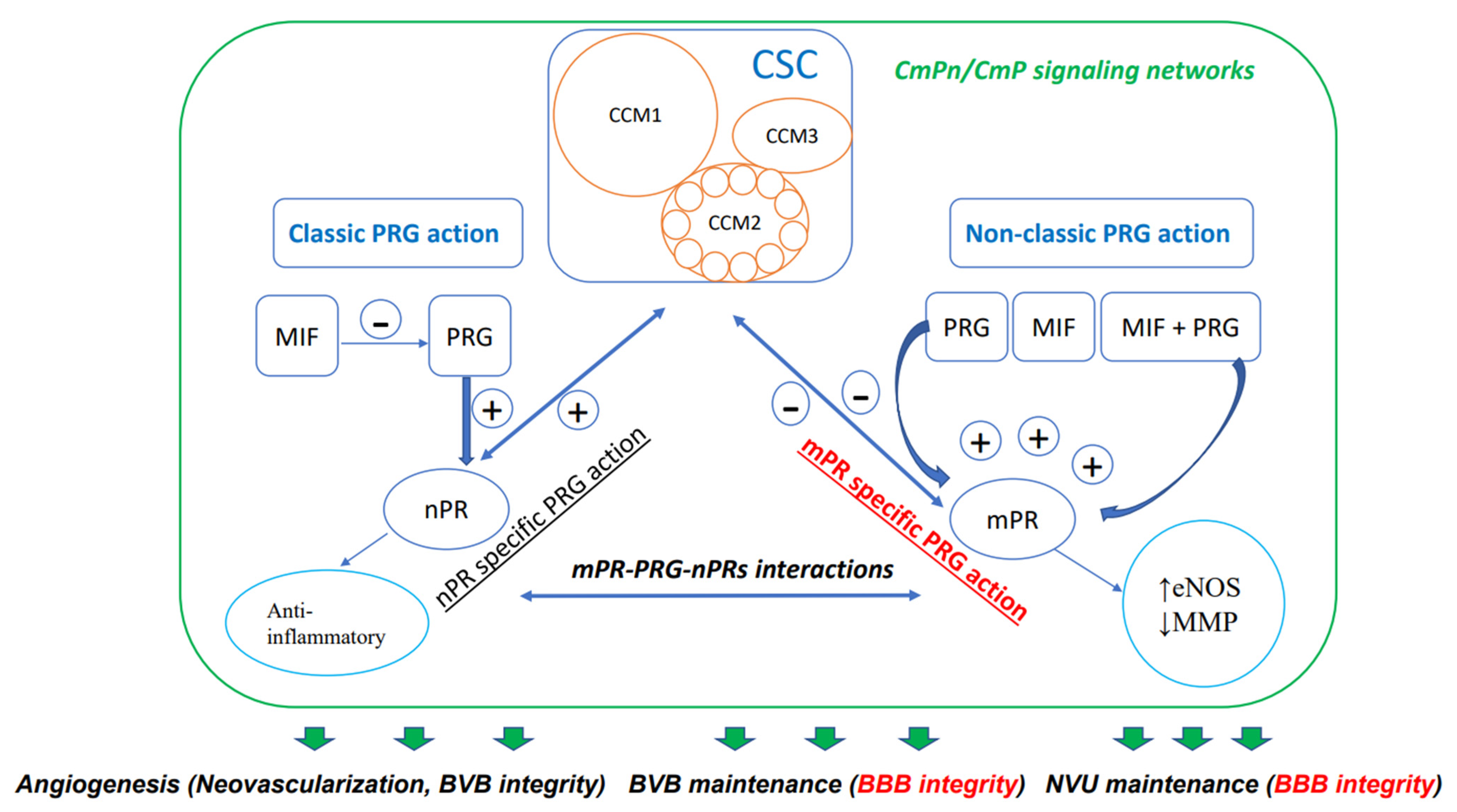
Steroids, which are either derived from dietary sources or synthesized within the body, are defined by their distinctive four-ring carbon structure and play vital roles as hormones in various physiological functions. These compounds have been employed in numerous therapeutic applications for a broad array of human disorders [38,39,40], such as vascular conditions, although their use continues to be a subject of debate [41]. Specifically, the importance of progesterone (PRG), a key female sex steroid, in preserving the integrity of the blood–brain barrier has been the focus of extensive research. The latest research suggests that the reciprocal modulation and coupling of both the classic nuclear PRG receptor (nPR) and the non-classic membrane PRG receptor (mPR) signaling occur through the CSC, which is a crucial modulator of the BBB [8,9,10,42,43,44,45,46]. According to these findings, a novel signaling network called the CSC-mPR-PRG-nPR/CSC-mPR-PRG (CmPn/CmP) operates within endothelial cells (ECs), while the CmPn signaling network is present in nPR(+) cells, and the CmP signaling network is also present in nPR(−) cells (Figure 1).This network is subject to dynamic modulation and fine-tuning through a range of feedback mechanisms under the influence of PRG actions, which are crucial for maintaining the BBB [8,46].
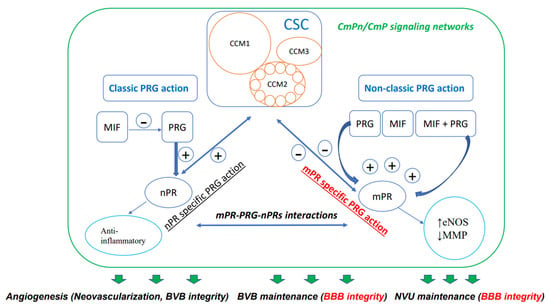
Figure 1.
Diagram demonstrating CmPn/CmP signaling network (largest square) where the CSC (CCM signaling complex, CSC square) couples both classic nPR (classic PRG action) and non-classic mPR (non-classic PRG action) signaling to exert their influence on the performance of vascular ECs. Multiple small circles within CCM2 indicates its essential role with binding status with both CCM1 and CCM3, through its multiple alternative spliced isoforms. Dual-directional arrows indicate bi-directional effects; bold green arrows indicate eventual effects of the CmPn/CmP signaling network on vascular angiogenesis, including neovascularization and dynamic regulation of the blood vessel barrier. Within the green frame of the CmPn/CmP signaling network, dark-blue arrows and frames are major components of the network, while light-blue circles are downstream cellular events modulated by the network. Plus symbol (+) indicates positive impact while minus symbol (−) for negative effect. Within the CSC, light-brown circles represent key components: CCM1, CCM2, and CCM3. The signaling pathway colored in red is important for BBB integrity. As mentioned in the article, this signaling network also affects the preservation of the blood–brain barrier (BBB) and neurovascular unit (NVU), both of which are essential for maintaining BBB integrity.
At present, tissue plasminogen activator (tPA) is the most effective medication recommended for treating ischemic strokes. tPA helps dissolve blood clots, restoring blood flow to the brain. Surgery remains the only viable option for treating hemorrhagic strokes, highlighting the importance of preventing and managing strokes [47,48,49,50]. While there is currently no available clinical agent or method for effectively repairing the blood–brain barrier (BBB), several epigenetic mechanisms and regulators that can either protect or disrupt the BBB have been identified. This suggests that the identification of both extracellular and intracellular factors in the future could hold therapeutic potential for addressing this challenge [51].
This article provides a comprehensive review of the interplay among steroids, EC junctions, and blood vessel permeability, including the impact of inflammation on BBB integrity and the effects of angiogenic factors on BBB integrity. It also discusses the potential of steroids as future therapeutics and examines the effects of sex steroids on neovascularization and downstream angiogenic factors of PRG signaling. Furthermore, it highlights PRG’s neuroprotective effects on the BBB and the actions of PRG and its derivatives as neurosteroids. Lastly, the article explores the impact of PRG-mediated signaling on endothelial cell function and the maintenance of BBB integrity by the newly defined CmPn/CmP signaling networks.
2. Key Factors Influencing Blood Vessel Permeability
Blood vessel permeability can be influenced by various factors, such as the integrity of endothelial cell junctions, the occurrence of inflammatory events, the expression of adhesion molecules, the activation of intracellular signaling pathways, and the release of angiogenic factors. Additionally, the presence of vascular pores or fenestrations, vesicular transport, and cell–cell communications can play a role in regulating blood vessel permeability. In simple terms, the permeability of blood vessels is controlled by molecular processes that regulate the EC junction to maintain stability in the vasculature [52,53]. Impaired EC stability contributes to various health issues. Similarly, numerous physiological and environmental factors, such as blood flow dynamics [54], heparin and glycocalyx [55,56], basement membrane [57], and pathological conditions including perturbed angiogenesis and lymphangiogenesis [58,59], cancers [58], diabetes [60], and aging [61,62], can all impact EC junction responses and result in increased permeability of blood vessels.
2.1. EC Junctions
As mentioned earlier, EC junctions mainly consist of TJs and AJs [63]. TJs consist of junction adhesion molecules (JAMs), claudins, and occludins [64], whereas AJs contain cadherin components such as VE-cadherin and N-cadherin [65]. Changes to the composition of both adherens junctions (AJs) and tight junctions (TJs) can potentially interfere with normal vascular permeability by affecting the transportation of molecules between adjacent cells. This can lead to a compromised vascular barrier, allowing immune cells and other substances to leak through. Endothelial cell junctions enable the flow of blood proteins, mediators, and immune cells through the vasculature. The regulation of endothelial cell permeability involves different mechanisms such as vascular pores or fenestrations, vesicular transport, and cell–cell communication [63]. Furthermore, damaged endothelial cells can lead to increased permeability of inflammatory cytokines and other factors such as vascular endothelial growth factor (VEGF), thrombin, and histamine [66,67]. If increased blood vessel permeability in the brain is present and reabsorption is not functioning adequately, this can result in the development of edema, which may worsen the conditions, leading to BBB dysfunction. Such complications can cause serious brain injuries, including hemorrhagic strokes [63].
2.2. Inflammation and BBB Integrity
Inflammation is widely recognized as a significant contributor to the BBB dysfunction [68,69]. Leukocytes can firmly attach and migrate through the ECs by utilizing the vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) [70]. When there is EC damage or EC junction dysregulation, immune cells release proinflammatory cytokines, which activate the endothelium and allow for selectin attachment to leukocytes [71]. The attachment and migration of leukocytes through the endothelium, facilitated by VCAM-1 and ICAM-1, can result in a delay in leukocyte circulation. This delay provides an opportunity for chemokines on the endothelial surface to interact with the leukocytes and activate integrin [72].
2.3. Angiogenic Factors and BBB Integrity
VEGF plays a crucial role in regulating vascularization from embryogenesis to adulthood, including the formation of adult blood vessels [73,74]. In mammals, there are three VEGF tyrosine kinase receptors that can bind to five VEGF ligands [73]. When VEGF receptors associate with their ligand, and this complex interacts with VE-cadherin, a component of AJs, BBB permeability can increase [75]. In addition, VEGF can induce the expression of integrin and selectin to initiate an inflammatory response [76].
3. Angiogenic Impacts of Steroids on Maintenance of Vasculature
3.1. Steroids Can Influence the BBB Integrity
Steroids have been found to influence the BBB through their regulation of VCAM-1 expression [77]. ESTs have been found to reduce the expression of VCAM-1 mRNA and protein, which is induced by lipopolysaccharide (LPS) and inhibits monocyte adhesion to ECs. In contrast, glucocorticoids (GCs), a related steroid, have the opposite effect by stabilizing VCAM-1 mRNA expression [77]. These findings suggest that the administration of GCs to ECs in the central nervous system (CNS) could potentially enhance the preservation of blood–brain barrier (BBB) integrity in various CNS disorders, particularly in cases where the BBB has been compromised [29]. Glucocorticoids (GCs) mainly exert their actions by binding their GC receptors (GRs), and they occasionally also bind to the mineralocorticoid receptor (MR) [78]. It has been reported that GCs increase the expression levels of occludin, claudin-5, cadherin-9, and VE-cadherin, key components of EC intercellular TJs and AJs, at both transcriptional and translational levels [29,41,79,80,81,82,83]. For example, GCs are known to induce the expression of occludin in the brain endothelium at the transcriptional level by binding to GC-responsive elements (GREs) in the occludin promoter [29,41,80,81,82], indicating their potential in restoring BBB integrity. However, the well-known fact that VCAM-1 promotes leukocyte attachment [84,85,86], has raised doubts regarding the effectiveness of GCs in maintaining endothelial cell junctions.
GCs are able to specifically have effects on the vasculature upon binding GRs through nitric oxide biosynthesis [87,88], further transmitting a signaling pathway that causes inflammation in ECs and affects angiogenesis [87,88]. The vascular inflammation caused by GCs has recently been found to be related to hypertension in mouse model studies [89]. Additionally, studies have also found that hypertension is in part mediated by EC GR expression [90]. When an individual undergoes a stress response, GC is released. As a ubiquitously expressed steroid receptor, GRs have two alternative splicing isoforms, dominant GRα and the less common GRβ, which drive a large spectrum of responses across various cell types and tissues [29,83,91,92]. GRβ has intrinsic activities and regulates a collection of genes related to inflammatory processes, cell communication, migration, and proliferation [29,83,92,93]. GRβ was also found to play a major role in maintaining BBB integrity [83]. Since evidence has suggested that GC/GR actions can improve BBB integrity by increasing the expression levels of many key components of TJs and AJs [29,41,79,80,81,82,83], attempts have been made to explore the therapeutic potential of GC/GR actions on neurovascular ECs in improving clinical symptoms of hemorrhagic strokes. However, a large clinical trial on the therapeutic efficacy of GC/GR actions on hemorrhagic strokes provided contradicting outcomes [94,95], as described below.
3.2. Steroids Might Play Major Roles for Compromised BBB
ESTs belong to another group of important regulatory hormones of BBB permeability. They protect the BBB before menopause, but may increase BBB permeability with aging [96,97,98]. Following the initial discoveries regarding the positive effects of EST treatment in stroke prevention, subsequent clinical evidence presented conflicting results regarding the impact of ESTs on blood–brain barrier (BBB) integrity [99,100], These findings highlighted the significant dependence of EST efficacy on the reproductive or postmenopausal age of women [99,100]. For example, hormone (mixture of ESTs + PRGs) replacement therapy (HRT) appears to increase stroke risk and worsen neurological outcomes [99,101,102,103,104]. More specifically, hormonal changes during pregnancy appear to be a major risk factor for stroke in women [105,106,107,108,109,110,111,112], suggesting that the EST-to-PRG ratio may be critical for hemorrhagic stroke susceptibility. Additionally, hemorrhagic stroke is the most dominant type (up to 74%) of strokes during pregnancy [113,114,115,116,117,118,119,120]. Although pregnancy as a hemorrhage risk factor in women with CCMs is under debate [121], CCM patients are more likely to bleed or to have lesion expansion during pregnancy, suggesting a potential influence of sex hormones in hemorrhagic events [8,46,122]. Therefore, clinical recommendations for asymptomatic CCM patients with pregnancy were made with conservative monitoring by magnetic resonance imaging (MRI) [123].
Evaluation of female hormone contraceptives, such as PRG and its derivatives, are yet to be explored for effects on hemorrhagic stroke management and prevention. A recent report indicated that mifepristone (MIF), a widely used contraceptive and a known antagonist of PRG-nPR signaling, can inhibit PRG actions through nuclear PRG receptors (nPRs) in cells that express them. However, in cells that lack nPR expression, MIF can function as a PRG agonist on its own or in combination with PRG to activate PRG signaling through non-classic membrane PRG receptors (mPRs), leading to specific mPR-mediated PRG actions [8,42,43,44,46,122,124,125].
Hence, MIF and PRG collaborate to amplify mPR-specific PRG action in this signaling cascade (Figure 1). Within nPR(−) ECs, the CmP signaling network reduces CSC stability through the adverse effects of mPR-specific PRG signaling actions on the CSC [PRG, MIF alone or combined (PRG/MIF)], in the absence of the positive impacts of nPR-specific PRG signaling actions (Figure 1) [8,42,43,44,45]. The balance between classic and non-classic PRG signaling impacts the CSC function and recognizes the CSC as an important mediator of nPR and mPR crosstalk in nPR(+) cells [8,42,43,44,45]. This observation is supported by a previous finding that activation of mPR-specific PRG signaling can potentiate expression of the hormone-activated nPR-2 isoform [126]. This observation was later duplicated in nPR(−) microvascular ECs, indicating existence of a common CmP signaling network in various nPR(−) cells [45]. Several studies have suggested that the ratio of estrogens (ESTs) to progestogens (PRGs) could have a more substantial impact on blood–brain barrier (BBB) maintenance [105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120]. Additionally, clinical investigations have provided further proof of this by demonstrating a link between prolonged exposure to PRGs, as seen in birth control regimes, and the development of more severe hemorrhagic cerebral cavernous malformations (CCMs) [46,127].
3.3. Steroids as Potential Therapeutics
When damage to the endothelium takes place, EST aids in repairing the EC by reestablishing the tight and adherens junctions, which results in a decreased likelihood of hemorrhagic stroke. This finding has contributed to the utilization of tamoxifen in treating vascular conditions [128,129]. A similar finding of GC/GR actions to improve BBB integrity has been employed as a medical therapy to treat vascular malformations [29,41,79,80,81,82,83]. This approach was first applied in the treatment of infantile hemangiomas, but with varying success rates [130,131]. Similar endeavors have also been made on cerebral cavernous malformations (CCMs). A study on animals revealed that GC therapy can effectively tackle the bleeding characteristics in Ccm mice by targeting GRs in the brain’s endothelial cells [132]. Subsequently, various crucial molecular and cellular processes were analyzed, and researchers also investigated the potential clinical uses of therapies derived from the effects of GC/GR interactions on CCMs [133,134]. However, despite numerous efforts, clinical trials have not shown evidence to support the use of GC/GR-based therapies in treating CCMs [133,134,135,136,137,138].
Like ESTs and GC, the effect of PRG and its metabolites/derivatives (defined as PRGs), as well as contraceptive derivatives (progestins), on BBB integrity has also been primitively explored, despite a lack of understanding that different progestins used in contraception exhibit differential off-target effects via steroid receptors [139]. PRGs were indeed subsequently found to be able to bind both GRs and MRs [140,141]. It was reported that PRGs can repress the inflammatory response through their binding to GRs in a dose-dependent manner at concentrations within the physiologically relevant range [141]. This suggests the same positive effect of PRGs on the BBB. Furthermore, the neuroprotective properties of PRGs against VEGF, a major angiogenic factor that can lead to vascular leakage, have been thoroughly investigated [142,143,144]. Many contradicting results were presented; it was found that, unlike EST, which mainly promotes neovascularization through activating classic ER-mediated signaling pathways, PRGs regulate a variety of downstream factors which can be either angiogenic or antiangiogenic [145]. This suggests that PRGs might exert their cellular effect on ECs through different receptors. These findings suggest that PRGs may exert their cellular effects on ECs through various receptors. While no correlation was identified between the parameters measured in classic nuclear progesterone receptors (nPRs) and neovascularization, there is strong evidence to suggest that progestin-responsive genes may solely exert their effects through a non-classical mechanism [142,143,144,146]. In fact, one report demonstrated that PRG stimulation of NO, through increased eNOS expression in human vascular ECs does not involve nPRs, but instead employs non-classical mPRα through the PI3K/Akt and MAPK pathways [147].
4. Impacts of Sex Steroids on Maintenance of Vasculature
4.1. Neovascularization
Neovascularization is the formation of new blood vessels, which typically occurs in specific physiological processes, such as the menstrual cycle and tumorigenesis [148,149]. Disruption of angiogenic regulation can lead to the acquisition of enhanced angiogenic capabilities by endothelial cells (ECs) in the vascular beds, which can result in the formation of new blood vessels from destabilized sites in the basement membrane [149]. Sex steroids, particularly PRGs, regulate neovascularization in a diverse fashion. Several studies have found a phenomenon that low concentrations of PRG potentially promote neovascularization through nPRs in a dose-dependent fashion, while high concentrations inhibit the original effect [150,151,152].
4.2. Downstream Angiogenic Factors of PRG Signaling
The uterus vasculature is sensitive to cytokines, growth factors, and sex hormones, affecting angiogenesis and tissue growth. VEGF distribution changes during the menstrual cycle may impact angiogenesis. Endometrial stromal cells and myometrium smooth muscle cells produce VEGF, which increases in rat uterine tissue due to PRGs and ESTs [153,154,155]. Glandular VEGF immunostaining increases during the menstrual cycle, while nPRs decrease and ERs remain unchanged. Contraceptives containing PRG and EST lead to decreased VEGF without affecting menstrual bleeding or EC density, supporting PRG regulation of glandular endometrial VEGF levels. Other regulators of neovascularization include bFGF, PD-ECGF, Ang, HIF1a, and NOS.
Moreover, research has demonstrated that PRGs can increase the actions of nitric oxide (NO) in vascular ECs exclusively through membrane progesterone receptor (mPR) signaling. In a study of human umbilical vein endothelial cells (HUVECs), the role of mPRs in angiogenesis was investigated [156]. As an mPR agonist, PRG–BSA conjugates increased NO levels in HUVECs, indicating that progestin-responsive genes are activated by mPRs at the cell membrane. The study also revealed that PRG–mPR interactions boost eNOS activity via eNOS phosphorylation [157]. Phosphoinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) inhibitors reversed this stimulation of eNOS phosphorylation brought on by the PRG–mPR action. Similarly, siRNA silencing of mPRα diminished the stimulatory signaling effects. However, knockout of nPR did not result in a similar blockage of stimulatory effects [158]. Therefore, mPRα-mediated signaling via PI3K/Akt and MAPK signaling facilitates the PRG-induced stimulation of NO synthesis in HUVECs. The abnormal expression of eNOS [159] and MMPs [160,161] in CCM-deficient mutant models has been widely investigated using systems biology methods [10] in both in vitro and in vivo models.
5. Angiogenic Impacts of PRG on the Neurovascular Unit (NVU)
5.1. Neuroprotective Impact of PRGs on the NVU
The NVU is composed of neurons, astrocytes, ECs, pericytes, microglia, and basement membranes [13,23,52,57]. PRG is known to have neuroprotective effects on neurons vulnerable to ischemic and excitotoxic damage [162,163,164]. Post-traumatic brain injury (TBI) edema can be a consequence that leads to excitotoxic effects on neurons. The administration of PRG has been found to decrease neuronal death and edema, resulting in improved behavioral recovery, as reported in studies [165,166]. Furthermore, reduced antioxidant enzyme activity leads to oxidative stress in neural tissues. A study demonstrated that low doses of PRG and/or EST can significantly increase superoxide dismutase activity and mitigate the negative impact of lipid peroxidation [167]. Lipid peroxidation, which can initiate cerebral edema, is a common occurrence after brain trauma. A study demonstrated that rats treated with PRG exhibited one-third of the lipid peroxidation observed in the controls [168].
In addition, PRG is known to regulate microglial activation, reduce the production of proinflammatory cytokines, increase the expression of antiapoptotic Bcl-2 protein, promote myelinization and myelin repair [169], and regulate neuronal plasticity via neurotrophins such as brain-derived neurotrophic factor (BDNF) by interacting with one class of non-classic PRG membrane receptors, PRG receptor membrane component 1 (PGRMC1) [170,171,172]. Therefore, neurological effects of long-term exposure to contraceptives (mixture of ESTs and PRG derivatives) on cognitive function have recently been noticed [173], although whether ESTs and PRGs coordinate or counteract during these neuroprotective steroid actions remains under debate [172].
5.2. Neuroprotective Effects of PRG and Its Derivatives on the BBB against Thrombin and Matrix Metalloproteinase (MMP)
Thrombin has the ability to impair both endothelial cell (EC) junctions and the extracellular matrix (ECM) [174,175]. Thrombin can compromise the BBB’s integrity in two ways. Firstly, it activates proteases via protease-activated receptor 1 (PAR-1), resulting in the internalization of the protein Claudin-5 present in the BBB’s tight junctions (TJs) [175]. Additionally, thrombin can cause necrosis and apoptosis in the brain due to ischemia and/or hypoxia, which can result in cerebral edema, stroke, and other CNS injuries [175,176].
Activated MMPs have been linked to a compromised blood–brain barrier (BBB). This is believed to be due to their ability to affect the integrity of the BBB by breaking down and restructuring the extracellular matrix that surrounds the capillaries in the brain, as well as by degrading the proteins responsible for tight junctions [177,178,179]. PRG has demonstrated neuroprotective properties by reducing the induction of MMP and edema in the BBB [180]. PRG and its derivatives [allopregnanolone and 5α-dihydroprogestrone (5α-DHP)] regulate MMPs through mPRs as neurosteroids. The inhibition of PRG conversion to 5α-DHP and allopregnanolone has been shown to block PRG’s neuroprotective effects [175]. Furthermore, PRG has been shown to partially inhibit the actions of thrombin in the myometrium [176,181].
5.3. Neuroprotective Effects of PRG on BBB against Inflammatory Pathway
Like EST, PRG can attenuate LPS-stimulated inflammation in a dose-dependent fashion by suppressing the LPS-induced NF-κB activation. These specific anti-inflammatory effects of PRG can be inhibited by MIF, suggesting PRG-nPR as a sole anti-inflammatory signaling pathway [182].
PRG can potentially regulate intracellular free calcium (Ca2+) levels by activating a specific membrane receptor called PGRMC1. This activation results in reduced nuclear accumulation of Ca2+-dependent nuclear factor of activated CD8+ T cells 1 (NFAT1), associated with T-cell development and activation. Therefore, PRG and its non-classic membrane receptor, PGRMC1, may directly suppress T-cell activation in inflammatory responses, indicating their involvement in non-classic PRG signaling [182]. PRG can also indirectly inhibit T-cell activation by stimulating IL-10-producing dendritic cells, while also inhibiting Th1 and Th17 differentiation of CD4+ T helper (Th) cells, shortening the active course of some chronic autoimmune inflammatory diseases in the brain [182].
5.4. Actions of PRG and Its Derivatives as Neurosteroids
Physiological impacts of PRG can be influenced by both mPRs and nPRs [183]. nPR-A and nPR-B are the most common nPR isoforms that mediate the response of PRG [163]. These nPRs are structurally different and have tissue-specific responses due to factors such as post-translational changes, in addition to various cofactors that lead to distinct responses for each isoform receptor [184]. Furthermore, mRNA transcripts of both nPR-A and nPR-B isoforms are found to colocalize in areas of the brain where nPRs are already known to be located [184]. Within the CNS, steroid levels are due to both secretions of endocrine glands and local metabolism. All neurons and glial cells can metabolize PRG; therefore, changes in the steroid hormone pool could indicate changes in the brain [185]. In fact, AP (3,5-tetrahydroxyprogesterone) is a PRG-derived neuro-metabolite that acts as an agonist for the receptor of neurotransmitter gamma-aminobutyric acid (GABA), the GABA-A receptor [186]. Therefore, PRGs have important physiologically antiepileptic, sedative, and regulatory effects on behavior and stress [186]. In sum, PRG and its derivatives, as neurosteroids, can indirectly influence the BBB integrity through their impact on neurons within the NVU.
6. Impacts of CmPn/CmP Networks on the BBB
6.1. The Impact of PRG-Mediated Signaling on Endothelial Cell (EC) Function in the Vasculature
PRG derivatives in hormone replacement therapy for postmenopausal women have complex cardiovascular effects due to various signaling pathways [187]. They promote endometrial safety and reduce blood pressure in hypertensive patients by increasing nitric oxide production via eNOS modulation; the cumulative impact of these PRG derivatives on the vasculature can ultimately provide protection against cerebral hemorrhage [188,189,190,191].
6.2. CmPn/CmP Signaling Ntworks Maintain the BBB Integrity
The intricate feedback regulation among the PRG-activated CmPn signaling network in nPR(+) cells can be summarized with a model of CSC-modulated classic and non-classic PRG signaling (Figure 1). As a sex steroid hormone, PRG binds to its nPR for classic PRG actions and mPR for non-classic PRG actions [125,192,193,194,195,196]. Additionally, recent findings demonstrated that CCM2 interacts with both CCM1 and CCM3 to form the CSC [1,10]. In this model, steroid actions are attained through the balanced efforts between nPR and mPR signaling pathways and are further fine-tuned by the CSC (Figure 1) [8,42,43,44,45,46,124,125]. The CSC can couple both classic nPR and non-classic mPR signaling to form the CmPn signaling network in nPR(+) cells and the CmP signaling network in nPR(−) cells [42,43,44,125,197]. The CmP signaling network was found to play a major role in maintaining BBB integrity in nPR(−) microvascular ECs within the NVU [8,46,125,198]. Additionally, within nPR(−) vascular ECs, the CSC is a master regulator governing homeostasis of PRG and its mediated signaling cascades in the more fragile CmP signaling network, indicating an important role of the CmPn/CmP signaling networks in angiogenesis and tumorigenesis [8,42,43,44,45,46,125,198]. Previous multi-omics data, which provided a global view of signal transduction modulated by the CSC, indicated that perturbed CSC leads to disruption of blood vessel cell junctions [8,46]. Comparative omics data across multiple models provided further supportive evidence that the perturbed CmP network in nPR(−) microvascular ECs leads to a compromised BBB with disrupted EC junctions, both in vivo and in vitro [8,45,46,198].
7. Conclusions
In this review, we attempted to summarize steroid signaling pathways that regulate BBB integrity through the impacts of certain steroid receptors on its key components, with special emphasis on the combined effect of PRG and CSC through the CmPn/CmP signaling network (Figure 1, Table 1).

Table 1.
This table summarizes the steroid signaling pathways that regulate BBB integrity via their effects on key components of EC junctions, as well as relevant genes and key findings discussed in the text. Additionally, a list of recent publications discussing each topic in the text, as well as the newly discovered roles of CmPn and CmP signaling networks in maintaining the BBB among vascular ECs, is included.
The BBB is an intricate interface between blood flow and brain tissue with significant contribution to brain injuries, such as hemorrhagic stroke, through its disruption. Understanding the mechanisms through which various pathologies alter BBB integrity will allow for future advances in therapeutics for neurovascular injuries. Steroids, especially sex steroids (mainly ESTs and PRGs), have shown some major impacts on EC repair, neovascularization, and maintenance of BBB integrity. PRG is perhaps the most significant factor involved in maintaining vasculature and protecting neurons and the BBB. The CSC’s essential role in coupling and modulating nPR and mPR signaling was found to be integral in maintaining the BBB integrity within the CmPn/CmP signaling networks. Perturbation of this network, especially in CmP signaling in cerebral ECs, disrupts EC junction components and leads to compromised BBB. In addition to PRG, we described the role of other steroids in BBB maintenance, as well as potential roles in hemorrhagic stroke treatment.
Author Contributions
J.Z., conceptualization, methodology, writing—original draft preparation, and writing—review and editing; R.G., writing—original draft preparation, writing—review and editing, and illustration; M.H., writing—original draft preparation and writing—review and editing; J.A., writing—original draft preparation and writing—review and editing; N.S., writing—original draft preparation and writing—review and editing; J.C., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Johnathan Abou-Fadel, Texas Tech University Health Science Center El Paso (TTUHSCEP), for technical help during the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5α-DHP | 5α-dihydroprogestrone |
| AJs | adherens junctions |
| ANG | angiopoietin |
| AP | 3,5-tetrahydroxyprogesterone |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| CCM | cerebral cavernous malformations |
| CmP | CSC-mPRs-PRG |
| CmPn | CSC-mPRs-PRG-nPRs |
| CNS | central nervous system |
| CSC | CCM signaling complex |
| CSF | cerebrospinal fluid |
| EC | endothelial cells |
| PCs | pericytes |
| BMs | basement membranes |
| AE | astrocyte endfeet |
| eNOS | endothelial nitric oxide synthase |
| EST | estrogen |
| fCCM | familial CCM |
| GC | glucocorticoid |
| GRs | glucocorticoid receptors |
| HIF1a | Hypoxia-inducible factor 1a |
| HRT | hormone replacement therapy |
| HUVEC | human umbilical vein endothelial cells |
| ICAM-1 | intercellular cell adhesion molecule 1 |
| JAMs | junction adhesion molecules |
| KO | knockout |
| LPS | lipopolysaccharide |
| MAPKs | mitogen-activated protein kinases |
| MEFs | mouse embryonic fibroblasts |
| MIF | mifepristone |
| MMPs | matrix metalloproteinases |
| mPRs | membrane PRG receptors |
| MR | mineralocorticoid receptor |
| MRI | magnetic resonance imaging |
| NF-κB | nuclear factor kappa B |
| NFAT | nuclear factor of activated T cells |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NVU | neurovascular unit |
| nPR–s | nuclear PRG receptors |
| PD-ECGF | platelet-derived EC growth factor |
| PI3Ks | phosphoinositide 3-kinases |
| PRG | progesterone |
| PGRMC1,2 | progesterone receptor membrane component 1,2 |
| siRNA | short interfering RNA |
| Th | T helper |
| TJs | tight junctions |
| TBI | post-traumatic brain injury |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| WT | wildtype |
References
- Padarti, A.; Zhang, J. Recent advances in cerebral cavernous malformation research. Vessel Plus 2018, 2, 21. [Google Scholar] [CrossRef]
- Zhang, J. Molecular biology of cerebral cavernous malformation. In Cavernous Malformations of the Nervous System; Rigamonti, D., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 31–40. [Google Scholar] [CrossRef]
- Zhang, J.; Clatterbuck, R.E.; Rigamonti, D.; Dietz, H.C. Cloning of the murine Krit1 cDNA reveals novel mammalian 5′ coding exons. Genomics 2000, 70, 392–395. [Google Scholar] [CrossRef]
- Laberge-le Couteulx, S.; Jung, H.H.; Labauge, P.; Houtteville, J.P.; Lescoat, C.; Cecillon, M.; Marechal, E.; Joutel, A.; Bach, J.F.; Tournier-Lasserve, E. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat. Genet. 1999, 23, 189–193. [Google Scholar] [CrossRef]
- Jiang, X.; Padarti, A.; Qu, Y.; Sheng, S.; Abou-Fadel, J.; Badr, A.; Zhang, J. Alternatively spliced isoforms reveal a novel type of PTB domain in CCM2 protein. Sci. Rep. 2019, 9, 15808. [Google Scholar] [CrossRef]
- Liquori, C.L.; Berg, M.J.; Siegel, A.M.; Huang, E.; Zawistowski, J.S.; Stoffer, T.; Verlaan, D.; Balogun, F.; Hughes, L.; Leedom, T.P.; et al. Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. Am. J. Hum. Genet. 2003, 73, 1459–1464. [Google Scholar] [CrossRef]
- Bergametti, F.; Denier, C.; Labauge, P.; Arnoult, M.; Boetto, S.; Clanet, M.; Coubes, P.; Echenne, B.; Ibrahim, R.; Irthum, B.; et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am. J. Hum. Genet. 2005, 76, 42–51. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Jiang, X.; Padarti, A.; Goswami, D.G.; Smith, M.; Grajeda, B.; Bhalli, M.; Le, A.; Walker, W.E.; Zhang, J. mPR-Specific Actions Influence Maintenance of the Blood-Brain Barrier (BBB). Int. J. Mol. Sci. 2022, 23, 9684. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Smith, M.; Falahati, K.; Zhang, J. Comparative omics of CCM signaling complex (CSC). Chin. Neurosurg. J. 2020, 6, 4. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Vasquez, M.; Grajeda, B.; Ellis, C.; Zhang, J. Systems-wide analysis unravels the new roles of CCM signal complex (CSC). Heliyon 2019, 5, e02899. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Zhang, J. Systems Wide Analysis of CCM Signaling Complex Alterations in CCM-Deficient Models Using Omics Approaches. Methods Mol. Biol. 2020, 2152, 325–344. [Google Scholar] [CrossRef]
- Yan, L.; Moriarty, R.A.; Stroka, K.M. Recent progress and new challenges in modeling of human pluripotent stem cell-derived blood-brain barrier. Theranostics 2021, 11, 10148–10170. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Correale, J.; Villa, A. Cellular elements of the blood-brain barrier. Neurochem. Res. 2009, 34, 2067–2077. [Google Scholar] [CrossRef]
- Liebner, S.; Czupalla, C.J.; Wolburg, H. Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 2011, 55, 467–476. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef]
- Manno, E.M.; Atkinson, J.L.; Fulgham, J.R.; Wijdicks, E.F. Emerging medical and surgical management strategies in the evaluation and treatment of intracerebral hemorrhage. Mayo Clin. Proc. 2005, 80, 420–433. [Google Scholar] [CrossRef]
- Gomes, J.A.; Manno, E. New developments in the treatment of intracerebral hemorrhage. Neurol. Clin. 2013, 31, 721–735. [Google Scholar] [CrossRef]
- Ballabh, P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 2014, 41, 47–67. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Vandenbroucke, E.; Mehta, D.; Minshall, R.; Malik, A.B. Regulation of endothelial junctional permeability. Ann. N. Y. Acad. Sci. 2008, 1123, 134–145. [Google Scholar] [CrossRef]
- Ono, S.; Egawa, G.; Kabashima, K. Regulation of blood vascular permeability in the skin. Inflamm. Regen. 2017, 37, 11. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef]
- Batra, S.; Lin, D.; Recinos, P.F.; Zhang, J.; Rigamonti, D. Cavernous malformations: Natural history, diagnosis and treatment. Nat. Rev. Neurol. 2009, 5, 659–670. [Google Scholar] [CrossRef]
- Witt, K.A.; Sandoval, K.E. Steroids and the blood-brain barrier: Therapeutic implications. Adv. Pharmacol. 2014, 71, 361–390. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Salvador, E.; Shityakov, S.; Forster, C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014, 355, 597–605. [Google Scholar] [CrossRef]
- Friden, P.M. Receptor-mediated transport of therapeutics across the blood-brain barrier. Neurosurgery 1994, 35, 294–298, discussion 298. [Google Scholar] [CrossRef]
- Roberts, R.L.; Fine, R.E.; Sandra, A. Receptor-mediated endocytosis of transferrin at the blood-brain barrier. J. Cell Sci. 1993, 104 Pt 2, 521–532. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2018, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Segaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef]
- Stanimirovic, D.B.; Sandhu, J.K.; Costain, W.J. Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy Across the Blood-Brain Barrier. BioDrugs 2018, 32, 547–559. [Google Scholar] [CrossRef]
- Gomes, P.A.; Bodo, C.; Nogueras-Ortiz, C.; Samiotaki, M.; Chen, M.; Soares-Cunha, C.; Silva, J.M.; Coimbra, B.; Stamatakis, G.; Santos, L.; et al. A novel isolation method for spontaneously released extracellular vesicles from brain tissue and its implications for stress-driven brain pathology. Cell Commun. Signal. 2023, 21, 35. [Google Scholar] [CrossRef]
- Serafini, U.; Masala, C. Corticosteroids and autoimmune diseases. Recenti Prog. Med. 1976, 61, 684–710. [Google Scholar]
- McWilliams, D.F.; Thankaraj, D.; Jones-Diette, J.; Morgan, R.; Ifesemen, O.S.; Shenker, N.G.; Walsh, D.A. The efficacy of systemic glucocorticosteroids for pain in rheumatoid arthritis: A systematic literature review and meta-analysis. Rheumatology 2021, 61, 76–89. [Google Scholar] [CrossRef]
- Euers, L.; Abughazaleh, S.; Glassner, K.; Gajula, P.; Jones-Pauley, M.; Ezeana, C.; Puppala, M.; Wang, L.; Wong, S.; Oglat, A.; et al. Risk Factors for and Frequency of CT Scans, Steroid Use, and Repeat Visits in Inflammatory Bowel Disease Patients Seen at a Single-Center Emergency Department: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 2679. [Google Scholar] [CrossRef]
- Forster, C.; Burek, M.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Drenckhahn, D. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J. Physiol. 2008, 586, 1937–1949. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Bhalli, M.; Grajeda, B.; Zhang, J. CmP Signaling Network Leads to Identification of Prognostic Biomarkers for Triple-Negative Breast Cancer in Caucasian Women. Genet. Test. Mol. Biomarkers 2022, 26, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Grajeda, B.; Jiang, X.; Cailing-De La, O.A.; Flores, E.; Padarti, A.; Bhalli, M.; Le, A.; Zhang, J. CmP signaling network unveils novel biomarkers for triple negative breast cancer in African American women. Cancer Biomark. 2022, 34, 607–636. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Jiang, X.; Grajeda, B.; Padarti, A.; Ellis, C.C.; Flores, E.; Cailing-De La, O.A.; Zhang, J. CCM signaling complex (CSC) couples both classic and non-classic Progesterone receptor signaling. Cell Commun. Signal. 2022, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Jiang, X.; Padarti, A.; Goswami, D.; Smith, M.; Grajeda, B.; Walker, W.; Zhang, J. CCM signaling complex (CSC) is a master regulator governing homeostasis of progestins and their mediated signaling cascades. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Abou-Fadel, J. Calm the raging hormone—A new therapeutic strategy involving progesterone-signaling for hemorrhagic CCMs. Vessel Plus 2021, 5, 23. [Google Scholar] [CrossRef]
- Manners, J.; Steinberg, A.; Shutter, L. Early management of acute cerebrovascular accident. Curr. Opin. Crit. Care 2017, 23, 556–560. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Blanco, M.; Castillo, J. Stroke in 2012: Major advances in the treatment of stroke. Nat. Rev. Neurol. 2013, 9, 68–70. [Google Scholar] [CrossRef]
- Ihezie, S.A.; Mathew, I.E.; McBride, D.W.; Dienel, A.; Blackburn, S.L.; Thankamani Pandit, P.K. Epigenetics in blood-brain barrier disruption. Fluids Barriers CNS 2021, 18, 17. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L. Vascular permeability—The essentials. Upsala J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Kwee, I.L. Fluid Dynamics Inside the Brain Barrier: Current Concept of Interstitial Flow, Glymphatic Flow, and Cerebrospinal Fluid Circulation in the Brain. Neuroscientist 2019, 25, 155–166. [Google Scholar] [CrossRef]
- Yang, R.; Chen, M.; Zheng, J.; Li, X.; Zhang, X. The Role of Heparin and Glycocalyx in Blood-Brain Barrier Dysfunction. Front. Immunol. 2021, 12, 754141. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.M.; Baluk, P. Imaging of angiogenesis in inflamed airways and tumors: Newly formed blood vessels are not alike and may be wildly abnormal: Parker B. Francis lecture. Chest 2005, 128, 602S–608S. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Lieth, E.; Barber, A.J.; Gardner, T.W. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin. Ophthalmol. 1999, 14, 240–248. [Google Scholar] [CrossRef]
- Shah, G.N.; Mooradian, A.D. Age-related changes in the blood-brain barrier. Exp. Gerontol. 1997, 32, 501–519. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef]
- Andriopoulou, P.; Navarro, P.; Zanetti, A.; Lampugnani, M.G.; Dejana, E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arter. Thromb. Vasc. Biol. 1999, 19, 2286–2297. [Google Scholar] [CrossRef]
- Rosenblum, S.L.; Kosman, D.J. Aberrant Cerebral Iron Trafficking Co-morbid With Chronic Inflammation: Molecular Mechanisms and Pharmacologic Intervention. Front. Neurol. 2022, 13, 855751. [Google Scholar] [CrossRef]
- Huber, J.D.; Witt, K.A.; Hom, S.; Egleton, R.D.; Mark, K.S.; Davis, T.P. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1241–H1248. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- McEver, R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015, 107, 331–339. [Google Scholar] [CrossRef]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Nam, J.O.; Jean, C.; Lawson, C.; Walsh, C.T.; Goka, E.; Lim, S.T.; Tomar, A.; Tancioni, I.; Uryu, S.; et al. VEGF-induced vascular permeability is mediated by FAK. Dev. Cell 2012, 22, 146–157. [Google Scholar] [CrossRef]
- Kim, I.; Moon, S.O.; Park, S.K.; Chae, S.W.; Koh, G.Y. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ. Res. 2001, 89, 477–479. [Google Scholar] [CrossRef]
- Simoncini, T.; Maffei, S.; Basta, G.; Barsacchi, G.; Genazzani, A.R.; Liao, J.K.; De Caterina, R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ. Res. 2000, 87, 19–25. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Oakley, R.H.; Cidlowski, J.A. Glucocorticoid Signaling and the Aging Heart. Front. Endocrinol. 2020, 11, 347. [Google Scholar] [CrossRef]
- Felinski, E.A.; Cox, A.E.; Phillips, B.E.; Antonetti, D.A. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp. Eye Res. 2008, 86, 867–878. [Google Scholar] [CrossRef]
- Harke, N.; Leers, J.; Kietz, S.; Drenckhahn, D.; Forster, C. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol. Cell. Endocrinol. 2008, 295, 39–47. [Google Scholar] [CrossRef]
- Forster, C.; Waschke, J.; Burek, M.; Leers, J.; Drenckhahn, D. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J. Physiol. 2006, 573, 413–425. [Google Scholar] [CrossRef]
- Forster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Westcott, R.; Chung, N.; Ghosh, A.; Ferguson, L.; Bingaman, W.; Najm, I.M.; Ghosh, C. Glucocorticoid Receptor beta Isoform Predominates in the Human Dysplastic Brain Region and Is Modulated by Age, Sex, and Antiseizure Medication. Int. J. Mol. Sci. 2022, 23, 4940. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, C.; Wang, Y.; Mu, L.; Li, X.; Qi, Y.; Yang, J.; Ma, C. Soluble Vascular Cell Adhesion Molecule-1 as an Inflammation-Related Biomarker of Coronary Slow Flow. J. Clin. Med. 2023, 12, 543. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Remuzgo-Martinez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.M.; Iturbe-Fernandez, D.; Lera-Gomez, L.; Sebastian Mora-Gil, M.; Prieto-Pena, D.; Portilla, V.; et al. Elevated VCAM-1, MCP-1 and ADMA serum levels related to pulmonary fibrosis of interstitial lung disease associated with rheumatoid arthritis. Front. Mol. Biosci. 2022, 9, 1056121. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef] [PubMed]
- Hafezi-Moghadam, A.; Simoncini, T.; Yang, Z.; Limbourg, F.P.; Plumier, J.C.; Rebsamen, M.C.; Hsieh, C.M.; Chui, D.S.; Thomas, K.L.; Prorock, A.J.; et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 2002, 8, 473–479. [Google Scholar] [CrossRef]
- Baum, M.; Moe, O.W. Glucocorticoid-mediated hypertension: Does the vascular smooth muscle hold all the answers? J. Am. Soc. Nephrol. 2008, 19, 1251–1253. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Zhang, J.; Geller, D.S. A critical role for vascular smooth muscle in acute glucocorticoid-induced hypertension. J. Am. Soc. Nephrol. 2008, 19, 1291–1299. [Google Scholar] [CrossRef]
- Oakley, R.H.; Sar, M.; Cidlowski, J.A. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J. Biol. Chem. 1996, 271, 9550–9559. [Google Scholar] [CrossRef]
- Ramos-Ramirez, P.; Tliba, O. Glucocorticoid Receptor beta (GRbeta): Beyond Its Dominant-Negative Function. Int. J. Mol. Sci. 2021, 22, 3649. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.; Peck, B.; Shek, E.; Stroup, S.; Hinson, J.; Arthur, S.; Marino, J.S. Overexpression of Glucocorticoid Receptor beta Enhances Myogenesis and Reduces Catabolic Gene Expression. Int. J. Mol. Sci. 2016, 17, 232. [Google Scholar] [CrossRef]
- Wintzer, S.; Heckmann, J.G.; Huttner, H.B.; Schwab, S. Dexamethasone in Patients with Spontaneous Intracerebral Hemorrhage: An Updated Meta-Analysis. Cerebrovasc. Dis. 2020, 49, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sundboll, J.; Horvath-Puho, E.; Schmidt, M.; Dekkers, O.M.; Christiansen, C.F.; Pedersen, L.; Botker, H.E.; Sorensen, H.T. Preadmission Use of Glucocorticoids and 30-Day Mortality After Stroke. Stroke 2016, 47, 829–835. [Google Scholar] [CrossRef]
- Maggioli, E.; McArthur, S.; Mauro, C.; Kieswich, J.; Kusters, D.H.M.; Reutelingsperger, C.P.M.; Yaqoob, M.; Solito, E. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav. Immun. 2016, 51, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Lenert, M.E.; Avona, A.; Garner, K.M.; Barron, L.R.; Burton, M.D. Sensory Neurons, Neuroimmunity, and Pain Modulation by Sex Hormones. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Kuruca, S.E.; Karadenizli, S.; Akgun-Dar, K.; Kapucu, A.; Kaptan, Z.; Uzum, G. The effects of 17beta-estradiol on blood brain barrier integrity in the absence of the estrogen receptor alpha; an in-vitro model. Acta Histochem. 2017, 119, 638–647. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 2014, 35, 8–30. [Google Scholar] [CrossRef]
- Sohrabji, F.; Bake, S.; Lewis, D.K. Age-related changes in brain support cells: Implications for stroke severity. Neurochem. Int. 2013, 63, 291–301. [Google Scholar] [CrossRef]
- Selvamani, A.; Sohrabji, F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol. Aging 2010, 31, 1618–1628. [Google Scholar] [CrossRef]
- Brinton, R.D. Investigative models for determining hormone therapy-induced outcomes in brain: Evidence in support of a healthy cell bias of estrogen action. Ann. N. Y. Acad. Sci. 2005, 1052, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.L.; Li, X.; Huber, J.D.; Rosen, C.L. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinology 2012, 153, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Viscoli, C.M.; Brass, L.M.; Kernan, W.N.; Sarrel, P.M.; Suissa, S.; Horwitz, R.I. A clinical trial of estrogen-replacement therapy after ischemic stroke. N. Engl. J. Med. 2001, 345, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Beal, C.C.; Faucher, M.A. Stroke and pregnancy: An integrative review with implications for neuroscience nurses. J. Neurosci. Nurs. 2015, 47, 76–84, quiz E71. [Google Scholar] [CrossRef]
- Carbillon, L. Pregnancy is an essential spontaneous screening stress test for the risk of early stroke in women. Stroke 2008, 39, e138. [Google Scholar] [CrossRef]
- Caso, V.; Falorni, A.; Bushnell, C.D.; Acciarresi, M.; Remohi, J.; Sprigg, N.; Gerli, S. Pregnancy, Hormonal Treatments for Infertility, Contraception, and Menopause in Women After Ischemic Stroke: A Consensus Document. Stroke 2017, 48, 501–506. [Google Scholar] [CrossRef]
- Cheng, C.A.; Lee, J.T.; Lin, H.C.; Lin, H.C.; Chung, C.H.; Lin, F.H.; Tsao, C.H.; Wu, Y.F.; Chien, W.C.; Chiu, H.W. Pregnancy increases stroke risk up to 1 year postpartum and reduces long-term risk. QJM 2017, 110, 355–360. [Google Scholar] [CrossRef]
- Feske, S.K. Stroke in pregnancy. Semin. Neurol. 2007, 27, 442–452. [Google Scholar] [CrossRef]
- James, A.H.; Bushnell, C.D.; Jamison, M.G.; Myers, E.R. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet. Gynecol. 2005, 106, 509–516. [Google Scholar] [CrossRef]
- Jeng, J.S.; Tang, S.C.; Yip, P.K. Stroke in women of reproductive age: Comparison between stroke related and unrelated to pregnancy. J. Neurol. Sci. 2004, 221, 25–29. [Google Scholar] [CrossRef]
- Sanders, B.D.; Davis, M.G.; Holley, S.L.; Phillippi, J.C. Pregnancy-Associated Stroke. J. Midwifery Womens Health 2018, 63, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ban, L.; Abdul Sultan, A.; Stephansson, O.; Tata, L.J.; Sprigg, N.; Nelson-Piercy, C.; Bath, P.M.; Ludvigsson, J.F. The incidence of first stroke in and around pregnancy: A population-based cohort study from Sweden. Eur. Stroke J. 2017, 2, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.C.; Feske, S.K.; Singhal, A.B. Stroke in Pregnancy: An Update. Neurol. Clin. 2019, 37, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Leffert, L.R.; Clancy, C.R.; Bateman, B.T.; Cox, M.; Schulte, P.J.; Smith, E.E.; Fonarow, G.C.; Schwamm, L.H.; Kuklina, E.V.; George, M.G. Patient Characteristics and Outcomes After Hemorrhagic Stroke in Pregnancy. Circ. Cardiovasc. Qual. Outcomes 2015, 8, S170–S178. [Google Scholar] [CrossRef]
- Liew, B.S.; Ghani, A.A.; You, X. Stroke in pregnancy. Med. J. Malaysia 2019, 74, 246–249. [Google Scholar] [PubMed]
- Liu, X.J.; Wang, S.; Zhao, Y.L.; Zhang, D.; Zhao, J.Z. A single-center study of hemorrhagic stroke caused by cerebrovascular disease during pregnancy and puerperium in China. Int. J. Gynaecol. Obstet. 2011, 113, 82–83. [Google Scholar] [CrossRef]
- Miller, E.C.; Sundheim, K.M.; Willey, J.Z.; Boehme, A.K.; Agalliu, D.; Marshall, R.S. The Impact of Pregnancy on Hemorrhagic Stroke in Young Women. Cerebrovasc. Dis. 2018, 46, 10–15. [Google Scholar] [CrossRef]
- Ulrich, N.D.; Lapeyre, E.R.; Moore, R.C. Hemorrhagic Stroke Resulting From Venous Malformation at 20 Weeks of Pregnancy. Ochsner J. 2016, 16, 542–544. [Google Scholar]
- Yoshida, K.; Takahashi, J.C.; Takenobu, Y.; Suzuki, N.; Ogawa, A.; Miyamoto, S. Strokes Associated With Pregnancy and Puerperium: A Nationwide Study by the Japan Stroke Society. Stroke 2017, 48, 276–282. [Google Scholar] [CrossRef]
- Witiw, C.D.; Abou-Hamden, A.; Kulkarni, A.V.; Silvaggio, J.A.; Schneider, C.; Wallace, M.C. Cerebral cavernous malformations and pregnancy: Hemorrhage risk and influence on obstetrical management. Neurosurgery 2012, 71, 626–630, discussion 631. [Google Scholar] [CrossRef]
- Zuurbier, S.M.; Santos, A.N.; Flemming, K.D.; Schmidt, B.; Jabbarli, R.; Lanzino, G.; Sure, U.; Dammann, P. Female Hormone Therapy and Risk of Intracranial Hemorrhage From Cerebral Cavernous Malformations: A Multicenter Observational Cohort Study. Neurology 2023, 100, e1673–e1679. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Nakase, H.; Nakagawa, I.; Nishimura, F.; Motoyama, Y.; Park, Y.S. Cavernous malformations in pregnancy. Neurol. Med. Chir. 2013, 53, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Renteria, M.; Belkin, O.; Aickareth, J.; Jang, D.; Hawwar, M.; Zhang, J. Zinc’s Association with the CmPn/CmP Signaling Network in Breast Cancer Tumorigenesis. Biomolecules 2022, 12, 1672. [Google Scholar] [CrossRef]
- Renteria, M.; Belkin, O.; Jang, D.; Aickareth, J.; Bhalli, M.; Zhang, J. CmPn signaling networks in the tumorigenesis of breast cancer. Front. Endocrinol. 2022, 13, 1013892. [Google Scholar] [CrossRef] [PubMed]
- Karteris, E.; Zervou, S.; Pang, Y.; Dong, J.; Hillhouse, E.W.; Randeva, H.S.; Thomas, P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: Potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 2006, 20, 1519–1534. [Google Scholar] [CrossRef]
- Flemming, K.D.; Kumar, S.; Brown, R.D., Jr.; Lanzino, G. Predictors of Initial Presentation with Hemorrhage in Patients with Cavernous Malformations. World Neurosurg. 2020, 133, e767–e773. [Google Scholar] [CrossRef]
- Zahreddine, R.; Davezac, M.; Smirnova, N.; Buscato, M.; Lhuillier, E.; Lupieri, A.; Solinhac, R.; Vinel, A.; Vessieres, E.; Henrion, D.; et al. Tamoxifen Accelerates Endothelial Healing by Targeting ERalpha in Smooth Muscle Cells. Circ. Res. 2020, 127, 1473–1487. [Google Scholar] [CrossRef]
- Zahreddine, R.; Davezac, M.; Buscato, M.; Smirnova, N.; Laffargue, M.; Henrion, D.; Adlanmerini, M.; Lenfant, F.; Arnal, J.F.; Fontaine, C. A historical view of estrogen effect on arterial endothelial healing: From animal models to medical implication. Atherosclerosis 2021, 338, 30–38. [Google Scholar] [CrossRef]
- Takagi, H. Diagnosis and management of cavernous hemangioma of the liver. Semin. Surg. Oncol. 1985, 1, 12–22. [Google Scholar] [CrossRef]
- Dourmishev, L.A.; Dourmishev, A.L. Craniofacial cavernous hemangioma: Succesful treatment with methylprednisolone. Acta Dermatovenerol. Alp. Pannonica Adriat. 2005, 14, 49–52. [Google Scholar]
- Tang, A.T.; Sullivan, K.R.; Hong, C.C.; Goddard, L.M.; Mahadevan, A.; Ren, A.; Pardo, H.; Peiper, A.; Griffin, E.; Tanes, C.; et al. Distinct cellular roles for PDCD10 define a gut-brain axis in cerebral cavernous malformation. Sci. Transl. Med. 2019, 11, eaaw3521. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Tee, J.W.; Trost, N.; McKelvie, P.; Wang, Y.Y. Anterior visual pathway cavernous malformations. J. Clin. Neurosci. 2015, 22, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Chu, K.; Jeong, S.W.; Park, H.K.; Bae, H.J.; Yoon, B.W. Cerebral cavernous malformations with dynamic and progressive course: Correlation study with vascular endothelial growth factor. Arch Neurol. 2003, 60, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, L.D.; Khan, A.A.; Niranjan, A.; Kano, H.; Flickinger, J.C.; Kondziolka, D. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J. Neurosurg. 2010, 113, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Abou-Al-Shaar, H.; Bahatheq, A.; Takroni, R.; Al-Thubaiti, I. Optic chiasmal cavernous angioma: A rare suprasellar vascular malformation. Surg. Neurol. Int. 2016, 7, S523–S526. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Sun, H.; Xu, J.G.; Li, Q.Y. Stereotactic radiosurgery of brainstem cavernous malformations: A systematic review and meta-analysis. J. Neurosurg. 2014, 120, 982–987. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, K.H.; Lee, M.H.; Lee, J.I. Stereotactic Radiosurgery for Brainstem Cavernous Malformations: An Updated Systematic Review and Meta-Analysis. World Neurosurg. 2019, 130, e648–e659. [Google Scholar] [CrossRef]
- Chakhtoura, Z.; Canonico, M.; Gompel, A.; Thalabard, J.C.; Scarabin, P.Y.; Plu-Bureau, G. Progestogen-only contraceptives and the risk of stroke: A meta-analysis. Stroke 2009, 40, 1059–1062. [Google Scholar] [CrossRef]
- Louw-du Toit, R.; Hapgood, J.P.; Africander, D. A direct comparison of the transcriptional activities of progestins used in contraception and menopausal hormone therapy via the mineralocorticoid receptor. Biochem. Biophys. Res. Commun. 2020, 526, 466–471. [Google Scholar] [CrossRef]
- Hapgood, J.P.; Ray, R.M.; Govender, Y.; Avenant, C.; Tomasicchio, M. Differential glucocorticoid receptor-mediated effects on immunomodulatory gene expression by progestin contraceptives: Implications for HIV-1 pathogenesis. Am. J. Reprod. Immunol. 2014, 71, 505–512. [Google Scholar] [CrossRef]
- Mueck, A.O.; Seeger, H. Progestogens and target tissues: Vascular systems. Maturitas 2009, 62, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, S.; Wong, B.C.; Archer, D.F. Effect of 17 beta-estradiol, progesterone, synthetic progestins, tibolone, and tibolone metabolites on vascular endothelial growth factor mRNA in breast cancer cells. Fertil. Steril. 2005, 84, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Charnock-Jones, D.S.; Macpherson, A.M.; Archer, D.F.; Leslie, S.; Makkink, W.K.; Sharkey, A.M.; Smith, S.K. The effect of progestins on vascular endothelial growth factor, oestrogen receptor and progesterone receptor immunoreactivity and endothelial cell density in human endometrium. Hum. Reprod. 2000, 15 (Suppl. 3), 85–95. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Xiao, J.; Dai, Z.; Chen, Q. Membrane progesterone receptor alpha (mPRalpha) enhances hypoxia-induced vascular endothelial growth factor secretion and angiogenesis in lung adenocarcinoma through STAT3 signaling. J. Transl. Med. 2022, 20, 72. [Google Scholar] [CrossRef]
- Neubauer, H.; Adam, G.; Seeger, H.; Mueck, A.O.; Solomayer, E.; Wallwiener, D.; Cahill, M.A.; Fehm, T. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric 2009, 12, 230–239. [Google Scholar] [CrossRef]
- Pang, Y.; Dong, J.; Thomas, P. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-alpha. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E899–E911. [Google Scholar] [CrossRef]
- Ribatti, D.; Pezzella, F. Overview on the Different Patterns of Tumor Vascularization. Cells 2021, 10, 639. [Google Scholar] [CrossRef]
- Demir, R.; Yaba, A.; Huppertz, B. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem. 2010, 112, 203–214. [Google Scholar] [CrossRef]
- Yu, P.; Li, S.; Zhang, Z.; Wen, X.; Quan, W.; Tian, Q.; Gao, C.; Su, W.; Zhang, J.; Jiang, R. Progesterone-mediated angiogenic activity of endothelial progenitor cell and angiogenesis in traumatic brain injury rats were antagonized by progesterone receptor antagonist. Cell Prolif 2017, 50, e12362. [Google Scholar] [CrossRef]
- Xia, Z.; Xiao, J.; Chen, Q. Solving the Puzzle: What Is the Role of Progestogens in Neovascularization? Biomolecules 2021, 11, 1686. [Google Scholar] [CrossRef]
- Matsubara, Y.; Matsubara, K. Estrogen and progesterone play pivotal roles in endothelial progenitor cell proliferation. Reprod. Biol. Endocrinol. 2012, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.M.; Stancel, G.M.; Chiappetta, C.; Murthy, L.; Boettger-Tong, H.L.; Makela, S. Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res. 1996, 56, 3954–3960. [Google Scholar]
- Cullinan-Bove, K.; Koos, R.D. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: Rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 1993, 133, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Arias-Loza, P.A.; Hu, K.; Widder, J.; Govindaraj, V.; von Poser-Klein, C.; Bauersachs, J.; Fritzemeier, K.H.; Hegele-Hartung, C.; Neyses, L.; et al. Ligand-dependent activation of ERbeta lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc. Res. 2008, 77, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Selles, J.; Polini, N.; Alvarez, C.; Massheimer, V. Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation. Life Sci. 2001, 69, 815–827. [Google Scholar] [CrossRef]
- Simoncini, T.; Mannella, P.; Fornari, L.; Caruso, A.; Willis, M.Y.; Garibaldi, S.; Baldacci, C.; Genazzani, A.R. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology 2004, 145, 5745–5756. [Google Scholar] [CrossRef]
- Lopez-Ramirez, M.A.; Lai, C.C.; Soliman, S.I.; Hale, P.; Pham, A.; Estrada, E.J.; McCurdy, S.; Girard, R.; Verma, R.; Moore, T.; et al. Astrocytes propel neurovascular dysfunction during cerebral cavernous malformation lesion formation. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Fujimura, M.; Watanabe, M.; Shimizu, H.; Tominaga, T. Expression of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinase (TIMP) in cerebral cavernous malformations: Immunohistochemical analysis of MMP-2, -9 and TIMP-2. Acta Neurochir. 2007, 149, 179–183, discussion 183. [Google Scholar] [CrossRef]
- Choquet, H.; Trapani, E.; Goitre, L.; Trabalzini, L.; Akers, A.; Fontanella, M.; Hart, B.L.; Morrison, L.A.; Pawlikowska, L.; Kim, H.; et al. Cytochrome P450 and matrix metalloproteinase genetic modifiers of disease severity in Cerebral Cavernous Malformation type 1. Free Radic. Biol. Med. 2016, 92, 100–109. [Google Scholar] [CrossRef]
- Xu, L.; Sapolsky, R.M.; Giffard, R.G. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp. Neurol. 2001, 169, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Cubeddu, A.; Giannini, A.; Merlini, S.; Cela, V.; Angioni, S.; Genazzani, A.R. Progestogens and brain: An update. Maturitas 2009, 62, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vidal, M.D.; Cervera-Gaviria, M.; Ruelas, R.; Escobar, A.; Morali, G.; Cervantes, M. Progesterone: Protective effects on the cat hippocampal neuronal damage due to acute global cerebral ischemia. Arch. Med. Res. 1998, 29, 117–124. [Google Scholar]
- Roof, R.L.; Duvdevani, R.; Braswell, L.; Stein, D.G. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp. Neurol. 1994, 129, 64–69. [Google Scholar] [CrossRef]
- Guo, Q.; Sayeed, I.; Baronne, L.M.; Hoffman, S.W.; Guennoun, R.; Stein, D.G. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp. Neurol. 2006, 198, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, K.; Yadav, U.C.; Siddiqui, M.R.; Mantha, A.K.; Basir, S.F.; Sharma, D.; Cowsik, S.M.; Baquer, N.Z. Effect of hormone replacement therapy in normalizing age related neuronal markers in different age groups of naturally menopausal rats. Biogerontology 2005, 6, 345–356. [Google Scholar] [CrossRef]
- Roof, R.L.; Hoffman, S.W.; Stein, D.G. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol. Chem. Neuropathol. 1997, 31, 1–11. [Google Scholar] [CrossRef]
- Singh, M.; Su, C. Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience 2013, 239, 84–91. [Google Scholar] [CrossRef]
- Su, C.; Cunningham, R.L.; Rybalchenko, N.; Singh, M. Progesterone increases the release of brain-derived neurotrophic factor from glia via progesterone receptor membrane component 1 (Pgrmc1)-dependent ERK5 signaling. Endocrinology 2012, 153, 4389–4400. [Google Scholar] [CrossRef]
- Bali, N.; Arimoto, J.M.; Morgan, T.E.; Finch, C.E. Progesterone antagonism of neurite outgrowth depends on microglial activation via Pgrmc1/S2R. Endocrinology 2013, 154, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.C.; Baudry, M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur. J. Neurosci. 2009, 29, 447–454. [Google Scholar] [CrossRef]
- Nobile, B.; Maimoun, L.; Jaussent, I.D.; Seneque, M.; Dupuis-Maurin, K.; Lefebvre, P.; Courtet, P.; Renard, E.; Guillaume, S. Effects of Hormonal Contraception Use on Cognitive Functions in Patients With Bulimia Nervosa. Front. Psychiatry 2021, 12, 658182. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.D.; Le, C.S.; Zhang, H.M.; Shang, D.S.; Tong, L.S.; Gao, F. Thrombin disrupts vascular endothelial-cadherin and leads to hydrocephalus via protease-activated receptors-1 pathway. CNS Neurosci. Ther. 2019, 25, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Ogawa, M.; Wada, H.; Nishikawa, S. Thrombin induces rapid disassembly of claudin-5 from the tight junction of endothelial cells. Exp. Cell Res. 2009, 315, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, F.; Mogami, H.; Moriuchi, K.; Chigusa, Y.; Mandai, M.; Kondoh, E. Mechanisms of thrombin-Induced myometrial contractions: Potential targets of progesterone. PLoS ONE 2020, 15, e0231944. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Guo, S.; Lok, J.; Navaratna, D.; Whalen, M.J.; Kim, K.W.; Lo, E.H. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr. Pharm. Des. 2012, 18, 3645–3648. [Google Scholar] [CrossRef] [PubMed]
- Lischper, M.; Beuck, S.; Thanabalasundaram, G.; Pieper, C.; Galla, H.J. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010, 1326, 114–127. [Google Scholar] [CrossRef]
- Feng, Z.H.; Hao, J.; Ye, L.; Dayao, C.; Yan, N.; Yan, Y.; Chu, L.; Shi, F.D. Overexpression of mu-calpain in the anterior temporal neocortex of patients with intractable epilepsy correlates with clinicopathological characteristics. Seizure 2011, 20, 395–401. [Google Scholar] [CrossRef]
- Ishrat, T.; Sayeed, I.; Atif, F.; Hua, F.; Stein, D.G. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp. Neurol. 2010, 226, 183–190. [Google Scholar] [CrossRef]
- Shynlova, O.; Tsui, P.; Jaffer, S.; Lye, S.J. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144 (Suppl. 1), S2–S10. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef]
- Mani, S. Progestin receptor subtypes in the brain: The known and the unknown. Endocrinology 2008, 149, 2750–2756. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Wahlstrom, G.; Backstrom, T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J. Steroid Biochem. Mol. Biol. 1997, 62, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Coirini, H.; McEwen, B.S. Regulation of high-affinity GABAA receptors in the dorsal hippocampus by estradiol and progesterone. Brain Res. 1989, 487, 178–183. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Mueck, A.O.; Seeger, H. Estrogens acting as cardiovascular agents: Direct vascular actions. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2004, 2, 35–42. [Google Scholar] [CrossRef]
- Cid, M.C.; Schnaper, H.W.; Kleinman, H.K. Estrogens and the vascular endothelium. Ann. N. Y. Acad. Sci. 2002, 966, 143–157. [Google Scholar] [CrossRef]
- White, W.B. Drospirenone with 17beta-estradiol in the postmenopausal woman with hypertension. Climacteric 2007, 10 (Suppl. 1), 25–31. [Google Scholar] [CrossRef]
- Imthurn, B.; Rosselli, M.; Jaeger, A.W.; Keller, P.J.; Dubey, R.K. Differential effects of hormone-replacement therapy on endogenous nitric oxide (nitrite/nitrate) levels in postmenopausal women substituted with 17 beta-estradiol valerate and cyproterone acetate or medroxyprogesterone acetate. J. Clin. Endocrinol. Metab. 1997, 82, 388–394. [Google Scholar] [CrossRef]
- Leonhardt, S.A.; Boonyaratanakornkit, V.; Edwards, D.P. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids 2003, 68, 761–770. [Google Scholar] [PubMed]
- Fu, X.D.; Flamini, M.; Sanchez, A.M.; Goglia, L.; Giretti, M.S.; Genazzani, A.R.; Simoncini, T. Progestogens regulate endothelial actin cytoskeleton and cell movement via the actin-binding protein moesin. Mol. Hum. Reprod. 2008, 14, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratanakornkit, V.; McGowan, E.; Sherman, L.; Mancini, M.A.; Cheskis, B.J.; Edwards, D.P. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol. Endocrinol. 2007, 21, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratanakornkit, V.; Edwards, D.P. Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones. Essays Biochem. 2004, 40, 105–120. [Google Scholar]
- Boonyaratanakornkit, V.; Bi, Y.; Rudd, M.; Edwards, D.P. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids 2008, 73, 922–928. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Qu, Y.; Gonzalez, E.; Smith, M.; Zhang, J. Emerging roles of CCM genes during tumorigenesis with potential application as novel biomarkers across major types of cancers. Oncol. Rep. 2020, 43, 1945–1963. [Google Scholar] [CrossRef]
- Zhang, J. Learn from the past, review the present, and look towards the future. Vessel Plus 2022, 6, 20. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).