Impact of Hypothermic Oxygenated Machine Perfusion on Hepatocellular Carcinoma Recurrence after Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Popolation and Design
2.2. Patient Management
2.3. Statistical Analysis
3. Results
3.1. HCC Recurrence and Postoperative Outcomes
3.2. Survival Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Boztetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Carcinomas in Patients With Cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef] [PubMed]
- Notarpaolo, A.; Layese, R.; Magistri, P.; Gambato, M.; Colledan, M.; Magini, G.; Miglioresi, L.; Vitale, A.; Vennarecci, G.; Ambrosio, C.D.; et al. Validation of the AFP Model as a Predictor of HCC Recurrence in Patients with Viral Hepatitis-Related Cirrhosis Who Had Received a Liver Transplant for HCC. J. Hepatol. 2017, 66, 552–559. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Halazun, K.J.; Tabrizian, P.; Najjar, M.; Florman, S.; Schwartz, M.; Michelassi, F.; Samstein, B.; Brown, R.S.; Emond, J.C.; Busuttil, R.W.; et al. Is It Time to Abandon the Milan Criteria? Results of a Bicoastal US Collaboration to Redefine Hepatocellular Carcinoma Liver Transplantation Selection Policies. Ann. Surg. 2018, 268, 690–699. [Google Scholar] [CrossRef]
- Halazun, K.J.; Rosenblatt, R.E.; Mehta, N.; Lai, Q.; Hajifathalian, K.; Gorgen, A.; Brar, G.; Sasaki, K.; Doyle, M.B.M.; Tabrizian, P.; et al. Dynamic α-Fetoprotein Response and Outcomes after Liver Transplant for Hepatocellular Carcinoma. JAMA Surg. 2021, 156, 559–567. [Google Scholar] [CrossRef]
- Tran, B.V.; Moris, D.; Markovic, D.; Zaribafzadeh, H.; Henao, R.; Lai, Q.; Florman, S.S.; Tabrizian, P.; Haydel, B.; Ruiz, R.M.; et al. Development and Validation of a REcurrent Liver CAncer Prediction ScorE (RELAPSE) Following Liver Transplantation in Patients with Hepatocellular Carcinoma: Analysis of the Us Multicenter Hcc Transplant Consortium. Liver Transplant. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Attia, M.; Silva, M.A.; Mirza, D.F. The Marginal Liver Donor—An Update. Transpl. Int. 2008, 21, 713–724. [Google Scholar] [CrossRef]
- Widmer, J.; Eden, J.; Carvalho, M.F.; Dutkowski, P.; Schlegel, A. Machine Perfusion for Extended Criteria Donor Livers: What Challenges Remain? J. Clin. Med. 2022, 11, 5218. [Google Scholar] [CrossRef]
- Doi, K.; Horiuchi, T.; Uchinami, M.; Tabo, T.; Kimura, N.; Yokomachi, J.; Yoshida, M.; Tanaka, K. Hepatic Ischemia-Reperfusion Promotes Liver Metastasis of Colon Cancer. J. Surg. Res. 2002, 105, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Horiuchi, T.; Uchinami, M.; Tabo, T.; Kimura, N.; Yokomachi, J.; Doi, K.; Nakamura, T.; Tamagawa, K.; Tanaka, K. Intermittent Hepatic Ischemia-Reperfusion Minimizes Liver Metastasis in Rats. J. Surg. Res. 2003, 111, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Shao, Y.; Ng, K.T.P.; Liu, X.B.; Ling, C.C.; Ma, Y.Y.; Geng, W.; Fan, S.T.; Lo, C.M.; Man, K. FTY720 Suppresses Liver Tumor Metastasis by Reducing the Population of Circulating Endothelial Progenitor Cells. PLoS ONE 2012, 7, e32380. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.C.; Ng, K.T.P.; Shao, Y.; Geng, W.; Xiao, J.W.; Liu, H.; Li, C.X.; Liu, X.B.; Ma, Y.Y.; Yeung, W.H.; et al. Post-Transplant Endothelial Progenitor Cell Mobilization via CXCL10/CXCR3 Signaling Promotes Liver Tumor Growth. J. Hepatol. 2014, 60, 103–109. [Google Scholar] [CrossRef]
- Oldani, G.; Crowe, L.A.; Orci, L.A.; Slits, F.; Rubbia-Brandt, L.; De Vito, C.; Morel, P.; Mentha, G.; Berney, T.; Vallée, J.P.; et al. Pre-Retrieval Reperfusion Decreases Cancer Recurrence after Rat Ischemic Liver Graft Transplantation. J. Hepatol. 2014, 61, 278–285. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Mori, A.; Fujimoto, Y.; Ito, T.; Iida, T.; Yagi, S.; Okajima, H.; Kaido, T.; Uemoto, S. Longer Warm Ischemia Can Accelerate Tumor Growth through the Induction of HIF-1α and the IL-6–JAK–STAT3 Signaling Pathway in a Rat Hepatocellular Carcinoma Model. J. Hepato-Biliary-Pancreat. Sci. 2016, 23, 771–779. [Google Scholar] [CrossRef]
- Orci, L.A.; Lacotte, S.; Oldani, G.; Slits, F.; De Vito, C.; Crowe, L.A.; Rubbia-Brandt, L.; Vallée, J.P.; Morel, P.; Toso, C. Effect of Ischaemic Preconditioning on Recurrence of Hepatocellular Carcinoma in an Experimental Model of Liver Steatosis. Br. J. Surg. 2016, 103, 417–426. [Google Scholar] [CrossRef]
- Wang, S.; Yang, F.J.; Wang, X.; Zhou, Y.; Dai, B.; Han, B.; Ma, H.C.; Ding, Y.T.; Shi, X.L. PARP-1 Promotes Tumor Recurrence after Warm Ischemic Liver Graft Transplantation via Neutrophil Recruitment and Polarization. Oncotarget 2017, 8, 88918–88933. [Google Scholar] [CrossRef]
- Orci, L.A.; Lacotte, S.; Delaune, V.; Slits, F.; Oldani, G.; Lazarevic, V.; Rossetti, C.; Rubbia-Brandt, L.; Morel, P.; Toso, C. Effects of the Gut–Liver Axis on Ischaemia-Mediated Hepatocellular Carcinoma Recurrence in the Mouse Liver. J. Hepatol. 2018, 68, 978–985. [Google Scholar] [CrossRef]
- Oldani, G.; Peloso, A.; Slits, F.; Gex, Q.; Delaune, V.; Orci, L.A.; van de Looij, Y.; Colin, D.J.; Germain, S.; de Vito, C.; et al. The Impact of Short-Term Machine Perfusion on the Risk of Cancer Recurrence after Rat Liver Transplantation with Donors after Circulatory Death. PLoS ONE 2019, 14, e0224890. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Ren, H.; Wang, J.; Shang, L.; Liu, Y.; Zhu, W.; Shi, X. Ischemia Reperfusion Injury Promotes Recurrence of Hepatocellular Carcinoma in Fatty Liver via ALOX12-12HETE-GPR31 Signaling Axis. J. Exp. Clin. Cancer Res. 2019, 38, 489. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Yang, X.X.; Wang, H.W.; Li, X.C.; Ng, K.T.P.; Lo, C.M.; Man, K. FTY720 Suppresses Liver Tumor Growth and Metastasis by Reducing Circulating Regulating T Cells and Enhancing the Anti-Tumor Effect of Rapamycin. Onco. Targets. Ther. 2020, 13, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bilt, J.D.W.; Kranenburg, O.; Nijkamp, M.W.; Smakman, N.; Veenendaal, L.M.; Te Velde, E.A.; Voest, E.E.; Van Diest, P.J.; Borel Rinkes, I.H.M. Ischemia/Reperfusion Accelerates the Outgrowth of Hepatic Micrometastases in a Highly Standardized Murine Model. Hepatology 2005, 42, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bilt, J.D.W.; Soeters, M.E.; Duyverman, A.M.M.J.; Nijkamp, M.W.; Witteveen, P.O.; Van Diest, P.J.; Kranenburg, O.; Rinkes, I.H.M.B. Perinecrotic Hypoxia Contributes to Ischemia/Reperfusion-Accelerated Outgrowth of Colorectal Micrometastases. Am. J. Pathol. 2007, 170, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Tashiro, H.; Miyata, Y.; Ushitora, Y.; Fudaba, Y.; Kobayashi, T.; Arihiro, K.; Okajima, M.; Asahara, T. Rho-Associated Kinase Inhibitor Reduces Tumor Recurrence after Liver Transplantation in a Rat Hepatoma Model. Am. J. Transplant. 2007, 7, 347–355. [Google Scholar] [CrossRef]
- Man, K.; Ng, K.T.; Lo, C.M.; Ho, J.W.; Sun, B.S.; Sun, C.K.; Lee, T.K.; Poon, R.T.P.; Fan, S.T. Ischemia-Reperfusion of Small Liver Remnant Promotes Liver Tumor Growth and Metastases—Activation of Cell Invasion and Migration Pathways. Liver Transplant. 2007, 13, 1669–1677. [Google Scholar] [CrossRef]
- Nicoud, I.B.; Jones, C.M.; Pierce, J.M.; Earl, T.M.; Matrisian, L.M.; Chari, R.S.; Gorden, D.L. Warm Hepatic Ischemia-Reperfusion Promotes Growth of Colorectal Carcinoma Micrometastases in Mouse Liver via Matrix Metalloproteinase-9 Induction. Cancer Res. 2007, 67, 2720–2728. [Google Scholar] [CrossRef]
- Man, K.; Lo, C.M.; Xiao, J.W.; Ng, K.T.; Sun, B.S.; Ng, I.O.; Cheng, Q.; Sun, C.K.; Fan, S.T. The Significance of Acute Phase Small-for-Size Graft Injury on Tumor Growth and Invasiveness after Liver Transplantation. Ann. Surg. 2008, 247, 1049–1057. [Google Scholar] [CrossRef]
- Ushitora, Y.; Tashiro, H.; Ogawa, T.; Tanimoto, Y.; Kuroda, S.; Kobayashi, T.; Miyata, Y.; Itamoto, T.; Asahara, T.; Ohdan, H. Suppression of Hepatocellular Carcinoma Recurrence after Rat Liver Transplantation by FTY720, a Sphingosine-1-Phosphate Analog. Transplantation 2009, 88, 980–986. [Google Scholar] [CrossRef]
- Man, K.; Co Shih, K.; Ng, K.T.P.; Xiao, J.W.; Guo, D.Y.; Sun, C.K.W.; Lim, Z.X.H.; Cheng, Q.; Liu, Y.; Fan, S.T.; et al. Molecular Signature Linked to Acute Phase Injury and Tumor Invasiveness in Small-for-Size Liver Grafts. Ann. Surg. 2010, 251, 1154–1161. [Google Scholar] [CrossRef]
- Ghinolfi, D.; Rreka, E.; De Tata, V.; Franzini, M.; Pezzati, D.; Fierabracci, V.; Masini, M.; Cacciatoinsilla, A.; Bindi, M.L.; Marselli, L.; et al. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transplant. 2019, 25, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Polak, W.G.; Muiesan, P.; Schlegel, A.; Verhoeven, C.J.; Scalera, I.; Deoliveira, M.L.; Kron, P.; Clavien, P.A. First Comparison of Hypothermic Oxygenated Perfusion versus Static Cold Storage of Human Donation after Cardiac Death Liver Transplants. Ann. Surg. 2015, 262, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Reznik, E.; Musat, C.; Lukose, T.I.; Ratner, L.E.; Brown, R.S.; Kato, T.; Emond, J.C. Hypothermic Machine Preservation Facilitates Successful Transplantation of “Orphan” Extended Criteria Donor Livers. Am. J. Transplant. 2015, 15, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-Transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE). Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]
- Ravaioli, M.; Germinario, G.; Dajti, G.; Sessa, M.; Vasuri, F.; Siniscalchi, A.; Morelli, M.C.; Serenari, M.; Del Gaudio, M.; Zanfi, C.; et al. Hypothermic Oxygenated Perfusion in Extended Criteria Donor Liver Transplantation—A Randomized Clinical Trial. Am. J. Transplant. 2022, 22, 2401–2408. [Google Scholar] [CrossRef]
- Schlegel, A.; Mueller, M.; Muller, X.; Eden, J.; Panconesi, R.; von Felten, S.; Steigmiller, K.; Sousa Da Silva, R.X.; de Rougemont, O.; Mabrut, J.Y.; et al. A Multicenter Randomized-Controlled Trial of Hypothermic Oxygenated Perfusion (HOPE) for Human Liver Grafts before Transplantation. J. Hepatol. 2023, 78, 783–793. [Google Scholar] [CrossRef]
- Patrono, D.; Lonati, C.; Romagnoli, R. Viability Testing during Liver Preservation. Curr. Opin. Organ Transplant. 2022, 27, 454–465. [Google Scholar] [CrossRef]
- Patrono, D.; De Carlis, R.; Gambella, A.; Farnesi, F.; Podestà, A.; Lauterio, A.; Tandoi, F.; De Carlis, L.; Romagnoli, R. Viability Assessment and Transplantation of Fatty Liver Grafts Using End-Ischemic Normothermic Machine Perfusion. Liver Transplant. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Fodor, M.; Cardini, B.; Peter, W.; Weissenbacher, A.; Oberhuber, R.; Hautz, T.; Otarashvili, G.; Margreiter, C.; Maglione, M.; Resch, T.; et al. Static Cold Storage Compared with Normothermic Machine Perfusion of the Liver and Effect on Ischaemic-Type Biliary Lesions after Transplantation: A Propensity Score-Matched Study. Br. J. Surg. 2021, 108, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Dondossola, D.; Ravaioli, M.; Lonati, C.; Maroni, L.; Pini, A.; Accardo, C.; Germinario, G.; Antonelli, B.; Odaldi, F.; Zanella, A.; et al. The Role of Ex Situ Hypothermic Oxygenated Machine Perfusion and Cold Preservation Time in Extended Criteria Donation After Circulatory Death and Donation After Brain Death. Liver Transplant. 2021, 27, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 6063. [Google Scholar] [CrossRef] [PubMed]
- Rayar, M.; Maillot, B.; Bergeat, D.; Camus, C.; Houssel-Debry, P.; Sulpice, L.; Meunier, B.; Boudjema, K. A Preliminary Clinical Experience Using Hypothermic Oxygenated Machine Perfusion for Rapid Recovery of Octogenarian Liver Grafts. Prog. Transplant. 2019, 29, 97–98. [Google Scholar] [CrossRef]
- Patrono, D.; Roggio, D.; Mazzeo, A.T.; Catalano, G.; Mazza, E.; Rizza, G.; Gambella, A.; Rigo, F.; Leone, N.; Elia, V.; et al. Clinical Assessment of Liver Metabolism during Hypothermic Oxygenated Machine Perfusion Using Microdialysis. Artif. Organs 2022, 46, 281–295. [Google Scholar] [CrossRef]
- Patrono, D.; Catalano, G.; Rizza, G.; Lavorato, N.; Berchialla, P.; Gambella, A.; Caropreso, P.; Mengozzi, G.; Romagnoli, R. Perfusate Analysis during Dual Hypothermic Oxygenated Machine Perfusion of Liver Grafts: Correlations with Donor Factors and Early Outcomes. Transplantation 2020, 104, 1929–1942. [Google Scholar] [CrossRef]
- Patrono, D.; Zanierato, M.; Vergano, M.; Magaton, C.; Diale, E.; Rizza, G.; Catalano, S.; Mirabella, S.; Cocchis, D.; Potenza, R.; et al. Normothermic Regional Perfusion and Hypothermic Oxygenated Machine Perfusion for Livers Donated After Controlled Circulatory Death With Prolonged Warm Ischemia Time: A Matched Comparison With Livers From Brain-Dead Donors. Transpl. Int. 2022, 35, 10390. [Google Scholar] [CrossRef]
- Patrono, D.; Cussa, D.; Sciannameo, V.; Montanari, E.; Panconesi, R.; Berchialla, P.; Lepore, M.; Gambella, A.; Rizza, G.; Catalano, G.; et al. Outcome of Liver Transplantation with Grafts from Brain-Dead Donors Treated with Dual Hypothermic Oxygenated Machine Perfusion, with Particular Reference to Elderly Donors. Am. J. Transplant. 2022, 22, 1382–1395. [Google Scholar] [CrossRef]
- Patrono, D.; Surra, A.; Catalano, G.; Rizza, G.; Berchialla, P.; Martini, S.; Tandoi, F.; Lupo, F.; Mirabella, S.; Stratta, C.; et al. Hypothermic Oxygenated Machine Perfusion of Liver Grafts from Brain-Dead Donors. Sci. Rep. 2019, 9, 9337. [Google Scholar] [CrossRef]
- Patrono, D.; Lavezzo, B.; Molinaro, L.; Rizza, G.; Catalano, G.; Gonella, F.; Salizzoni, M.; Romagnoli, R. Hypothermic Oxygenated Machine Perfusion for Liver Transplantation: An Initial Experience. Exp. Clin. Transplant. 2017, 16, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.T.P.R.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD Liver Transplantation Using Organs Treated by Hypothermic Oxygenated Perfusion before Implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rayar, M.; Beaurepaire, J.M.; Bajeux, E.; Hamonic, S.; Renard, T.; Locher, C.; Desfourneaux, V.; Merdrignac, A.; Bergeat, D.; Lakehal, M.; et al. Hypothermic Oxygenated Perfusion Improves Extended Criteria Donor Liver Graft Function and Reduces Duration of Hospitalization Without Extra Cost: The PERPHO Study. Liver Transplant. 2021, 27, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Otarashvili, G.; Meszaros, A.; Ebner, S.; Weissenbacher, A.; Cardini, B.; Oberhuber, R.; Resch, T.; Öfner, D.; Schneeberger, S.; et al. Restoring Mitochondrial Function While Avoiding Redox Stress: The Key to Preventing Ischemia/Reperfusion Injury in Machine Perfused Liver Grafts? Int. J. Mol. Sci. 2020, 21, 3132. [Google Scholar] [CrossRef]
- Mueller, M.; Kalisvaart, M.; O’Rourke, J.; Shetty, S.; Parente, A.; Muller, X.; Isaac, J.; Muellhaupt, B.; Muiesan, P.; Shah, T.; et al. Hypothermic Oxygenated Liver Perfusion (HOPE) Prevents Tumor Recurrence in Liver Transplantation From Donation After Circulatory Death. Ann. Surg. 2020, 272, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a Current Definition of Early Allograft Dysfunction in Liver Transplant Recipients and Analysis of Risk Factors. Liver Transplant. 2010, 16, 943–949. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron—Clin. Pract. 2012, 120, 179–184. [Google Scholar] [CrossRef]
- Agopian, V.G.; Markovic, D.; Klintmalm, G.B.; Saracino, G.; Chapman, W.C.; Vachharajani, N.; Florman, S.S.; Tabrizian, P.; Haydel, B.; Nasralla, D.; et al. Multicenter Validation of the Liver Graft Assessment Following Transplantation (L-GrAFT) Score for Assessment of Early Allograft Dysfunction. J. Hepatol. 2021, 74, 881–892. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The Comprehensive Complication Index: A Novel Continuous Scale to Measure Surgical Morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef]
- Rao, K.V.; Anderson, W.R.; Kasiske, B.L.; Dahl, D.C. Value of Liver Biopsy in the Evaluation and Management of Chronic Liver Disease in Renal Transplant Recipients. Am. J. Med. 1993, 94, 241–250. [Google Scholar] [CrossRef] [PubMed]
- de Vries, Y.; von Meijenfeldt, F.A.; Porte, R.J. Post-Transplant Cholangiopathy: Classification, Pathogenesis, and Preventive Strategies. Biochim. Biophys. Acta—Mol. Basis Dis. 2018, 1864, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Goodrich, N.P.; Bragg-Gresham, J.L.; Dykstra, D.M.; Punch, J.D.; DebRoy, M.A.; Greenstein, S.M.; Merion, R.M. Characteristics Associated with Liver Graft Failure: The Concept of a Donor Risk Index. Am. J. Transplant. 2006, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, R.; Schlegel, A.; Frassoni, S.; Olivieri, T.; Ravaioli, M.; Camagni, S.; Patrono, D.; Bassi, D.; Pagano, D.; Di Sandro, S.; et al. How to Preserve Liver Grafts from Circulatory Death with Long Warm Ischemia? A Retrospective Italian Cohort Study with Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation 2021, 105, 2385–2396. [Google Scholar] [CrossRef]
- Denz, R.; Klaaßen-Mielke, R.; Timmesfeld, N. A Comparison of Different Methods to Adjust Survival Curves for Confounders. Stat. Med. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Kaplan, D.; Chen, J. Bayesian Model Averaging for Propensity Score Analysis. Multivariate Behav. Res. 2014, 49, 505–517. [Google Scholar] [CrossRef]
- Silverstein, J.; Roll, G.; Dodge, J.L.; Grab, J.D.; Yao, F.Y.; Mehta, N. Donation After Circulatory Death Is Associated With Similar Posttransplant Survival in All but the Highest-Risk Hepatocellular Carcinoma Patients. Liver Transplant. 2020, 26, 1100–1111. [Google Scholar] [CrossRef]
- Doi, K.; Horiuchi, T.; Uchinami, M.; Tabo, T.; Kimura, N.; Yokomachi, J.; Yoshida, M.; Tanaka, K. Neutrophil Elastase Inhibitor Reduces Hepatic Metastases Induced by Ischaemia-Reperfusion in Rats. Eur. J. Surg. 2002, 168, 507–510. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, T.; Ju, W.; Li, F.; Zhang, Q. Ischemic-Free Liver Transplantation Reduces the Recurrence of Hepatocellular Carcinoma After Liver Transplantation. Front. Oncol. 2021, 11, 773535. [Google Scholar] [CrossRef]
- Kornberg, A.; Witt, U.; Kornberg, J.; Friess, H.; Thrum, K. Extended Ischemia Times Promote Risk of HCC Recurrence in Liver Transplant Patients. Dig. Dis. Sci. 2015, 60, 2832–2839. [Google Scholar] [CrossRef]
- Kornberg, A.; Witt, U.; Kornberg, J.; Friess, H.; Thrum, K. Treating Ischaemia-Reperfusion Injury with Prostaglandin E1 Reduces the Risk of Early Hepatocellular Carcinoma Recurrence Following Liver Transplantation. Aliment. Pharmacol. Ther. 2015, 42, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Yoshida, A.; Facciuto, M.; Moonka, D.; Abouljoud, M.S.; Schwartz, M.E.; Florman, S.S. Ischemia Time Impacts Recurrence of Hepatocellular Carcinoma after Liver Transplantation. Hepatology 2015, 61, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.A.; Berney, T.; Majno, P.E.; Lacotte, S.; Oldani, G.; Morel, P.; Mentha, G.; Toso, C. Donor Characteristics and Risk of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Br. J. Surg. 2015, 102, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, S.E.; Yip, V.S.; Cortes, M.; Jassem, W.; Quaglia, A.; O’Grady, J.; Heneghan, M.; Aluvihare, V.; Agarwal, K.; Menon, K.; et al. Does Donation after Cardiac Death Utilization Adversely Affect Hepatocellular Cancer Survival? Transplantation 2016, 100, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Grat, M.; Krawczyk, M.; Wronka, K.M.; Stypułkowski, J.; Lewandowski, Z.; Wasilewicz, M.; Krawczyk, P.; Grat, K.; Patkowski, W.; Zieniewicz, K. Ischemia-Reperfusion Injury and the Risk of Hepatocellular Carcinoma Recurrence after Deceased Donor Liver Transplantation. Sci. Rep. 2018, 8, 8935. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ling, C.C.; Yeung, W.H.O.; Pang, L.; Liu, J.; Zhou, J.; Zhang, W.Y.; Liu, X.B.; Ng, T.P.K.; Yang, X.X.; et al. Monocytic MDSC Mobilization Promotes Tumor Recurrence after Liver Transplantation via CXCL10/TLR4/MMP14 Signaling. Cell Death Dis. 2021, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Ghinolfi, D.; Lai, Q.; Dondossola, D.; De Carlis, R.; Zanierato, M.; Patrono, D.; Baroni, S.; Bassi, D.; Ferla, F.; Lauterio, A.; et al. Machine Perfusions in Liver Transplantation: The Evidence-Based Position Paper of the Italian Society of Organ and Tissue Transplantation. Liver Transplant. 2020, 26, 1298–1315. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; De La Mata, M.; Burroughs, A.K. Liver Transplantation: Immunosuppression and Oncology. Curr. Opin. Organ Transplant. 2014, 19, 253–260. [Google Scholar] [CrossRef]
- Yan, X.; Huang, S.; Yang, Y.; Lu, Z.; Li, F.; Jiang, L.; Jiang, Y.; Liu, J. Sirolimus or Everolimus Improves Survival After Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Transplant. 2022, 28, 1063–1077. [Google Scholar] [CrossRef]
- Abrahamsson, J.; Sternby Eilard, M.; Rizell, M.; Bennett, W.; Åberg, F. Reduced Calcineurin Inhibitor Exposure with Antibody Induction and Recurrent Hepatocellular Carcinoma after Liver Transplantation. Scand. J. Gastroenterol. 2022, 57, 325–332. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.H.; Yi, N.J.; Kim, H.S.; Lee, H.S.; Lee, B.K.; Kim, H.; Choi, Y.R.; Hong, G.; Lee, K.W.; et al. Impact of Immunosuppressant Therapy on Early Recurrence of Hepatocellular Carcinoma after Liver Transplantation. Clin. Mol. Hepatol. 2014, 20, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Viveiros, A.; Iesari, S.; Vitale, A.; Mennini, G.; Onali, S.; Hoppe-Lotichius, M.; Colasanti, M.; Manzia, T.M.; Mocchegiani, F.; et al. Prognostic Factors for 10-Year Survival in Patients With Hepatocellular Cancer Receiving Liver Transplantation. Front. Oncol. 2022, 27, 877107. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zou, J.; Sun, K.; Yang, J.; Lei, T.; Xu, L.; Liu, J.; Yin, S.; Li, G. Global Research Status and Frontiers on Microvascular Invasion of Hepatocellular Carcinoma: A Bibliometric and Visualized Analysis. Front. Oncol. 2022, 12, 1037145. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 326) | SCS (n = 246) | D-HOPE (n = 80) | p Value | ||
|---|---|---|---|---|---|
| Recipient and donor features | |||||
| Recipient age | 59.0 [55.3, 63.4] | 58.9 [55.1, 62.8] | 60.1 [55.7, 63.6] | 0.147 | |
| Recipient gender | F | 52 (16) | 41 (17) | 11 (14) | 0.658 |
| M | 274 (84) | 205 (83) | 69 (86) | ||
| Indication | Alcoholic cirrhosis | 55 (17) | 41 (17) | 14 (18) | 0.668 |
| Autoimmune hepatitis | 2 (1) | 2 (1) | 0 (0) | ||
| Cholestatic liver disease | 4 (1) | 2 (1) | 2 (2) | ||
| NASH | 12 (4) | 8 (3) | 4 (5) | ||
| Viral hepatitis | 222 (68) | 171 (70) | 51 (64) | ||
| Other | 31 (10) | 22 (9) | 9 (11) | ||
| Waiting time (days) | 35.0 [16.5, 90.5] | 35.0 [17.0, 86.0] | 32.0 [14.5, 94.5] | 0.713 | |
| Recipient BMI | 25.8 [23.5, 28.1] | 25.7 [23.4, 28.3] | 26.0 [23.9, 27.8] | 0.548 | |
| MELD | 10.0 [8.0, 14.0] | 10.0 [8.0, 14.0] | 10.0 [8.0, 14.0] | 0.872 | |
| Prev. abdominal surgery | 127 (39) | 90 (37) | 37 (46) | 0.159 | |
| Donor age | 68.7 [57.7, 77.4] | 67.9 [55.7, 76.5] | 71.8 [60.7, 82.4] | 0.003 | |
| Donor gender | F | 134 (41) | 105 (43) | 29 (36) | 0.376 |
| M | 192 (59) | 141 (57) | 51 (64) | ||
| Donor type | DBD | 312 (96) | 246 (100) | 66 (82) | <0.001 |
| DCD cat. II | 1 (0) | 0 (0) | 1 (1) | ||
| DCD cat. III | 13 (4) | 0 (0) | 13 (16) | ||
| Donor BMI | 25.7 [23.5, 28.4] | 25.4 [23.0, 27.7] | 27.2 [24.5, 29.4] | 0.001 | |
| Macrosteatosis % | 2.0 [0.0, 10.0] | 2.0 [0.0, 10.0] | 3.0 [0.0, 10.0] | 0.070 | |
| Macrosteatosis ≥ 15% | 57 (18) | 39 (16) | 18 (22) | 0.266 | |
| D-MELD | 674 [537, 916] | 659 [527, 897] | 715 [558, 968] | 0.078 | |
| BAR | 5.0 [3.0, 5.0] | 5.0 [3.0, 5.0] | 5.0 [3.0, 5.0] | 0.113 | |
| DRI | 1.8 [1.4, 2.3] | 1.7 [1.4, 2.3] | 2.1 [1.4, 2.4] | 0.137 | |
| Total preservation time (min) | 417 [364, 471] | 403 [354, 452] | 474 [411, 519] | <0.001 | |
| Rec. warm ischemia time (min) | 23 [20, 27] | 23 [21, 27] | 22 [20, 27] | 0.300 | |
| D-HOPE time (min) | 0 [0, 0] | 0 [0, 0] | 144 [117, 180] | <0.001 | |
| HCC characteristics | |||||
| N. nodes at LT | 0 | 3 (1) | 1 (0) | 2 (2) | 0.322 |
| 1 | 133 (41) | 99 (40) | 34 (42) | ||
| 2–3 | 92 (28) | 73 (30) | 19 (24) | ||
| 3–4 | 67 (21) | 48 (20) | 19 (24) | ||
| ≥5 | 31 (10) | 25 (10) | 6 (8) | ||
| Max diam. at LT (mm) | 20.0 [14.0, 30.0] | 20.0 [15.0, 30.0] | 20.0 [14.0, 30.0] | 0.901 | |
| Tot. diam. at LT (mm) | 32.0 [21.0, 50.0] | 34.0 [21.0, 52.0] | 30.0 [18.8, 48.0] | 0.298 | |
| AFP at LT (ng/mL) | 4.5 [2.9, 11.7] | 4.5 [2.9, 12.2] | 4.3 [3.0, 8.8] | 0.776 | |
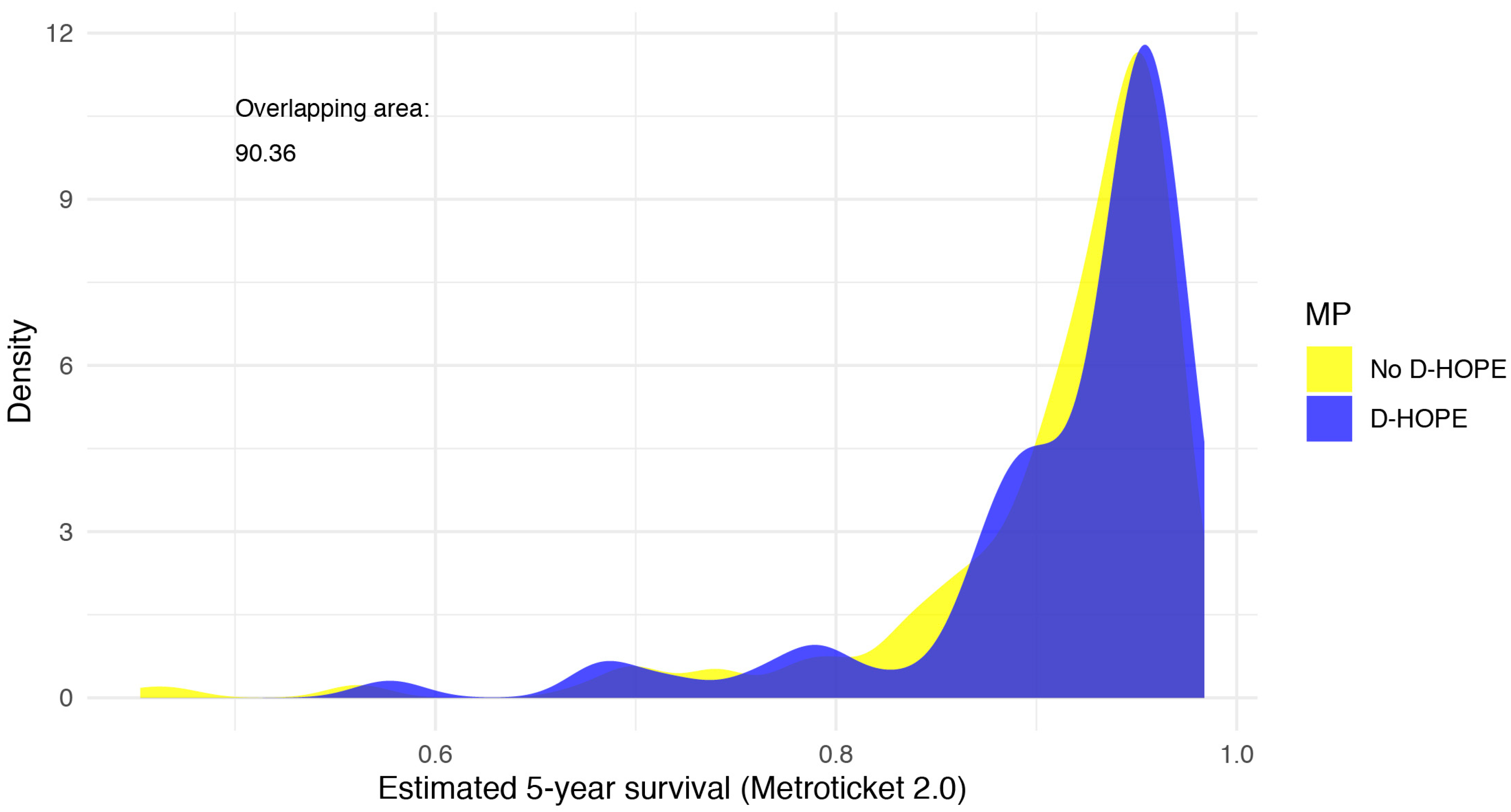
| Estimated 5-year survival * | 0.9 [0.9, 1.0] | 0.9 [0.9, 1.0] | 0.9 [0.9, 1.0] | 0.232 | |
| Estimated 5-year survival * | <80% | 28 (9) | 20 (9) | 8 (11) | |
| 80–85% | 12 (4) | 11 (5) | 1 (1) | ||
| 85–90% | 46 (15) | 31 (14) | 15 (20) | ||
| 90–95% | 109 (37) | 89 (40) | 20 (27) | ||
| >95% | 103 (35) | 72 (32) | 31 (41) | 0.118 | |
| Downstaging (Y/N) | 264 (81) | 201 (82) | 63 (79) | 0.673 | |
| Downstaging (n. procedures) | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 0.605 | |
| Locoregional | 247 (76) | 191 (78) | 56 (70) | 0.217 | |
| SBRT | 33 (10) | 19 (8) | 14 (18) | 0.021 | |
| Liver resection | 16 (5) | 13 (5) | 3 (4) | 0.799 | |
| AFP maximum level (ng/mL) | 7.0 [3.5, 26.4] | 7.0 [3.2, 28.2] | 6.7 [3.9, 22.9] | 0.894 | |
| N. nodes at pathology | 0 | 3 (1) | 2 (1) | 1 (1) | |
| 1 | 111 (34) | 88 (36) | 23 (29) | ||
| 2–3 | 78 (24) | 57 (23) | 21 (27) | ||
| 3–4 | 74 (23) | 51 (21) | 23 (29) | ||
| ≥5 | 56 (17) | 45 (19) | 11 (14) | 0.452 | |
| Max diam. pathology (mm) | 25.0 [18.0, 35.0] | 25.0 [18.0, 35.5] | 26.0 [17.5, 34.0] | 0.827 | |
| Tot. diam. pathology (mm) | 42.0 [28.0, 63.0] | 40.0 [29.2, 61.8] | 45.0 [25.5, 73.0] | 0.627 | |
| Grading | G1–G2 | 174 (72) | 136 (72) | 38 (69) | 0.764 |
| G3–G4 | 69 (28) | 52 (28) | 17 (31) | ||
| Microvascular invasion (%) | 55 (17) | 44 (18) | 11 (14) | 0.502 | |
| Immunosuppression and rejection | |||||
| Induction (basiliximab) | 93 (29) | 50 (20) | 43 (54) | <0.001 | |
| TAC start day | 0 | 49 (38) | 34 (40) | 15 (33) | |
| 1 | 46 (35) | 32 (38) | 14 (30) | ||
| 2 | 19 (15) | 9 (11) | 10 (22) | ||
| 3 | 7 (5) | 5 (6) | 2 (4) | ||
| 4 | 6 (5) | 3 (4) | 3 (7) | ||
| 5 | 2 (2) | 1 (1) | 1 (2) | ||
| 7 | 1 (1) | 0 (0) | 1 (2) | 0.405 | |
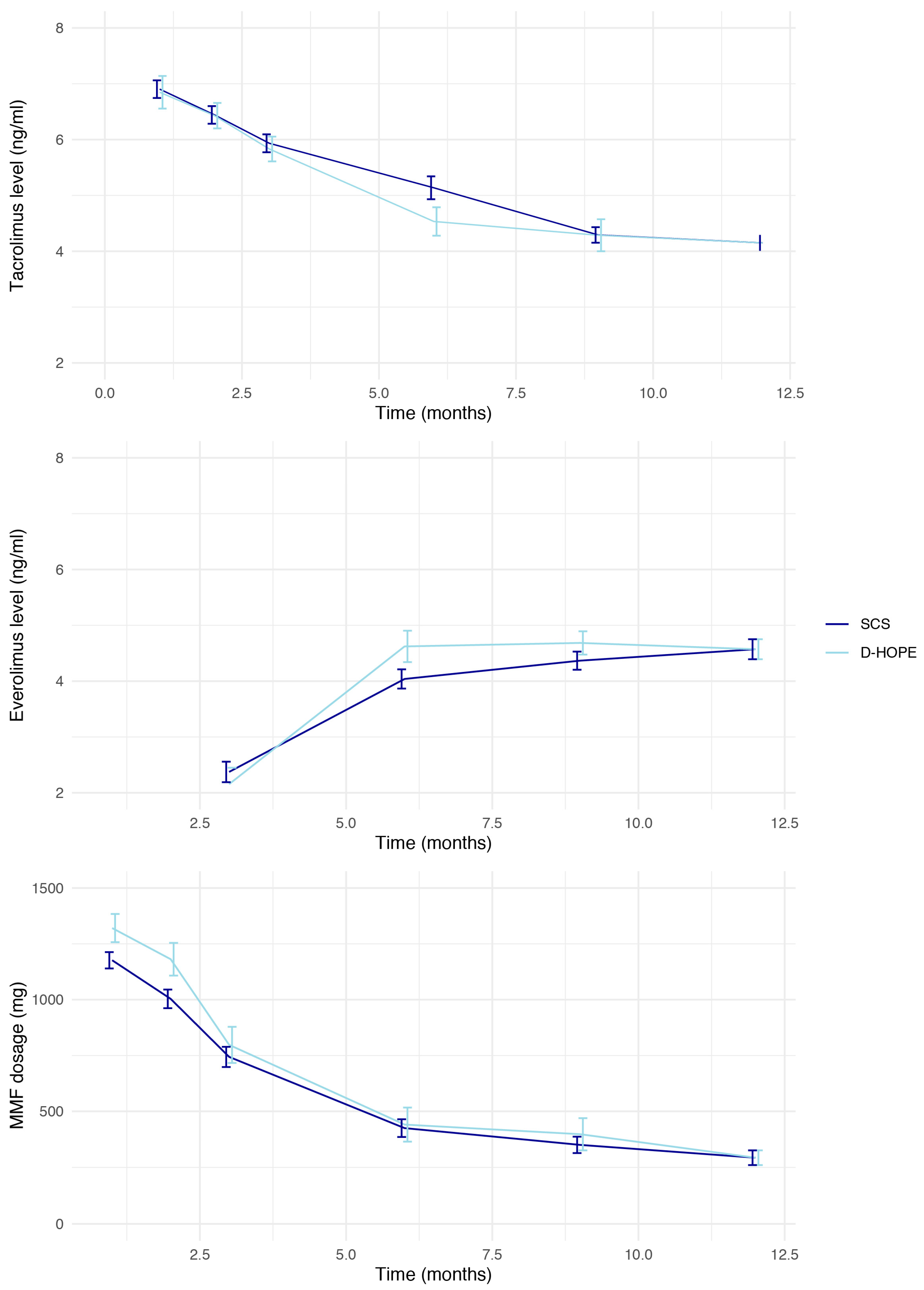
| 12-month TAC AUC (mg) | 55.1 [41.6, 69.5] | 55.6 [42.9, 69.6] | 49.3 [40.0, 68.8] | 0.307 | |
| MMF mean dose | 395 [166, 875] | 416 [166, 875] | 375 [250, 916] | 0.324 | |
| Switch to EVE (Y/N) | 218 (71) | 165 (71) | 53 (71) | 1.000 | |
| 12-month EVE AUC (mg) | 40.4 [23.9, 54.6] | 40.1 [22.2, 54.9] | 43.2 [32.1, 52.2] | 0.640 | |
| Early rejection (y/n) | 32 (10) | 24 (10) | 8 (10) | 1.000 | |
| Steroid pulses | 29 (9) | 23 (9) | 6 (8) | 0.780 | |
| Thymoglobulin | 2 (1) | 2 (1) | 0 (0) | 1.000 | |
| Late rejection | 18 (6) | 10 (4) | 8 (11) | 0.079 | |
| Overall (n = 326) | SCS (n = 246) | D-HOPE (n = 80) | p Value | ||
|---|---|---|---|---|---|
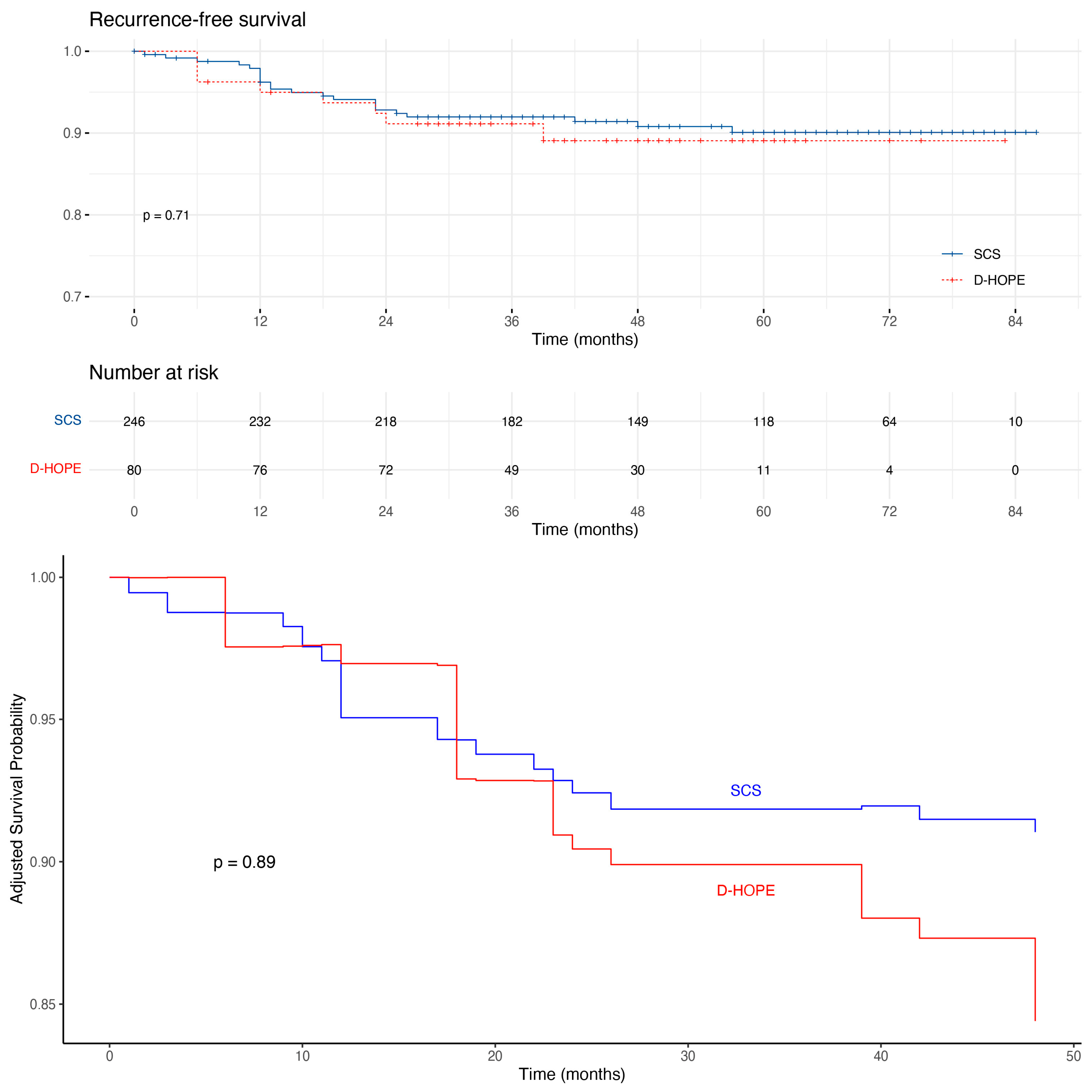
| HCC recurrence | 30 (9) | 22 (9) | 8 (10) | 0.951 | |
| AST peak | 1089.0 [671.0, 1782.0] | 1140.0 [719.0, 1831.0] | 903.0 [561.8, 1570.0] | 0.022 | |
| ALT peak | 699.0 [420.0, 1135.0] | 742.0 [454.0, 1163.0] | 496.5 [273.8, 984.2] | 0.002 | |
| EAD | 87 (27) | 66 (27) | 21 (26) | 1.000 | |
| AKI stage | no | 120 (37) | 93 (38) | 27 (34) | 0.081 |
| 1 | 126 (39) | 90 (37) | 36 (45) | ||
| 2 | 56 (17) | 48 (20) | 8 (10) | ||
| 3 | 24 (7) | 15 (6) | 9 (11) | ||
| Complications (Clavien–Dindo) | 0 | 32 (10) | 18 (7) | 14 (18) | 0.143 |
| 1 | 75 (23) | 61 (25) | 14 (18) | ||
| 2 | 167 (51) | 127 (52) | 40 (50) | ||
| 3a | 6 (2) | 5 (2) | 1 (1) | ||
| 3b | 23 (7) | 17 (7) | 6 (8) | ||
| 4a | 15 (5) | 12 (5) | 3 (4) | ||
| 4b | 4 (1) | 2 (1) | 2 (2) | ||
| 5 | 4 (1) | 4 (2) | 0 (0) | ||
| Clavien–Dindo ≥ 3 complications | 52 (16) | 40 (16) | 12 (15) | 0.927 | |
| CCI at discharge | 20.9 [8.7, 29.6] | 20.9 [8.7, 29.6] | 20.9 [8.7, 29.6] | 0.243 | |
| ICU stay (days) | 3.0 [2.0, 4.0] | 3.0 [2.0, 4.0] | 3.0 [2.0, 5.0] | 0.720 | |
| Hospital stay (days) | 10.0 [8.0, 15.8] | 10.0 [8.0, 14.8] | 11.0 [8.0, 18.0] | 0.967 | |
| Biliary complications (overall) | 63 (19) | 49 (20) | 14 (18) | 0.754 | |
| Anastomotic complications | 58 (18) | 44 (18) | 14 (18) | 1.000 | |
| Ischemic cholangiopathy | 7 (2) | 7 (3) | 0 (0) | 0.280 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95% CI for HR) | p Value | HR (95% CI for HR) | p Value | |
| N. nodes at LT | 1.1 (0.94–1.4) | 0.17 | ||
| Max diam. at LT | 1 (0.98–1) | 0.85 | ||
| AFP at TL | 1 (1–1) | 0.21 | ||
| Downstaging | 0.72 (0.31–1.7) | 0.45 | ||
| Grading G3–G4 | 5 (2.1–12) | <0.001 | 3.2 (1.3–7.9) | 0.10 |
| Microvascular invasion | 5.2 (2.6–11) | <0.001 | 5 (2–11.9) | <0.001 |
| Donor age (years) | 1 (0.98–1) | 0.66 | ||
| Donor BMI | 0.98 (0.91–1.1) | 0.7 | ||
| DCD donor | 3.9 × 10−8 (0-Inf) | 1 | ||
| D-HOPE | 1.2 (0.52–2.6) | 0.71 | 1.34 (0.5–3.4) | 0.54 |
| PRBC transfusion (units) | 0.98 (0.92–1) | 0.57 | ||
| Lactate end of LT (mmol/L) | 1.1 (0.88–1.5) | 0.32 | ||
| Severe PRS | 0.5 (0.12–2.1) | 0.35 | ||
| AST peak (IU/L) | 1 (1–1) | 0.32 | ||
| ALT peak (IU/L) | 1 (1–1) | 0.9 | ||
| L-GrAFT (risk %) | 1 (0.99–1) | 0.2 | ||
| CCI at discharge | 0.99 (0.97–1) | 0.66 | ||
| Tacrolimus AUC (mg) | 1 (0.97–1) | 0.74 | ||
| Everolimus AUC (mg) | 0.99 (0.97–1) | 0.41 | ||
| p Inclusion | HR | CI 95% | pd | |
|---|---|---|---|---|
| Microvascular invasion | 100.0 | 5.08 | 2.09; 12.362 | 1.00 |
| Grading G3–G4 | 97.2 | 3.25 | 1.323; 7.997 | 0.99 |
| DCD donor | 18.4 | 0.00 | 0; Inf | 0.50 |
| Macrovesicular steatosis (%) | 17.5 | 0.97 | 0.917; 1.031 | 0.83 |
| L-GrAFT score | 16.4 | 1.01 | 0.989; 1.035 | 0.84 |
| D-HOPE | 13.3 | 1.37 | 0.528; 3.568 | 0.74 |
| Donor age (years) | 12.9 | 1.01 | 0.982; 1.034 | 0.72 |
| Donor gender (male) | 10.6 | 0.82 | 0.345; 1.938 | 0.68 |
| Cold ischemia time (min) | 9.3 | 1.00 | 0.996; 1.006 | 0.62 |
| Recipient BMI | 9.1 | 1.01 | 0.891; 1.157 | 0.59 |
| Packed red blood cells transfusion (units) | 8.5 | 1.01 | 0.955; 1.067 | 0.63 |
| Donor BMI | 8.2 | 1.00 | 0.919; 1.097 | 0.54 |
| Author, Year | Animal | Model | Ischemia | Tumor | Findings |
|---|---|---|---|---|---|
| Doi et al., 2002 [11] | Rat | Partial IRI | 30 vs. 60 min | Colorectal liver metastases |
|
| Doi et al., 2002 [68] | Rat | Partial IRI | 60 min | Colorectal liver metastases |
|
| Yoshida et al., 2003 [12] | Rat | Partial IRI vs. intermittent clamping | 60 min | Colorectal liver metastases |
|
| van der Bilt et al., 2005 [23] | Mouse | Partial IRI | 45 min | Colorectal liver metastases |
|
| van der Bilt et al., 2007 [24] | Mouse | Partial IRI | 45 min | Colorectal liver metastases |
|
| Ogawa et al., 2007 [25] | Rat | LT | - | HCC |
|
| Man et al., 2007 [26] | Rat | Partial IRI +/− major hepatectomy | 60 min | HCC |
|
| Nicoud et al., 2007 [27] | Mouse | Partial IRI | 30 min | Colorectal liver metastases |
|
| Man et al., 2008 [28] | Rat | Standard vs. small-for-size graft LT | - | HCC |
|
| Ushitora et al., 2009 [29] | Rat | LT | - | HCC |
|
| Man et al., 2010 [30] | Rat | Standard vs. small-for-size graft LT | - | HCC |
|
| Li et al., 2012 [13] | Rat | Partial IRI +/− major hepatectomy | 30 min | HCC |
|
| Ling et al., 2014 [14] | Rat Mouse | Standard vs. small-for-size graft LT Partial IRI + major hepatectomy | 45 min | HCC |
|
| Oldani et al., 2014 [15] | Rat | DCD LT | 10 or 30 min | HCC |
|
| Hamaguchi et al., 2016 [16] | Rat | Major hepatectomy | 5 vs. 10 vs. 15 min | HCC |
|
| Orci et al., 2016 [17] | Mouse | Partial IRI | 30 min | HCC |
|
| Wang et al., 2017 [18] | Rat Mouse | DCD LT Partial IRI | 30 min 15 vs. 30 vs. 60 min | HCC |
|
| Orci et al., 2018 [19] | Mouse | Partial IRI | 60 min | HCC |
|
| Oldani et al., 2019 [20] | Rat | DCD LT | 60 min | HCC |
|
| Yang et al., 2019 [21] | Mouse | Partial IRI | 60 min | HCC |
|
| Li et al., 2020 [22] | Rat | Partial IRI +/− major hepatectomy | 30 min | HCC |
|
| Author, Year | Study | n | Donor | Intervention | Recurrence | Findings |
|---|---|---|---|---|---|---|
| Ling et al., 2014 [14] | Retrospective, single center | 115 | DBD | Standard (n = 37) vs. small-for-size graft (n = 78) LT | 8% vs. 24.4% | Patients with small-for-size liver graft had higher HCC recurrence, accompanied with increased circulating EPCs and CXCL10 levels |
| Kornberg et al., 2015 [70] | Retrospective, single center | 103 | DBD | - | 23.3% | WIT > 50 min identified as an independent predictor of HCC recurrence |
| Kornberg et al., 2015 [71] | Retrospective, single center | 106 | DBD | Post-operative PGE1 therapy | 23.6% | - PGE1 therapy identified as an independent prognostic factor for early HCC recurrence (within 12 months) - PGE1 therapy identified as an independent prognostic factor for recurrence-free survival in Milan-out patients |
| Nagai et al., 2015 [72] | Retrospective, multicenter | 391 | DBD | - | 15.3% | CIT > 10 h and WIT > 50 min identified as independent predictors of HCC recurrence |
| Orci et al., 2015 [73] | Retrospective, UNOS registry | 9724 | DBD DCD | - | - | Donor age > 60 y, BMI > 35, diabetes, steatosis > 60% and WIT > 19 min associated with an increased HCC recurrence risk |
| Khorsandi et al., 2016 [74] | Retrospective, single center | 347 | DBD DCD | - | 12.1% | DCDs had same HCC recurrence rates than DBDs |
| Grat et al., 2018 [75] | Retrospective, single center | 195 | DBD | - | 13.8% | Post-reperfusion AST < 1896 U/L and LDH < 4670 U/L increased HCC recurrence-free survival after LT in Milan-in patients |
| Silverstein et al., 2020 [67] | Retrospective, UNOS registry | 7563 | DBD DCD | - | 6.4% vs. 7.6% | DCD donor was an independent predictor of post-LT mortality. After stratifying for risk of HCC recurrence, only subgroups at higher risk for HCC recurrence had lower survival rates. |
| Mueller et al., 2020 [55] | Retrospective, multicenter | 280 | DBD DCD | HOPE- treated DCDs vs. SCS- DCDs/DBDs | 5.7% (DCD-HOPE, center A); 25.7% (DBD, center A); 14.3% (DCD, center B); 17.1% (DBD, center B) | DCD grafts exposed to 2 h of end-ischemic HOPE had lower HCC recurrence compared to cold-stored DBD grafts from the same center |
| Liu et al., 2021 [76] | Retrospective, single center | 329 | DBD | Standard (n = 149) vs. small-for-size graft (n = 180) LT | 10% vs. 19.4% | Patients with small-for-size liver graft had higher HCC recurrence, accompanied with increased circulating MDSCs and CXCL10 levels |
| Tang et al., 2021 [69] | Retrospective, single center | 226 | DBD | Ischemia-free LT (n = 30) vs. SCS-LT (n = 196) | - | Ischemia-free LT was associated with higher HCC recurrence-free survival rates than conventional LT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigo, F.; De Stefano, N.; Patrono, D.; De Donato, V.; Campi, L.; Turturica, D.; Doria, T.; Sciannameo, V.; Berchialla, P.; Tandoi, F.; et al. Impact of Hypothermic Oxygenated Machine Perfusion on Hepatocellular Carcinoma Recurrence after Liver Transplantation. J. Pers. Med. 2023, 13, 703. https://doi.org/10.3390/jpm13050703
Rigo F, De Stefano N, Patrono D, De Donato V, Campi L, Turturica D, Doria T, Sciannameo V, Berchialla P, Tandoi F, et al. Impact of Hypothermic Oxygenated Machine Perfusion on Hepatocellular Carcinoma Recurrence after Liver Transplantation. Journal of Personalized Medicine. 2023; 13(5):703. https://doi.org/10.3390/jpm13050703
Chicago/Turabian StyleRigo, Federica, Nicola De Stefano, Damiano Patrono, Victor De Donato, Ludovico Campi, Diana Turturica, Teresa Doria, Veronica Sciannameo, Paola Berchialla, Francesco Tandoi, and et al. 2023. "Impact of Hypothermic Oxygenated Machine Perfusion on Hepatocellular Carcinoma Recurrence after Liver Transplantation" Journal of Personalized Medicine 13, no. 5: 703. https://doi.org/10.3390/jpm13050703
APA StyleRigo, F., De Stefano, N., Patrono, D., De Donato, V., Campi, L., Turturica, D., Doria, T., Sciannameo, V., Berchialla, P., Tandoi, F., & Romagnoli, R. (2023). Impact of Hypothermic Oxygenated Machine Perfusion on Hepatocellular Carcinoma Recurrence after Liver Transplantation. Journal of Personalized Medicine, 13(5), 703. https://doi.org/10.3390/jpm13050703









