Chlorogenic Acid Attenuates Doxorubicin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Dityrosine Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
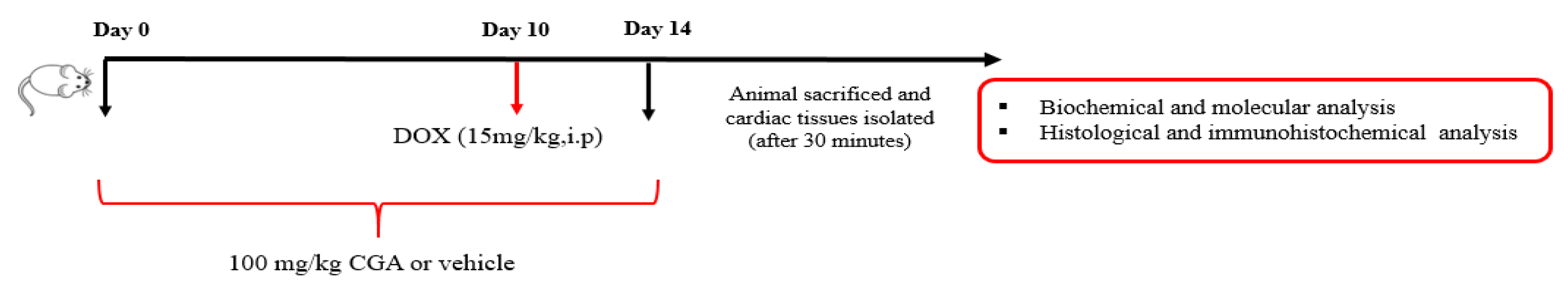
2.3. Experimental Design
2.4. Serum Collection and Tissue Preparation
2.5. Estimation of Serum Biochemical Parameters
2.6. Histopathological Examinations
2.7. Lipid Peroxidation (LPO) Assay
2.8. Estimation of Endogenous Antioxidants
2.9. Molecular Analysis
2.10. Immunofluorescent Staining
2.11. Statistical Analyses
3. Results
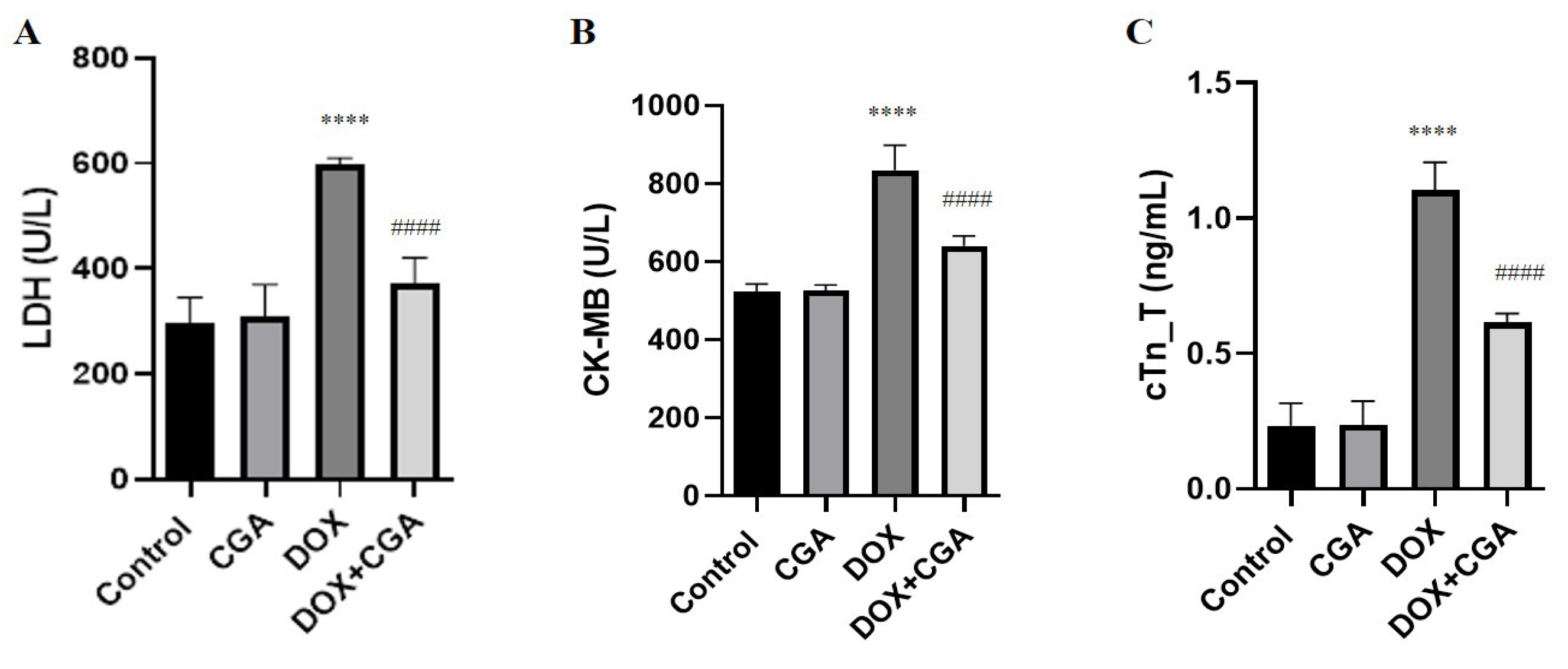
3.1. Biochemical Myocardial Injury Markers
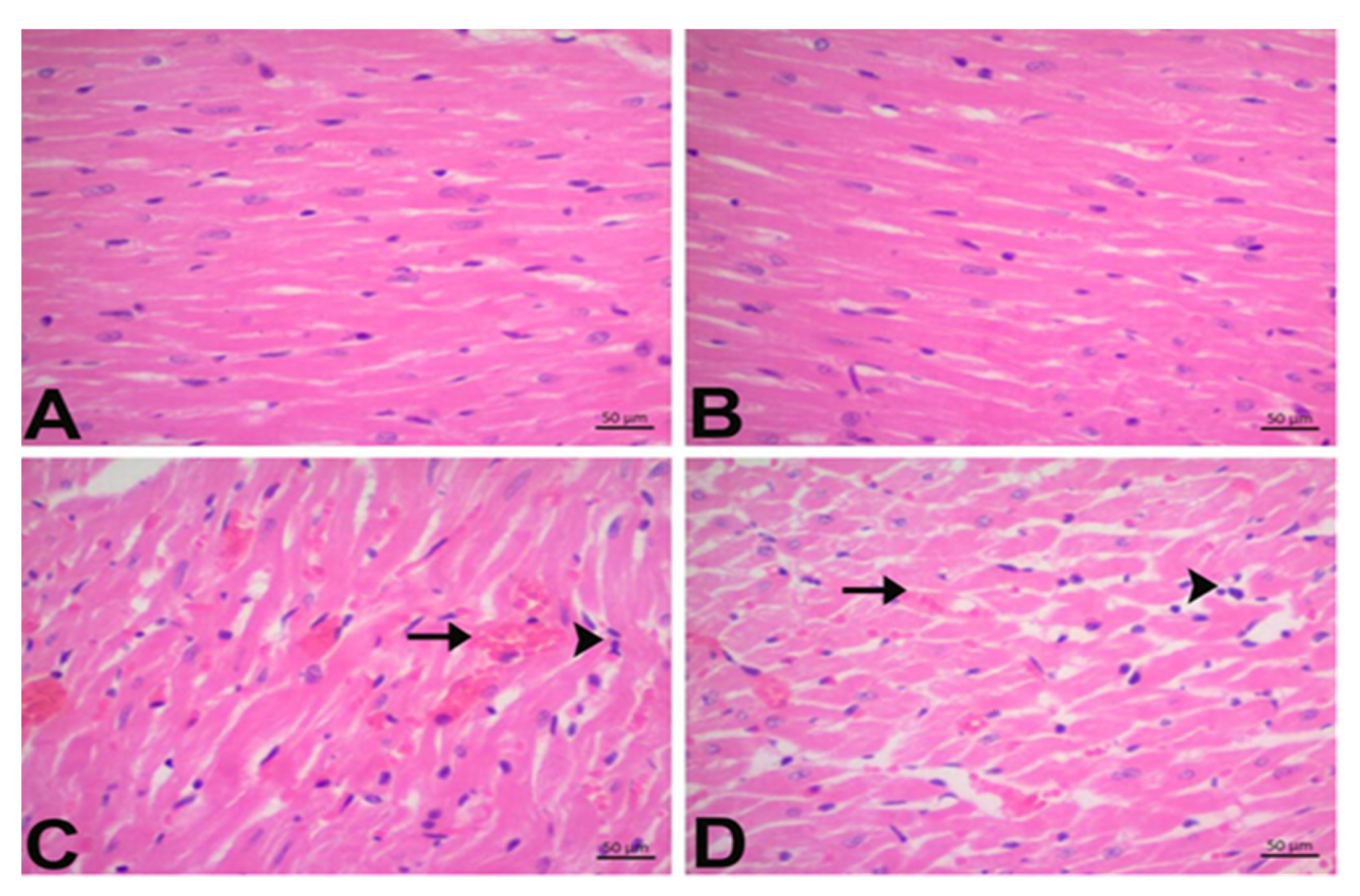
3.2. CGA Prevents DOX-Induced Cardiac Injury
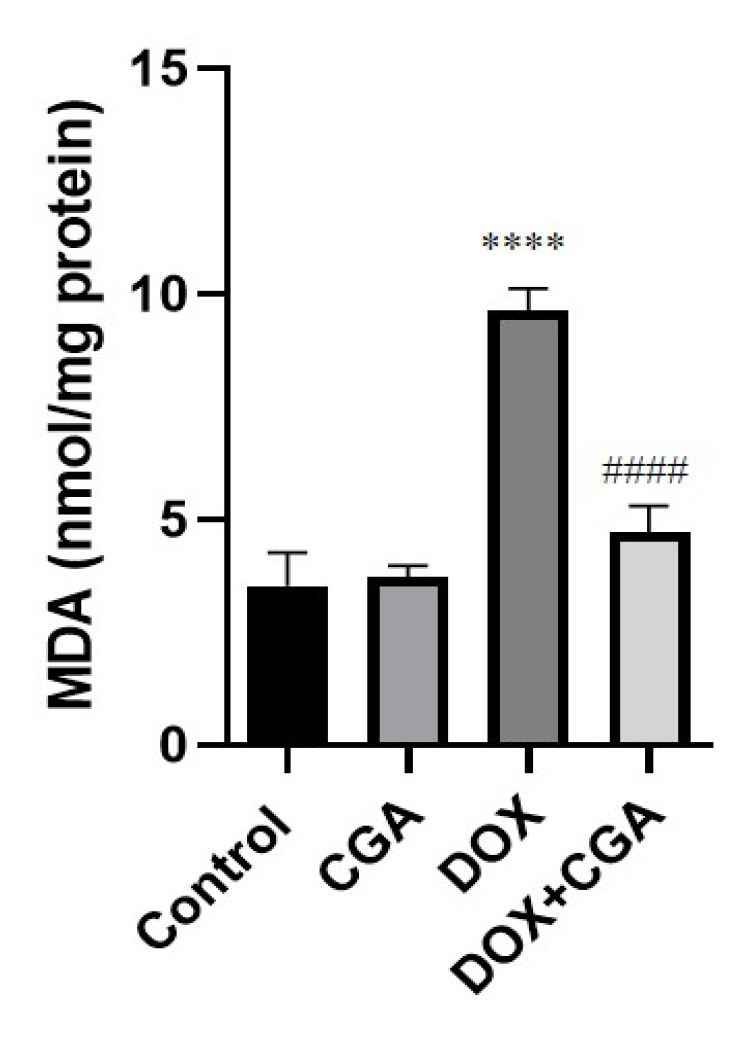
3.3. CGA Attenuates DOX-Induced LPO
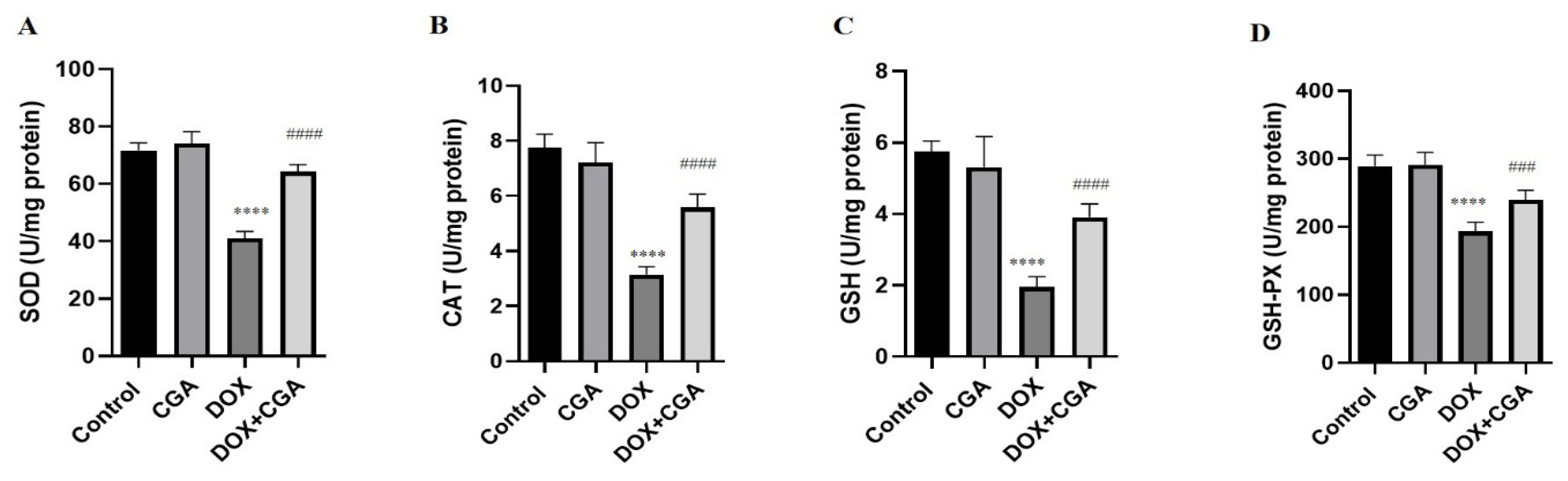
3.4. CGA Alleviates DOX-Induced Oxidative Stress
3.5. CGA Activates the Nrf2/HO-1 Pathway in DOX-Treated Cardiac Tissue
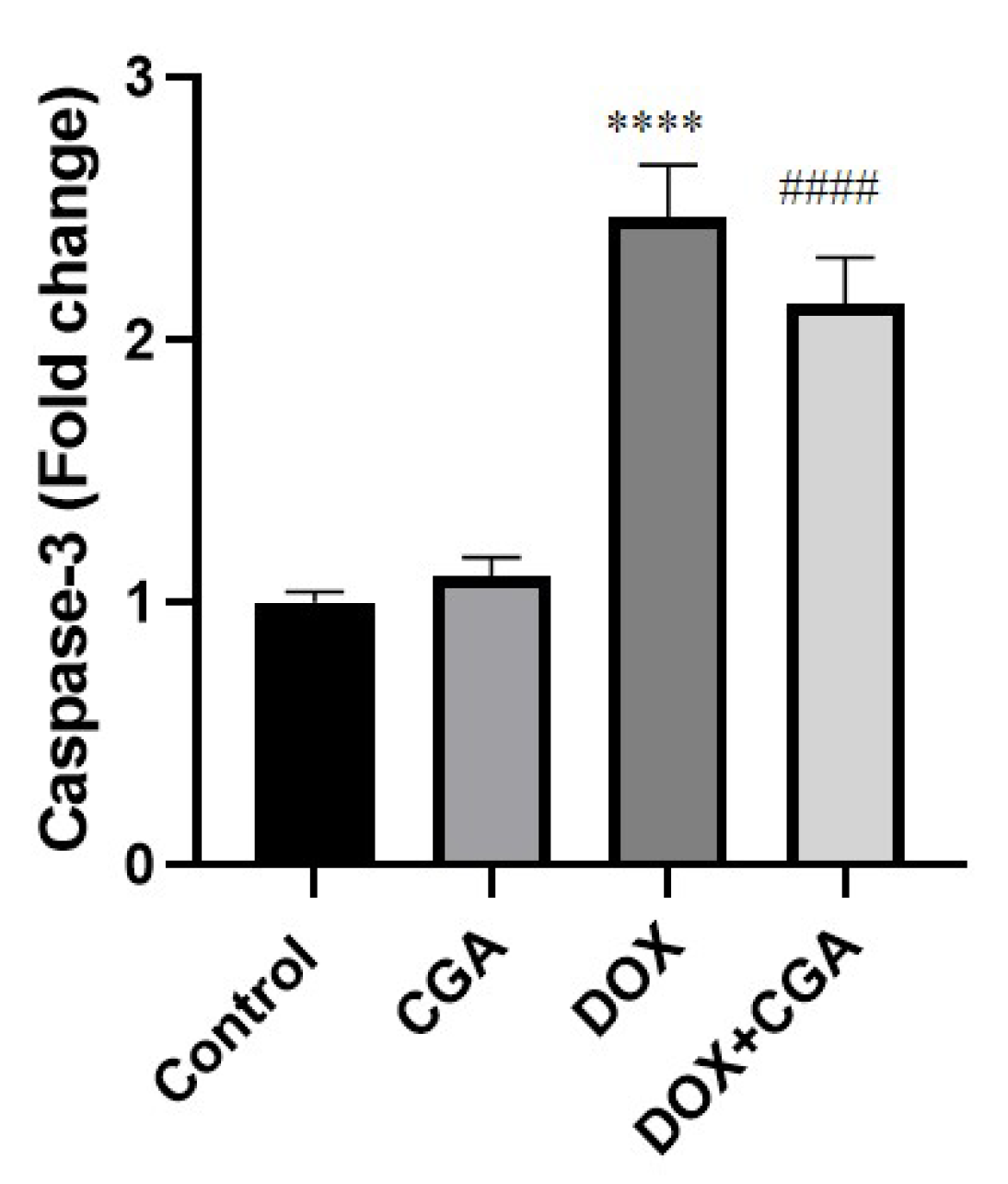
3.6. CGA Reduces DOX-Induced Caspase-3 Expression
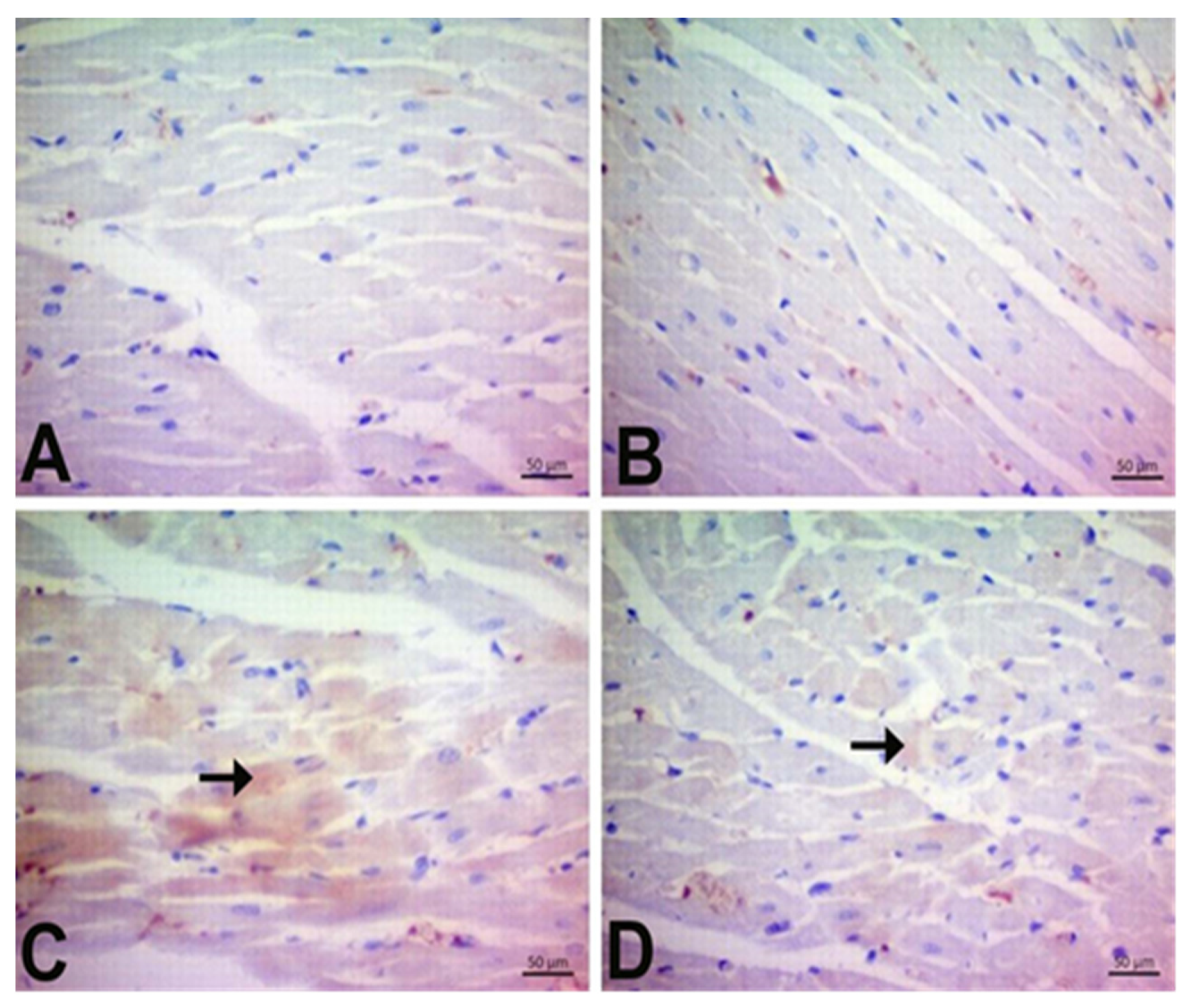
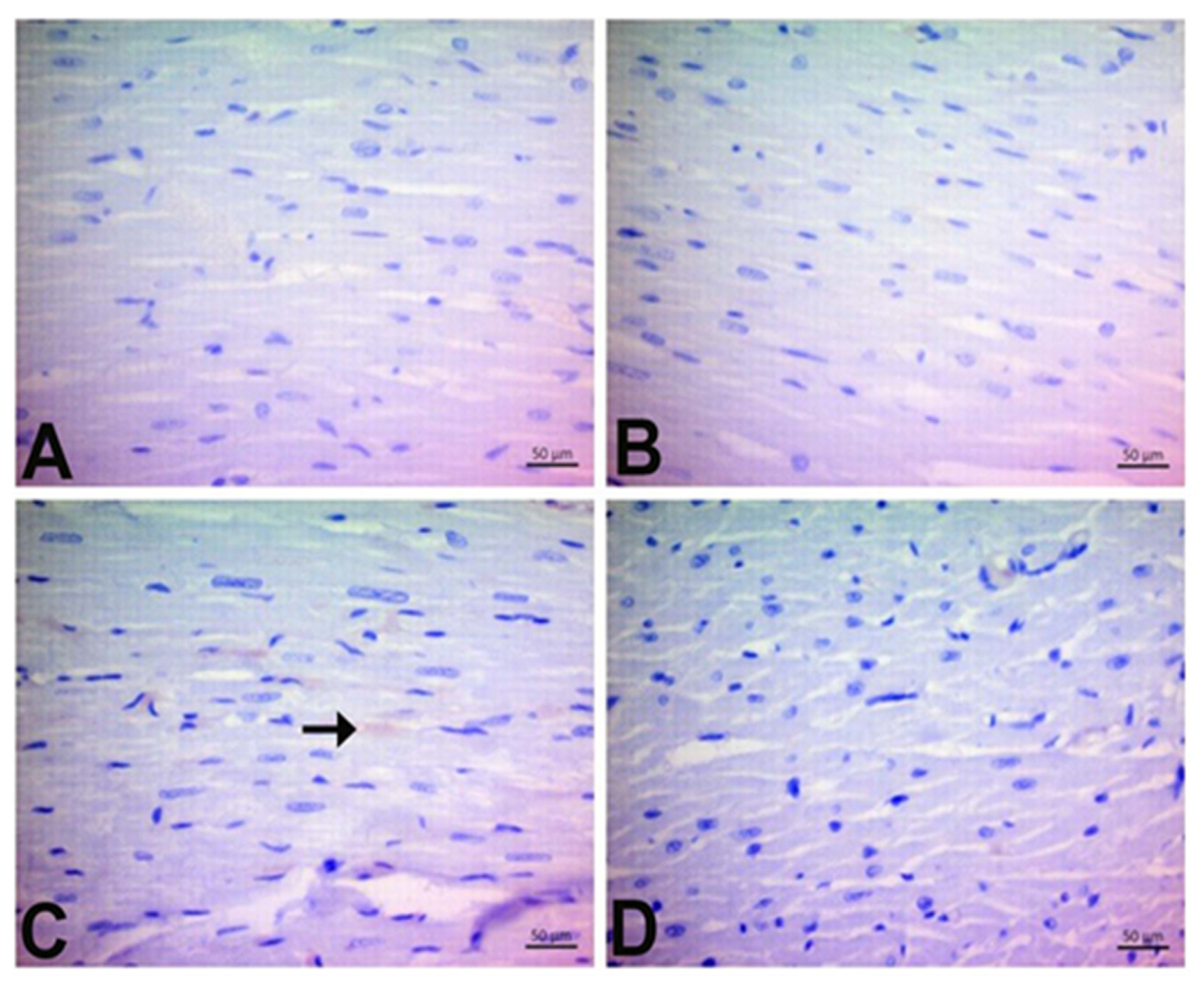
3.7. Immunohistochemical Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Amaral, D.G.; Witter, M.P. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 1989, 31, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, J.; Qu, L.; Liu, S.; Qin, A.; Liu, H.; Wang, T.; Li, W.; Zou, W. Exploring the role of ferroptosis in the doxorubicin-induced chronic cardiotoxicity using a murine model. Chem.-Biol. Interact. 2022, 363, 110008. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Dan, X.; Yu, S.; Zhang, L.; Li, X. Lapatinib induces mitochondrial dysfunction to enhance oxidative stress and ferroptosis in doxorubicin-induced cardiomyocytes via inhibition of PI3K/AKT signaling pathway. Bioengineered 2022, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Bardas, E.; Arslan, Y.K.; Polat, S.; Erisir, M.; Uslu, G.A.; Cetin, N.; Cicek, B. Vitamin E and selenium reduce prednisolone side effects in rat hearts. Int. J. Vitam. Nutr. Res. 2020, 90, 309–317. [Google Scholar] [CrossRef]
- Germanakis, I.; Kalmanti, M.; Parthenakis, F.; Nikitovic, D.; Stiakaki, E.; Patrianakos, A.; Vardas, P.E. Correlation of plasma N-terminal pro-brain natriuretic peptide levels with left ventricle mass in children treated with anthracyclines. Int. J. Cardiol. 2006, 108, 212–215. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Khalifa, H.A.; Ahmed, A.A. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol. 2017, 80, 745–753. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Taghizadehghalehjoughi, A.; Yildirim, S.; Ozkaraca, M.; Genc, S.; Yeni, Y.; Mokresh, M.Y.; Hacimuftuoglu, A.; Tsatsakis, A.; Tsaroushas, K. Deteriorated Vascular Homeostasis in Hypertension: Experimental Evidence from Aorta, Brain, and Pancreatic Vasculature. J. Pers. Med. 2022, 12, 1602. [Google Scholar] [CrossRef]
- Qi, W.; Boliang, G.; Xiaoxi, T.; Guoqiang, F.; Jianbo, X.; Gang, W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed. Pharmacother. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Zhai, J.; Tao, L.; Zhang, S.; Gao, H.; Zhang, Y.; Sun, J.; Song, Y.; Qu, X. Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1–NOD-like receptor protein 3 pathway. Phytother. Res. 2020, 34, 649–659. [Google Scholar] [CrossRef]
- Cicek, B.; Genc, S.; Yeni, Y.; Kuzucu, M.; Cetin, A.; Yildirim, S.; Bolat, I.; Kantarci, M.; Hacimuftuoglu, A.; Lazopoulos, G.; et al. Artichoke (Cynara Scolymus) Methanolic Leaf Extract Alleviates Diethylnitrosamine-Induced Toxicity in BALB/c Mouse Brain: Involvement of Oxidative Stress and Apoptotically Related Klotho/PPARγ Signaling. J. Pers. Med. 2022, 12, 2012. [Google Scholar] [CrossRef]
- Nikitovic, D.; Juranek, I.; Wilks, M.F.; Tzardi, M.; Tsatsakis, A.; Tzanakakis, G.N. Anthracycline-dependent cardiotoxicity and extracellular matrix remodeling. Chest 2014, 146, 1123–1130. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Ansari, M.A.; Raish, M.; Alkharfy, K.M.; Ahad, A.; Al-Jenoobi, F.I.; Haq, N.; Khan, M.R.; Ahmad, A. Sinapic acid ameliorates oxidative stress, inflammation, and apoptosis in acute doxorubicin-induced cardiotoxicity via the NF-κB-mediated pathway. BioMed Res. Int. 2020, 2020, 3921796. [Google Scholar] [CrossRef] [PubMed]
- Bostan, H.B.; Rezaee, R.; Valokala, M.G.; Tsarouhas, K. Cardiotoxicity of nano-particles. Life Sci. 2016, 165, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, N.; Tsaroushas, K.; Tsitsimpikou, C.; Vardavas, A.; Rezaee, R.; Germanakis, I.; Tsatsakis, A.; Stagos, D.; Kouretas, D. Pesticides and cardiotoxicity. Where do we stand? Toxicol. Appl. Pharmacol. 2018, 353, 1–14. [Google Scholar] [CrossRef]
- Ahiskalioglu, A.; Ince, I.; Aksoy, M.; Ahiskalioglu, E.O.; Comez, M.; Dostbil, A.; Celik, M.; Alp, H.H.; Coskun, R.; Taghizadehghalehjoughi, A.; et al. Comparative investigation of protective effects of metyrosine and metoprolol against ketamine cardiotoxicity in rats. Cardiovasc. Toxicol. 2015, 15, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Asilaki, F.; Tsitsimpikou, C.; Tsarouhas, K.; Germanakis, I.; Tzardi, M.; Kavvalakis, M.; Ozcagli, E.; Kouretas, D.; Tsatsakis, A. Cardiotoxicity in rabbits after long-term nandrolone decanoate administration. Toxicol. Lett. 2016, 241, 143–151. [Google Scholar] [CrossRef]
- Refaie, M.M.; Shehata, S.; Ibrahim, R.A.; Bayoumi, A.M.A.; Abdel-Gaber, S.A. Dose-dependent cardioprotective effect of hemin in doxorubicin-induced cardiotoxicity via Nrf-2/HO-1 and TLR-5/NF-κB/TNF-α signaling pathways. Cardiovasc. Toxicol. 2021, 21, 1033–1044. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, D. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Avci, S.; Gunaydin, S.; Ari, N.S.; Sulukoglu, E.K.; Erol-Polat, O.; Gecili, I.; Yeni, Y.; Yilmaz, A.; Genc, S.; Hacimuftuoglu, A.; et al. Cerebrolysin Alleviating Effect on Glutamate-Mediated Neuroinflammation Via Glutamate Transporters and Oxidative Stress. J. Mol. Neurosci. 2022, 72, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, K.K.; Wallace, K.B. Disruption of the keap1/nrf2-antioxidant response system After chronic doxorubicin exposure In vivo. Cardiovasc. Toxicol. 2020, 20, 557–570. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, D.; Xing, R.; Song, H.; Tian, X.; Yan, C.; Han, Y. Orosomucoid 1 attenuates doxorubicin-induced oxidative stress and apoptosis in cardiomyocytes via Nrf2 signaling. BioMed Res. Int. 2020, 2020, 5923572. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Pröpper, S.; Ritz-Timme, S. Dityrosine, a protein product of oxidative stress, as a possible marker of acute myocardial infarctions. Int. J. Leg. Med. 2014, 128, 787–794. [Google Scholar] [CrossRef]
- DiMarco, T.; Giulivi, C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom. Rev. 2007, 26, 108–120. [Google Scholar] [CrossRef]
- Benzer, F.; Kandemir, F.M.; Ozkaraca, M.; Kucukler, S.; Caglayan, C. Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J. Biochem. Mol. Toxicol. 2018, 32, e22030. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Todorovic, V.; Sobajic, S.; Mahajna, J.; Geric, M.; Tur, J.A.; Bartoszek, A. Natural products counteracting cardiotoxicity during cancer chemotherapy: The special case of doxorubicin, a comprehensive review. Int. J. Mol. Sci. 2021, 22, 10037. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ismail, A.; Abdo-Salem, A.M.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Willis, M.S.; Parry, T.L.; Brown, D.I.; Mota, R.I.; Huang, W.; Beak, J.Y.; Sola, M.; Zhou, C.; Hicks, S.T.; Caughey, M.C.; et al. Doxorubicin exposure causes subacute cardiac atrophy dependent on the striated muscle–specific ubiquitin ligase MuRF1. Circ. Heart Fail. 2019, 12, e005234. [Google Scholar] [CrossRef] [PubMed]
- Luminari, S.; Montanini, A.; Caballero, D.; Bologna, S.; Notter, M.; Dyer, M.J.S.; Chiappella, A.; Briones, J.; Petrini, M.; Barbato, A. Nonpegylated liposomal doxorubicin (Myocet™) combination (R-COMP) chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL): Results from the phase II EUR018 trial. Ann. Oncol. 2010, 21, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Martinoni, A.; Tricca, A.; Civelli, M.; Lamantia, G.; Cinieri, S.; Martinelli, G.; Fiorentini, C. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J. Am. Coll. Cardiol. 2000, 36, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, W.; Zhong, C.; Hong, Q.; Huang, G.; Que, D.; Wang, Y.; Yang, Y.; Rui, B.; Zhuang, Z. Elabela ameliorates doxorubicin-induced cardiotoxicity by promoting autophagic flux through TFEB pathway. Pharmacol. Res. 2022, 178, 106186. [Google Scholar] [CrossRef]
- Kwatra, M.; Kumar, V.; Jangra, A.; Mishra, M.; Ahmed, S.; Ghosh, P.; Vohora, D.; Khanam, R. Ameliorative effect of naringin against doxorubicin-induced acute cardiac toxicity in rats. Pharm. Biol. 2016, 54, 637–647. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Lv, H.; Pang, Z.; Li, D.; Yao, Z.; Wang, S. Chlorogenic acid prevents acute myocardial infarction in rats by reducing inflammatory damage and oxidative stress. Biomed. Pharmacother. 2020, 132, 110773. [Google Scholar] [CrossRef]
- Yeni, Y.; Cakir, Z.; Hacimuftuoglu, A.; Taghizadehghalehjoughi, A.; Okkay, U.; Genc, S.; Yildirim, S.; Saglam, Y.S.; Calina, D.; Tsatsakis, A. A selective histamine H4 receptor antagonist, JNJ7777120, role on glutamate transporter activity in chronic depression. J. Pers. Med. 2022, 12, 246. [Google Scholar] [CrossRef]
- Celebi, D.; Taghizadehghalehjoughi, A.; Baser, S.; Genc, S.; Yilmaz, A.; Yeni, Y.; Yesilyurt, F.; Yildirim, S.; Bolat, I.; Kordali, S. Effects of boric acid and potassium metaborate on cytokine levels and redox stress parameters in a wound model infected with methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2022, 26, 294. [Google Scholar] [CrossRef]
- Genc, S.; Pennisi, M.; Yeni, Y.; Yildirim, S.; Gattuso, G.; Altinoz, M.A.; Taghizadehghalehjoughi, A.; Bolat, I.; Tsatsakis, A.; Hacımüftüoğlu, A. Potential neurotoxic effects of glioblastoma-derived exosomes in primary cultures of cerebellar neurons via oxidant stress and glutathione depletion. Antioxidants 2022, 11, 1225. [Google Scholar] [CrossRef]
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardio-Oncology 2019, 5, 18. [Google Scholar] [CrossRef]
- Sobczuk, P.; Czerwińska, M.; Kleibert, M.; Cudnoch-Jędrzejewska, A. Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system—From molecular mechanisms to therapeutic applications. Heart Fail. Rev. 2022, 27, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Bhayana, V.; Henderson, A.R. Biochemical markers of myocardial damage. Clin. Biochem. 1995, 28, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, S.M. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wu, X.; Nie, X.; Wu, Y.; Zhang, C.; Lee, S.-Y.; Lv, K.; Leung, G.-H.; Fu, C.; Zhang, J. Natural compound glycyrrhetinic acid protects against doxorubicin-induced cardiotoxicity by activating the Nrf2/HO-1 signaling pathway. Phytomedicine 2022, 106, 154407. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, Y.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Jinyong Peng, J. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018, 15, 284–296. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Yuan, Y.-P.; Zhang, X.; Kong, C.-Y.; Song, P.; Ma, G.-Z.; Tang, Q.-Z. Protection against doxorubicin-induced cytotoxicity by geniposide involves AMPKα signaling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7901735. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Kong, C.; Song, P.; Wu, H.; Xu, S.; Yuan, Y.; Deng, W.; Ma, Z.; Tang, Q. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020, 27, 540–555. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; Lio, C.; Sun, W.; Lai, K.; Liu, Y.; Zhang, Z.; Liang, J.; Zhou, H.; Liu, L. A chlorogenic acid-phospholipid complex ameliorates post-myocardial infarction inflammatory response mediated by mitochondrial reactive oxygen species in SAMP8 mice. Pharmacol. Res. 2018, 130, 110–122. [Google Scholar] [CrossRef]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef]
- Cappetta, D.; Angelis, D.A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxidative Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Qin, Y.; Xie, J.; Zheng, R.; Li, Y.; Wang, H. Oleoylethanolamide as a New Therapeutic Strategy to Alleviate Doxorubicin-Induced Cardiotoxicity. Front. Pharmacol. 2022, 13, 1344. [Google Scholar] [CrossRef] [PubMed]
- Hada, Y.; Uchida, A.H.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The protective effect of chlorogenic acid on vascular senescence via the Nrf2/HO-1 pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Niu, T.; Wang, H.; Li, B.; Shao, L.; Lai, W.; Li, W.; Janicki, S.J.; Wang, L.X.; et al. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxidative Med. Cell. Longev. 2014, 2014, 748524. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.; Chen, B.; Fang, Y.; Lin, W.; Zhang, T.; Feng, X.; Tao, X.; Wu, Y.; Fu, X.; et al. Chlorogenic acid exerts neuroprotective effect against hypoxia-ischemia brain injury in neonatal rats by activating Sirt1 to regulate the Nrf2-NF-κB signaling pathway. Cell Commun. Signal. 2022, 20, 84. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Nawal, M.; Al-Rasheed, L.M.; Faddah, A.M.; Mohamed, R.A.; Mohammad, M.A.-A. Potential impact of silymarin in combination with chlorogenic acid and/or melatonin in combating cardiomyopathy induced by carbon tetrachloride. Saudi J. Biol. Sci. 2014, 21, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bowen, L.; Yuhui, Y.; Yinyi, D.; Yueting, G.; Yuncong, X.; Yanli, X.; Yonghui, S.; Guowei, L. Dityrosine administration induces myocardium injury and inflammatory response in mice. Wei Sheng Yan Jiu J. Hyg. Res. 2018, 47, 345–351. [Google Scholar]
- Nikolaos, G.; Konstantinos, T.; Ramin, R.; Haritini, N.; George, E.N.; Kass Jean-Lou, C.M.D.; Dimitrios, S.; Konstantinos, T.; Demetrios, A.S.; Dimitrios, K.; et al. What is considered cardiotoxicity of anthracyclines in animal studies. Oncol. Rep. 2020, 44, 798–818. [Google Scholar] [CrossRef]


| MN cell infiltrations | Absent (0) | |
| Mild (1) | <10 MN cells | |
| Moderate (2) | 10–30 MN cells | |
| Severe (3) | >30 MN cells | |
| Hemorrhage | Absent (0) | |
| Mild (1) | <3 hemorrhage focus | |
| Moderate (2) | 3–6 hemorrhage focus | |
| Severe (3) | >7 hemorrhage focus |
| Genes | Forward Sequence (5′-3′) | Reverse Sequence (3′-5′) |
|---|---|---|
| Nfr2 | TGTAGTGCGAGGAAGAGGTATGA | GGAGGGAAAGGAGAGGAAGG |
| HO-1 | AAGAGGCTAAGACCGCCTTC | GCATAAATTCCCACTGCCAC |
| Caspase-3 | TGGACAACAACGAAACCTCC | CTCTTGCCTCAGTCATCAGC |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| Mn Cell Infiltration | Hemorrhage | |
|---|---|---|
| Control | 0.16 ± 0.40 | 0.16 ± 0.40 |
| CGA | 0.33 ± 0.40 | 0.16 ± 0.40 |
| DOX | 2 ± 0.00 * | 2.66 ± 0.51 * |
| DOX + CGA | 1.12 ± 0.40 # | 1.33 ± 0.51 # |
| 4-HNE | 8-OHdG | DT | |
|---|---|---|---|
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| CGA | 0.00 ± 0.00 | 0.16 ± 0.40 | 0.00 ± 0.00 |
| DOX | 1.00 ± 0.00 * | 2.83 ± 0.40 * | 0.83 ± 0.40 * |
| DOX + CGA | 0.16 ± 0.40 # | 1.12 ± 0.40 # | 0.16 ± 0.40 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicek, B.; Hacimuftuoglu, A.; Yeni, Y.; Danisman, B.; Ozkaraca, M.; Mokhtare, B.; Kantarci, M.; Spanakis, M.; Nikitovic, D.; Lazopoulos, G.; et al. Chlorogenic Acid Attenuates Doxorubicin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Dityrosine Signaling. J. Pers. Med. 2023, 13, 649. https://doi.org/10.3390/jpm13040649
Cicek B, Hacimuftuoglu A, Yeni Y, Danisman B, Ozkaraca M, Mokhtare B, Kantarci M, Spanakis M, Nikitovic D, Lazopoulos G, et al. Chlorogenic Acid Attenuates Doxorubicin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Dityrosine Signaling. Journal of Personalized Medicine. 2023; 13(4):649. https://doi.org/10.3390/jpm13040649
Chicago/Turabian StyleCicek, Betul, Ahmet Hacimuftuoglu, Yesim Yeni, Betul Danisman, Mustafa Ozkaraca, Behzad Mokhtare, Mecit Kantarci, Marios Spanakis, Dragana Nikitovic, Georgios Lazopoulos, and et al. 2023. "Chlorogenic Acid Attenuates Doxorubicin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Dityrosine Signaling" Journal of Personalized Medicine 13, no. 4: 649. https://doi.org/10.3390/jpm13040649
APA StyleCicek, B., Hacimuftuoglu, A., Yeni, Y., Danisman, B., Ozkaraca, M., Mokhtare, B., Kantarci, M., Spanakis, M., Nikitovic, D., Lazopoulos, G., Tsarouhas, K., Tsatsakis, A., & Taghizadehghalehjoughi, A. (2023). Chlorogenic Acid Attenuates Doxorubicin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Dityrosine Signaling. Journal of Personalized Medicine, 13(4), 649. https://doi.org/10.3390/jpm13040649










