A Novel Clinical Nomogram for Predicting Overall Survival in Patients with Emergency Surgery for Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Variables and Definitions
2.3. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. 1-Year, 3-Year and 5-Year Overall Survival
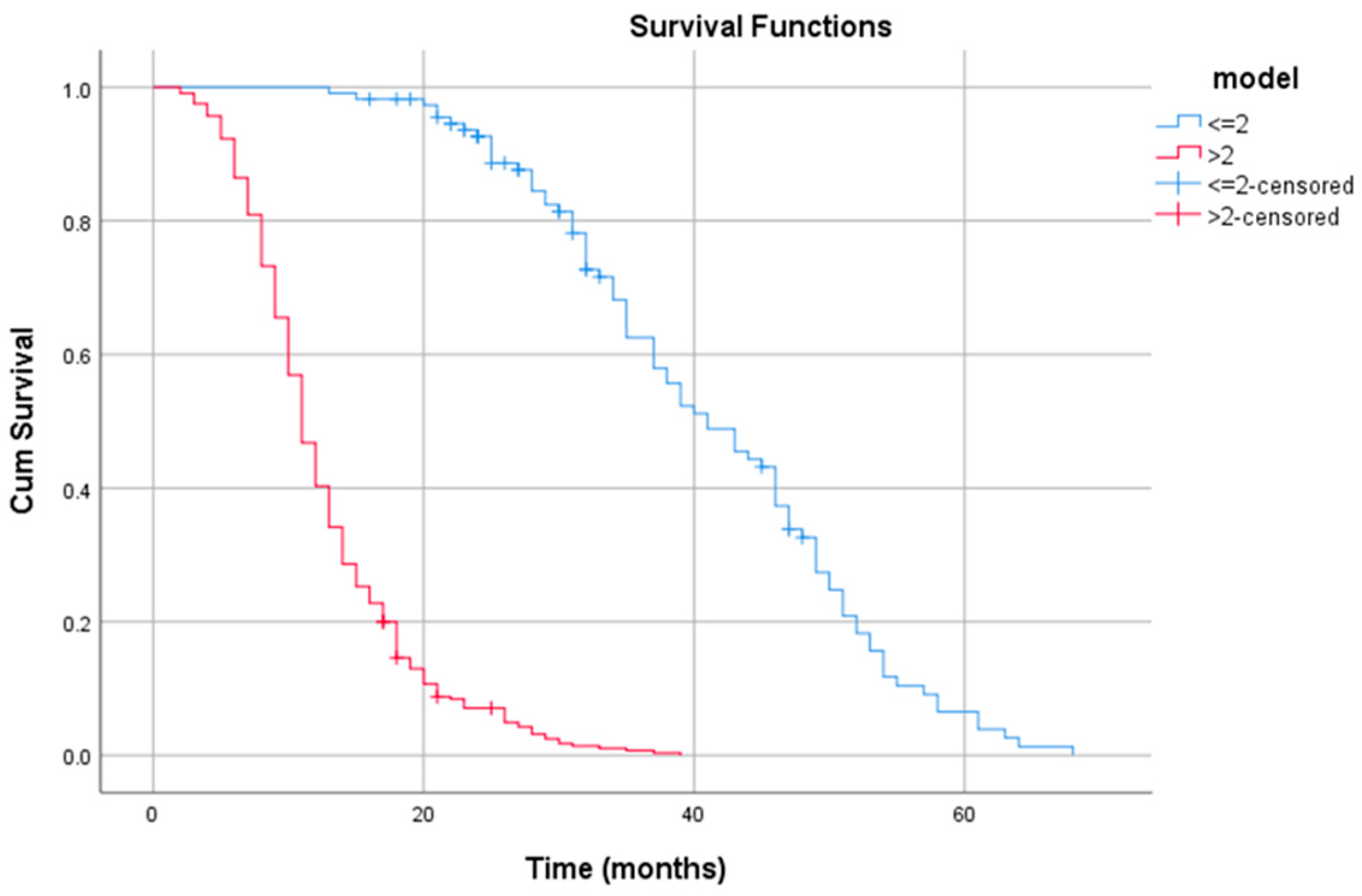
3.3. Prognosis Factors
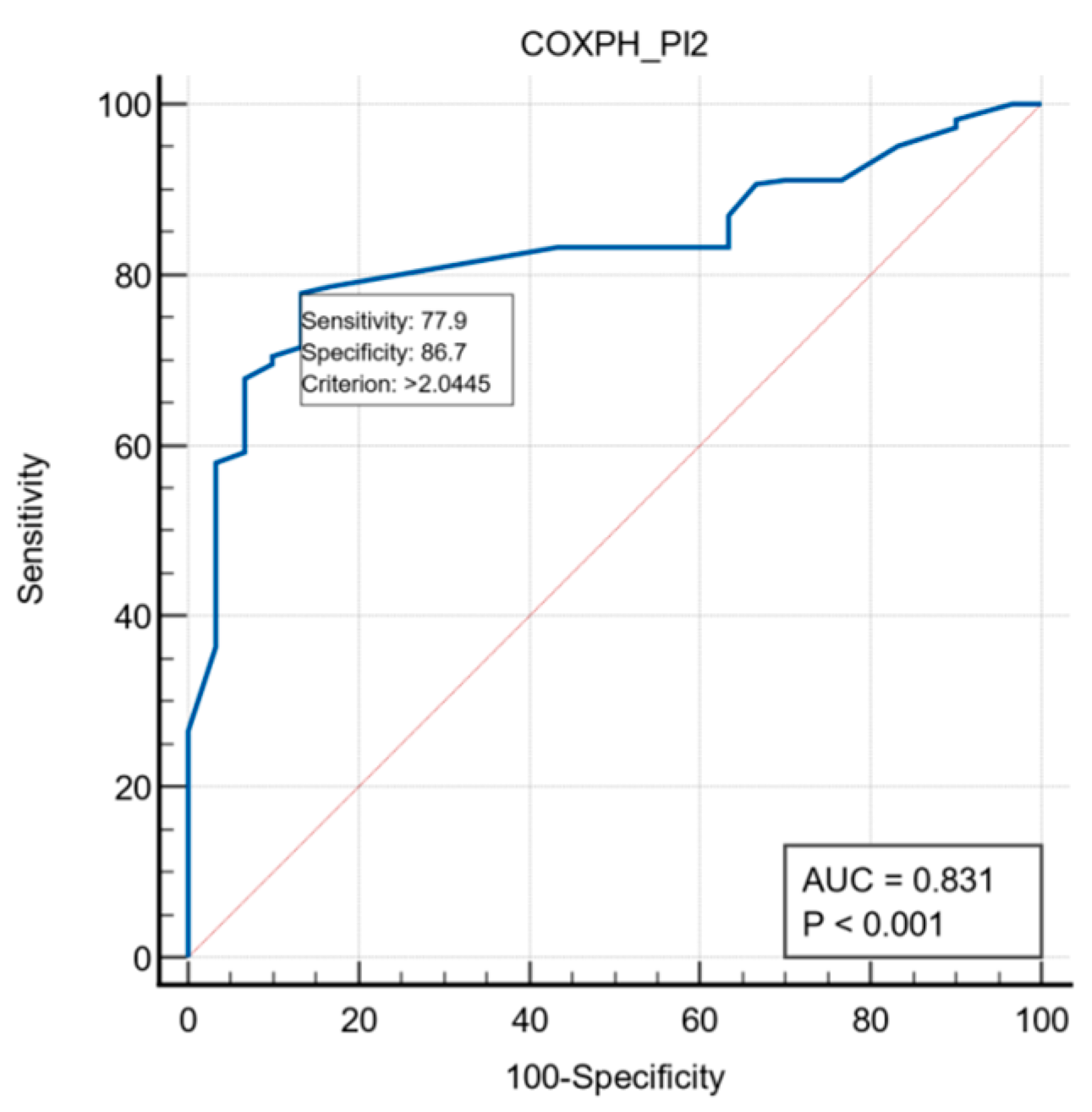
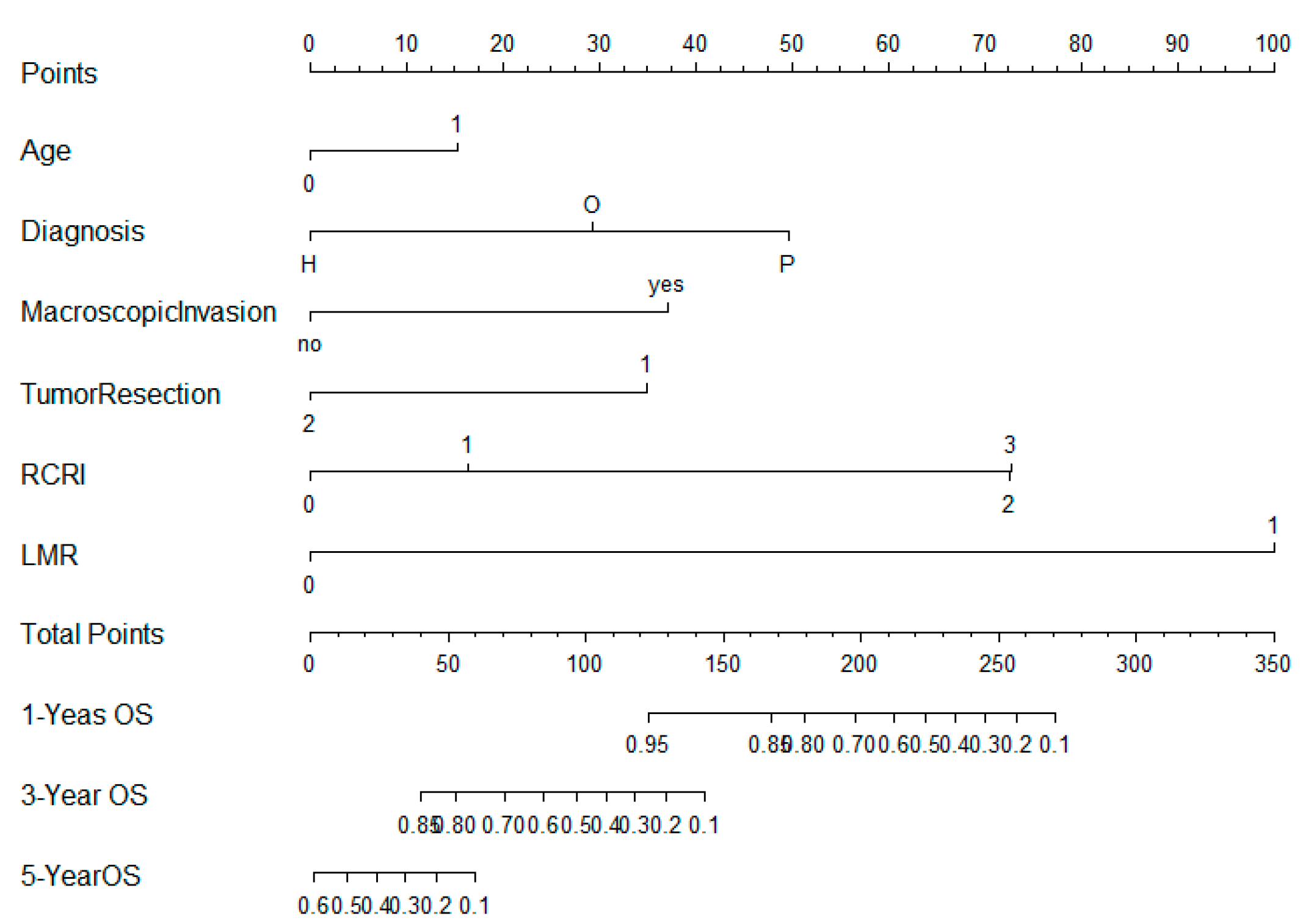
3.4. Nomogram Construction
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colon. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/8-Colon-fact-sheet.pdf (accessed on 11 March 2023).
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Jestin, P.; Nilsson, J.; Heurgren, M.; Pahlman, L.; Glimelius, B.; Gunnarsson, U. Emergency surgery for colonic cancer in a defined population. Br. J. Surg. 2005, 92, 94–100. [Google Scholar] [CrossRef]
- Nascimbeni, R.; Ngassa, H.; Di Fabio, F.; Valloncini, E.; Di Betta, E.; Salerni, B. Emergency surgery for complicated colorectal cancer. A two-decade trend analysis. Dig. Surg. 2008, 25, 133–139. [Google Scholar] [CrossRef]
- Mohd, S.; Tan, W.L.; Soelar, S.A.; Ismail, I.; Abu, H. Intestinal obstruction: Predictor of poor prognosis in colorectalcarcinoma? Epidemiol. Health 2015, 30, e2015017. [Google Scholar] [CrossRef]
- Ali, N.D.; Donohue, K.; Zandieh, S.; Chen, C.; Moore, D.; Poplin, E.; Shah, M.M.; Nosher, J.; Gui, B.; Jabbour, S.K.; et al. Conditional Survival Analysis of Metastatic Colorectal Cancer Patients Living ≥ 24 Months: A Single Institutional Study. Am. J. Clin. Oncol. 2019, 42, 512–518. [Google Scholar] [CrossRef] [PubMed]
- McArdle, C.S.; Hole, D.J. Emergency presentation of colorectal cancer is associated with poor 5-year survival. Br. J. Surg. 2004, 91, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazeq, A.S.; Scott, N.; Thorn, C.; Verbeke, C.S.; Ambrose, N.S.; Botelli, I.D. The impact of spontaneous tumor perforation on outcome following colon cancer surgery. Colorectal. Dis. 2008, 10, 775–780. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Sharma, V.; Kumar, S.; Kumar, A.; Deo, S.; Pathy, S.; Shukla, N.K.; Pramanik, R.; Raina, V.; et al. Long-Term Survivors of Metastatic Colorectal Cancer: A Tertiary Care Centre Experience. South Asian J. Cancer 2021, 10, 87–91. [Google Scholar] [CrossRef]
- Wong, S.K.; Jalaludin, B.B.; Morgan, M.J.; Berthelsen, A.S.; Morgan, A.; Gatenby, A.H.; Fulham, S.B. Tumor pathology and long-term survival in emergency colorectal cancer. Dis. Colon Rectum. 2008, 51, 223–230. [Google Scholar] [CrossRef]
- Xu, Z.; Becerra, A.Z.; Aquina, C.T.; Hensley, B.J.; Justiniano, C.F.; Boodry, C.; Swanger, A.A.; Arsalanizadeh, R.; Noyes, K.; Monson, J.R.; et al. Emergent Colectomy Is Independently Associated with Decreased Long-Term Overall Survival in Colon Cancer Patients. J. Gastrointest Surg. 2017, 21, 543–553. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Eman, A.E.M. Emergency curative resection of colorectal cancer, do it with caution. A comparative case series. Ann. Med. Surg. 2020, 55, 70–76. [Google Scholar]
- Golder, A.M.; McMillan, D.C.; Horgan, P.G.; Roxburgh, C.S.D. Determinants of emergency presentation in patients with colorectal cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 4366. [Google Scholar] [CrossRef] [PubMed]
- Amri, R.; Bordeianou, L.G.; Sylla, P.; Berger, D.L. Colon cancer surgery following emergency presentation: Effects on admission and stage-adjusted outcomes. Am. J. Surg. 2015, 209, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, W.; Reingruber, B.; Merkel, S. Surgery for colon cancer. Scand. J. Surg. 2003, 92, 45–52. [Google Scholar] [CrossRef]
- Weixler, B.; Warschkow, R.; Ramser, M.; Droeser, R.; von Holzen, U.; Oertli, D.; Kettelhack, C. Urgent surgery after emergency presentation for colorectal cancer has no impact on overall and disease-free survival: A propensity score analysis. BMC Cancer 2016, 16, 208. [Google Scholar] [CrossRef]
- Antony, P.; Harnoss, J.C.; Warschkow, R.; Schmied, B.M.; Schneider, M.; Tarantino, I.; Ulrich, A. Urgent surgery in colon cancer has no impact on survival. J. Surg. Oncol. 2019, 119, 1170–1178. [Google Scholar] [CrossRef]
- Joël, L.L.; Lukas, V.; Tobias, H.; Inti, Z.; Lukas, E.B.; Candinas, D.; Schnüriger, B. Oncologic Long-TermOutcomes of Emergency Versus Elective Resection for Colorectal Cancer. In Proceedings of the European Congress for Trauma &Emergency Surgery, Valencia, Spain, 6–8 May 2018. [Google Scholar]
- Cauley, C.E.; Panizales, M.T.; Reznor, G.; Haynes, A.B.; Havens, J.M.; Kelley, E.; Mosenthal, A.C.; Cooper, Z. Outcomes after emergency abdominal surgery in patients with advanced cancer: Opportunities to reduce complications and improve palliative care. J. Trauma Acute Care Surg. 2015, 79, 399–406. [Google Scholar] [CrossRef]
- Brown, S.R.; Mathew, R.; Keding, A.; Marshall, H.C.; Brown, J.M.; Jayne, D.G. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann. Surg. 2014, 259, 916–923. [Google Scholar] [CrossRef]
- Paul Olson, T.J.; Pinkerton, C.; Brasel, K.J.; Schwarze, M.L. Palliative surgery for malignant bowel obstruction from carcinomatosis: A systematic review. JAMA Surg. 2014, 149, 383–392. [Google Scholar] [CrossRef]
- Iversen, L.H. Aspects of survivalfromcolorectal cancer in Denmark. Dan Med. J. 2012, 59, B4428. [Google Scholar]
- Luque-Fernandez, M.A.; Redondo-Sanchez, D.; Rodriguez-Barranco, M. The pattern of comorbidities and multimorbidity among colorectal cancer patients in Spain: CoMCoR study. bioRxiv 2019, 526673. [Google Scholar]
- Fearon, K.C.H.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.F.; Santos Dos-Reis, L.C.; Esteves BorgethTeixeira, B.; Andrade, I.M.; Sulzbach, J.S.; Leal, R.A. Cirurgiano cancer de colon empacientes operados de emergencia. Rev. Col. Bras. Cir. 2017, 44, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Borazan, E.; Balık, A.A.; Bozdağ, Z. Assessment of the relationship between neutrophil lymphocyte ratio and prognostic factors in non-metastatic colorectal cancer. Turk. J. Surg. 2017, 33, 185–189. [Google Scholar] [CrossRef]
- Ding, P.R.; An, X.; Zhang, R.X.; Fang, Y.J.; Li, L.R.; Chen, G. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int. J. Colorectal. Dis. 2010, 25, 1427–1433. [Google Scholar] [CrossRef]
- Chen, X.Q.; Xue, C.R.; Hou, P.; Lin, B.Q.; Zhang, J.R. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer. World J. Gastroenterol. 2019, 25, 4970–4984. [Google Scholar] [CrossRef]
- Palin, R.P.; Devine, A.T.; Hicks, G.; Burke, D. Association of pretreatment neutrophil-lymphocyte ratio and outcome in emergency colorectal cancer care. Ann. R Coll. Surg. Engl. 2018, 100, 308–315. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kim, S.H.; Oh, S.Y.; Lee, S.; Lee, J.H.; Choi, H.J.; Park, K.J.; Roh, M.S.; Kim, S.G.; Kim, H.J.; et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012, 17, 216–222. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Sun, P.; Xiao, C.; Xu, Y.; Li, R. Preoperative platelet-lymphocyte ratio is an independent prognostic factor for resectable colorectal cancer. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2013, 33, 70–73. [Google Scholar]
- Emir, S.; Aydin, M.; Can, G.; Bali, I.; Yildirim, O.; Öznur, M.; Yildiz, Z.D.; Sözen, S.; Gürel, A. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3613–3618. [Google Scholar]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Luvian-Morales, J.; Gonzalez-Trejo, S.; Herrera-Goepfert, R. Association of the prognostic nutritional index and overall survival in patients with colorectal cancer: A STROBE compliant retrospective cohortstudy. Cancer Med. 2019, 8, 3379–3388. [Google Scholar] [CrossRef] [PubMed]
- Jian-Hui, C.; Iskandar, E.A.; Cai, S.; Chen, C.Q.; Wu, H.; He, Y.L. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: A large cohort study in a single Chinese institution. Tumour Biol. 2016, 37, 3277–3283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, P.; Kang, H.; Lin, S.; Wang, M.; Yang, P.; Dai, C.; Liu, X.; Liu, K.; Zheng, Y.; et al. Prognostic nutritional index as a prognostic biomarker for survival in digestive system carcinomas. Oncotarget 2016, 7, 86573–86583. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Tomasello, G.; Borgonovo, K. Prognostic Survival Associated with Left-Sided vs. Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2017, 3, 211–219. [Google Scholar] [CrossRef]
- Cai, X.; Gu, D.; Chen, M. The effect of the primary tumor location on the survival of colorectal cancer patients after radical surgery. Int. J. Med. Sci. 2018, 15, 1640–1647. [Google Scholar] [CrossRef]
- Sheth, K.R.; Clary, B.M. Management of hepatic metastases from colorectal cancer. Clin. Colon Rectal Surg. 2005, 18, 215–223. [Google Scholar] [CrossRef]
- Tardu, A.; Kayaalp, C.; Yilmaz, S.; Tolan, K.; Ersan, V.; Karagul, S.; Ertugrul, I.; Kirmizi, S. Resection of colorectal liver metastasis with vena cava resection. Case Rep. Surg. 2016, 1, 8173048. [Google Scholar] [CrossRef]
- Valderrama-Treviño, A.I.; Barrera-Mera, B.; Ceballos-Villalva, J.C.; Montalvo-Javé, E.E. Hepatic Metastasis from Colorectal Cancer. Euroasian J. Hepatogastroenterol. 2017, 7, 166–175. [Google Scholar] [CrossRef]
- Compton, C.C.; Fnoglio-Preiser, C.M.; Pettigrew, N.; Fielding, L.P. American Joint Committee on Cancer Prognostic Factors consensus conferences: Colorectal Working Group. Cancer 2000, 88, 1739–1757. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheng, S.C.; Tung, H.Y.; Chang, S.C.; Ching, C.Y.; Wu, S.F. The Risk Factors Affecting Survival in Colorectal Cancer in Taiwan. Iran. J. Public Health 2018, 47, 519–530. [Google Scholar] [PubMed]
- Littlechild, J.; Junejo, M.; Simons, A.M.; Curran, F.; Subar, D. Emergency resection surgery for colorectal cancer: Patterns of recurrent disease and survival. World J. Gastrointest Pathophysiol. 2018, 9, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.; Samaha, G.; Burke, J.; Chang, K.H.; Condon, E.; Waldron, D.; Calvin, C.J. Emergency Presenting Colon Cancer Is an Independent Predictor of Adverse Disease-Free Survival. Int. Surg. 2015, 100, 77–86. [Google Scholar] [CrossRef]
- Poornakala, S.; Prema, N.S. A study of morphological prognostic factors in colorectal cancer and survival analysis. Indian J. Pathol. Microbiol. 2019, 62, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhang, Q.W.; Huang, J. Additional lymphadenectomy might not improve survival of patients with resectable metastatic colorectal adenocarcinoma of T4 stage, proximal location, poor/undifferentiation, or N3/N4 stages: A large population-based study. J. Cancer 2018, 9, 2428–2435. [Google Scholar] [CrossRef]
- O’Boyle, S.; Stephenson, K. More is better: Lymph node harvesting in colorectal cancer. Am. J. Surg. 2017, 213, 926–930. [Google Scholar] [CrossRef]
- Aquina, C.T.; Becerra, A.Z.; Xu, Z.; Boscoe, F.P.; Schymura, M.J.; Noyes, K. Nonelective colon cancer resection: A continued public health concern. Surgery 2017, 161, 1609–1618. [Google Scholar] [CrossRef]
- De Salvo, G.L.; Gava, C.; Pucciarelli, S.; Lise, M. Curative surgery for obstruction from primary left colorectal carcinoma: Primary or staged resection? Cochrane Database Syst. Rev. 2004, 2, CD002101. [Google Scholar] [CrossRef]
- Amelung, F.J.; Consten, E.C.J.; Siersema, P.D.; Tanis, P.J. A population-based analysis of three treatment modalities for malignant obstruction of the proximal colon: Acute resection versus stent or stoma as a bridge to surgery. Ann. Surg. Oncol. 2016, 23, 3660–3668. [Google Scholar] [CrossRef]
- Andreano, M.; D’Ambrosio, V.; Coretti, G.; Bianco, P.; Castriconi, M. Primary anastomosis in emergency surgery of left colon cancer. Ann. Ital. Chir. 2016, 87, 438–441. [Google Scholar]
- Tebala, G.D.; Natili, A.; Gallucci, A. Emergency treatment of complicated colorectal cancer. Cancer Manag. Res. 2018, 10, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, V.B.; Lausen, I.M.; Frederiksen, H.J.; Kjaergaard, J. Hartmann’s procedure in the treatment of acute obstructive left-sided colonic cancer. Ugeskr Laeger 1993, 155, 3816–3818. [Google Scholar]
- Charbonnet, P.; Gervaz, P.; Andres, A. Results of emergency Hartmann’s operation for obstructive or perforated left-sided colorectal cancer. World J. Surg. Oncol. 2008, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- denDulk, M.; Smit, M.; Peeters, K.C.; Kranenbarg, E.M.; Rutten, H.J.; Wiggers, T.; Putter, H.; Velde, C.J. Dutch Colorectal Cancer Group: A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: A retrospective study. Lancet Oncol. 2007, 8, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kosumi, K.; Mima, K.; Morito, A.; Yumoto, S.; Matsumoto, T.; Inoue, M.; Mizumoto, T.; Kubota, T.; Miyanari, N.; Baba, H. Patient Age and Long-term Survival in Colorectal Cancer Patients Who Undergo Emergency Surgery. Anticancer Res. 2021, 41, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.; Lin, W.; Sultana, R.; Foo, F.J.; Tang, C.L.; Chew, M.H. A prognostic score predicting survival following emergency surgery in patients with metastatic colorectal cancer. ANZ J. Surg. 2021, 91, 2493–2498. [Google Scholar] [CrossRef]
- Biondo, S.; Gálvez, A.; Ramírez, E.; Frago, R.; Kreisler, E. Emergency surgery for obstructing and perforated colon cancer: Patterns of recurrence and prognostic factors. Tech. Coloproctol. 2019, 23, 1141–1161. [Google Scholar] [CrossRef]
- Lee, T.H.; Marcantonio, E.R.; Mangione, C.M.; Thomas, E.J.; Polanczyk, C.A.; Cook, E.F.; Sugarbaker, D.J.; Donaldson, M.C.; Poss, R.; Ho, K.K.L.; et al. Derivation and Prospective Validation of a Simple Index for Prediction of Cardiac Risk of Major Noncardiac Surgery. Circulation 1999, 100, 1043–1049. [Google Scholar] [CrossRef]
- Caio Mazzonetto, T.; Mendonça Corrêa, L.; Procópio, R.J.; Lopes do Carmo, G.; Pinho Navarro, T. Tools and scores for general and cardiovascular perioperative risk assessment: A narrative review. Rev. Col Bras. Cir. 2022, 49, e20223124. [Google Scholar]
- AhlHulme, R.; Forssten, M.P.; Pourlotfi, A.; Cao, Y.; Bass, G.A.; Matthiessen, P.; Mohseni, S. The Association Between Revised Cardiac Risk Index and Postoperative Mortality Following Elective Colon Cancer Surgery: A Retrospective Nationwide Cohort Study. Scand. J. Surg. 2022, 1, 14574969211037588. [Google Scholar]
- Bass, G.A.; Forssten, M.; Pourlotfi, A.; AhlHulme, R.; Cao, Y.; Matthiessen, P.; Mohseni, S. Cardiac risk stratification in emergency resection for colonic tumours. BJS Open 2021, 5, zrab057. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Chan, D.L.; Diakos, C.I.; Engel, A.; Pavlakis, N.; Gill, A.; Clarke, S.J. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann. Surg. 2017, 265, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocytetomonocyteratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.P.; Cairns, V.; Jones, M.J.; Masood, M.M.; Nana, G.R.; Mann, C.D. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med. Oncol. 2015, 32, 144. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Y.; Jia, Y. A nomogram model for predicting prognosis of obstructive colorectal cancer. World J. Surg. Oncol. 2021, 19, 337. [Google Scholar] [CrossRef]
- Kao, Y.K.; Chen, H.P.; Liu, K.W.; Song, L.C.; Chen, Y.C.; Lin, Y.C.; Chen, C.I. Impact on inadequate lymph node harvest on survival in T4N0 colorectal cancer: A would-be medical center experience in Taiwan. Medicine 2022, 101, e32497. [Google Scholar] [CrossRef]
- Campos, F.G.; Calijuri-Hamra, M.C.; Imperiale, A.R. Locally advanced colorectal cancer: Results of surgical treatment and prognostic factors. Arq. Gastroenterol. 2011, 48, 270–275. [Google Scholar] [CrossRef]
- Perron, L.; Daigle, J.M.; Vandal, N.; Guertin, M.H.; Brisson, J. Characteristics affecting survival after locally advanced colorectal cancer in Quebec. Curr. Oncol. 2015, 22, e485–e492. [Google Scholar] [CrossRef]
- Sokolov, M. Surgical approach in locally advanced colorectal cancer-combined, extended and compound surgery. Khirurgiia 2013, 4, 29–50. [Google Scholar]
- Park, S.; Lee, Y.S. Analysis of the prognostic effectiveness of a multivisceral resection for locally advanced colorectal cancer. J. Korean Soc. Coloproctol. 2011, 27, 21–26. [Google Scholar] [CrossRef]
- Kim, C.N. Emergent Colorectal Surgery: What Should Be Considered? Ann. Coloproctol. 2016, 32, 124–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Xu, Y.; Xu, G. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Sunami, E.; Yamaguchi, H.; Ishihara, S.; Kazama, S.; Nozawa, H.; Hata, K.; Kiyomatsu, T.; Tanaka, J.; Tanaka, T.; et al. Nomograms for colorectal cancer: A systematic review. World J. Gastroenterol. 2015, 21, 11877–11886. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, M.; Xu, G. The incidence, associated factors, and predictive nomogram for early death in stage IV colorectal cancer. Int. J. Colorectal. Dis. 2019, 34, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
| No Deaths (N = 30) | Deaths (N = 407) | p-Value (Test) | |
|---|---|---|---|
| Sex | 0.99577 (1) | ||
| F | 12/30 (40%) | 163/407 (40%) | |
| M | 18/30 (60%) | 244/407 (60%) | |
| 0.00008 (2) | |||
| Age | 59.6 [51.75, 64.25] | 70.0 [60.0, 78.0] | |
| Age | 0.00001 (1) | ||
| ≤63 | 23/30 (76.7%) | 147/407 (36.1%) | |
| >63 | 7/30 (23.3%) | 260/407 (63.9%) | |
| 0.55101 (1) | |||
| History of surgery—yes | 10/30 (33.3%) | 158/407 (38.8%) | |
| 1.00000 (3) | |||
| History of colon disease—yes | 1/30 (3.3%) | 18/407 (4.4%) | |
| 0.02922 (1) | |||
| Cardiovascular comorbidity—yes | 4/30 (13.3%) | 132/407 (32.4%) | |
| 0.15289 (3) | |||
| Cardiac rhythm disorders—yes | 0/30 (0%) | 31/407 (7.6%) | |
| 0.39433 (3) | |||
| Hepatic comorbidity—yes | 1/30 (3.3%) | 6/407 (1.5%) | |
| Davies Score | 0.00000 (4) | ||
| 1 | 8/30 (26.7%) | 12/407 (2.9%) | |
| 2 | 18/30 (60.0%) | 151/407 (37.1%) | |
| 3 | 4/30 (13.3%) | 180/407 (44.2%) | |
| 4 | 0/30 (0%) | 64/407 (15.7%) | |
| 0.00002 (2) | |||
| Charlsonscore | 4.0 [3.0, 4.0] | 4.0 [4.0, 5.0] | |
| Charlsonscore | 0.00167 (1) | ||
| ≤4 | 25/30 (83.3%) | 219/407 (53.8%) | |
| >4 | 5/30 (16.7%) | 188/407 (46.2%) | |
| Age-adjusted Charlson score | 0.00000 (2) | ||
| 6.0 [5.0, 6.0] | 7.0 [6.0, 9.0] | ||
| Age-adjusted Charlson score | 0.00000 (1) | ||
| ≤6 | 24/30 (80.0%) | 137/407 (33.7%) | |
| >6 | 6/30 (20.0%) | 270/407 (66.3%) | |
| RCRI | 0.00000 (4) | ||
| 0 | 11/30 (36.7%) | 17/407 (4.2%) | |
| 1 | 17/30 (56.7%) | 137/407 (33.7%) | |
| 2 | 2/30 (6.7%) | 245/407 (60.2%) | |
| 3 | 0/30 (0%) | 8/407 (2%) | |
| 0.78329 (3) | |||
| Anemia—yes | 21/30 (70.0%) | 275/407 (67.6%) | |
| 0.56493 (3) | |||
| WBC-aN | 5/30 (16.7%) | 50/407 (12.3%) | |
| 0.39433 (3) | |||
| Platelets-aN | 1/30 (3.3%) | 6/407 (1.5%) | |
| 0.00000 (2) | |||
| NLR | 2.00 [1.71, 2.42] | 2.95 [2.43, 3.43] | |
| NLR | 0.00000 (1) | ||
| ≤2.61 | 28/30 (93.3%) | 124/407 (30.5%) | |
| >2.61 | 2/30 (6.7%) | 283/407 (69.5%) | |
| 0.00088 (2) | |||
| PLR | 139.6 [130.1, 152.2] | 151.3 [140.1, 182.2] | |
| PLR | 0.00000 (1) | ||
| ≤140 | 19/30 (63.3%) | 85/407 (20.9%) | |
| >140 | 11/30 (36.7%) | 322/407 (79.1%) | |
| 0.63807 (2) | |||
| LMR | 1.58 [1.22, 3.01] | 1.98 [1.36, 2.71] | |
| LMR | 0.00612 (1) | ||
| >2.96 | 11/30 (36.7%) | 68/407 (16.7%) | |
| ≤2.96 | 19/30 (63.3%) | 339/407 (83.3%) | |
| 0.02550 (2) | |||
| PNI | 35.1 [32.7, 44.5] | 38.4 [35.9, 42.9] | |
| PNI | 0.00000 (3) | ||
| >34.9 | 15/30 (50.0%) | 371/407 (91.2%) | |
| ≤34.9 | 15/30 (50.0%) | 36/407 (8.8%) | |
| 0.14105 (3) | |||
| BMI<18.5 kg/m2—yes | 4/30 (13.3%) | 103/407 (25.3%) | |
| Diagnosis | 0.55628 (4) | ||
| Hemorrhage | 5/30 (16.7%) | 41/407 (10.1%) | |
| Obstruction | 22/30 (73.3%) | 327/407 (80.3%) | |
| Peritonitis | 3/30 (10.0%) | 39/407 (9.6%) | |
| Tumoral site | 0.00537 (4) | ||
| A | 3/30 (10.0%) | 56/407 (13.8%) | |
| T | 4/30 (13.3%) | 27/407 (6.6%) | |
| D | 2/30 (6.7%) | 62/407 (15.2%) | |
| S | 17/30 (56.7%) | 116/407 (28.5%) | |
| R | 4/30 (13.3%) | 146/407 (35.9%) | |
| 0.09647 (3) | |||
| Synchronous tumors—yes | 1/13 (7.7%) | 2/379 (0.5%) | |
| Macroscopic invasion of | 0.00045 (1) | ||
| The adjacent organs—yes | 0/30 (0%) | 121/407 (29.7%) | |
| 0.03144 (1) | |||
| Synchronous metastasis—yes | 1/30 (3.3%) | 77/407 (18.9%) | |
| 0.61228 (3) | |||
| Carcinomatosis—yes | 0/30 (0%) | 14/407 (3.4%) | |
| Surgery—tumor resection | 0.00058 (1) | ||
| No | 5/30 (16.7%) | 200/407 (49.1%) | |
| Yes | 25/30 (83.3%) | 207/407 (50.9%) | |
| 0.102401 (1) | |||
| Lymph node dissection—yes | 9/30 (30.0%) | 73/407 (17.9%) | |
| Stage (pTNM) | 0.00115 (1) | ||
| 2 | 16/30 (53.3%) | 97/407 (23.8%) | |
| 3 | 12/30 (40.0%) | 222/407 (54.5%) | |
| 4 | 2/30 (6.7%) | 88/407 (21.6%) | |
| Grading | 0.52042 (1) | ||
| 1 | 8/30 (26.7%) | 110/407 (27%) | |
| 2 | 21/30 (70.0%) | 259/407 (63.6%) | |
| 3 | 01/30 (3.3%) | 38/407 (9.3%) | |
| 0.00250 (1) | |||
| Stoma reversal—yes | 11/26 (42.3%) | 49/278 (17.6%) | |
| 0.01836 (1) | |||
| Staged surgery—yes | 11/26 (42.3%) | 72/328 (22%) |
| N (%) of Survivors 1-Year (N = 243–50.9%) | N (%) of Survivors 3-Years (N = 83–17.4%) | N (%) of Survivors 5-Years (N = 35–7.3%) | |
|---|---|---|---|
| Age | |||
| ≤63 | 111/170 (65.3%) | 45/170 (26.5%) | 26/170 (15.3%) |
| >63 | 132/267 (49.4%) | 38/267 (14.2%) | 9/267 (3.4%) |
| LMR ≤ 2.96 | |||
| >2.96 | 79/79 (100%) | 61/79 (77.2%) | 16/79 (20.3%) |
| ≤2.96 | 164/358 (45.8%) | 22/358 (6.1%) | 19/358 (5.3%) |
| BMI < 18.5 kg/m2 | |||
| yes | 37/107 (34.6%) | 9/107 (8.4%) | 4/107 (3.7%) |
| no | 206/313 (62.4%) | 74/313 (22.4%) | 31/313 (9.4%) |
| Diagnosis | |||
| H | 27/46 (58.7%) | 10/46 (21.7%) | 6/46 (13.0%) |
| O | 200/349 (57.3%) | 68/349 (19.5%) | 26/349 (7.4%) |
| P | 16/42 (38.1%) | 5/42 (11.9%) | 3/42 (7.1%) |
| Tumor site | |||
| S | 101/133 (75.9%) | 36/133 (27.1%) | 19/133 (14.3%) |
| A | 38/59 (64.4%) | 17/59 (28.8%) | 4/59 (6.8%) |
| T | 17/31 (54.8%) | 7/31 (22.6%) | 4/31 (12.9%) |
| D | 32/64 (50.0%) | 7/64 (10.9%) | 2/64 (3.1%) |
| R | 55/150 (36.7%) | 16/150 (10.7%) | 6/150 (4.0%) |
| Macroscopic invasion | |||
| yes | 20/121 (16.5%) | 0/121 (0.0%) | 0/121 (0.0%) |
| no | 223/316 (70.6%) | 83/316 (26.3%) | 35/316 (11.1%) |
| Surgery—tumor resection | |||
| no | 57/205 (27.8%) | 16/205 (7.8%) | 7/205 (3.4%) |
| yes | 186/232 (80.2%) | 67/232 (28.9%) | 28/232 (12.1%) |
| Lymph node dissection | |||
| yes | 75/82 (91.5%) | 33/82 (40.2%) | 10/82 (12.2%) |
| no | 168/355 (47.3%) | 50/355 (14.1%) | 25/355 (0.07%) |
| Stage (pTNM) | |||
| 2 | 113/113 (100%) | 69/113 (61.1%) | 21/113 (18.6%) |
| 3 | 122/234 (52.1%) | 12/234 (5.1%) | 12/234 (5.1%) |
| 4 | 8/90 (8.9%) | 2/90 (2.2%) | 2/90 (2.2%) |
| Charlsonscore | |||
| ≤4 | 195/244 (79.9%) | 174/244 (30.3%) | 29/244 (11.9%) |
| >4 | 48/193 (24.8%) | 9/193 (4.7%) | 6/193 (3.1%) |
| Revised cardiac risk index (RCRI) | |||
| 0 | 28/28 (100%) | 26/28 (92.9%) | 14/28 (50.0%) |
| 1 | 136/154 (88.3%) | 53/154 (34.4%) | 19/154 (12.3%) |
| 2 | 78/247 (31.6%) | 3/247 (1.2%) | 2/247 (0.8%) |
| 3 | 1/8 (12.5%) | 1/8 (12.5%) | 0/8 (0.0%) |
| N (%) of Survivors | Kaplan–Meier Means (95%CI) | Kaplan –Meier p-Value (Log Rank) | Univariate Cox | Multivariate Cox | |||
|---|---|---|---|---|---|---|---|
| p-Value | HR [95% CI] | p-Value | HR [95% CI] | ||||
| Sex | 0.289791 | 0.30600 | 1.11 [0.90, 1.35] | ||||
| M [Ref] | 12/175 (6.9%) | 19.3 [17.1, 21.5] | |||||
| F | 18/262 (6.9%) | 20.5 [18.6, 22.4] | |||||
| Age | 0.00001 | 0.00002 | 1.57 [1.28, 1.92] | 0.00034 | 1.48 [1.19, 1.84] | ||
| ≤63 [Ref] | 23/170 (13.5%) | 24.2 [21.6, 26.8] | |||||
| >63 | 7/267 (2.6%) | 17.4 [15.8, 19] | |||||
| History of surgery | 0.02854 | 0.03452 | 1.11 [1.00, 1.23] | ||||
| Yes | 10/168 (6.0%) | 18.2 [15.9, 20.5] | |||||
| No [Ref] | 20/269 (7.4%) | 21.1 [19.3, 23] | |||||
| Cardiovascularcomorbidity | 0.00000 | 0.00000 | 1.29 [1.16, 1.43] | ||||
| Yes | 4/136 (2.9%) | 15.4 [13.3, 17.4] | |||||
| No [Ref] | 26/301 (8.6%) | 22.1 [20.3, 23.9] | |||||
| Cardiac rhythm disorders | 0.00191 | 0.00300 | 1.32 [1.09, 1.58] | ||||
| Yes | 0/31 (0.0%) | 13.5 [9.3, 17.8] | |||||
| No [Ref] | 30/406 (7.4%) | 20.5 [19.0, 22.0] | |||||
| Hepatic comorbidity | 0.39752 | 0.41584 | 1.18 [0.78, 1.77] | ||||
| Yes | 1/7 (14.3%) | 13.1 [9.89, 16.4] | |||||
| No [Ref] | 29/430 (6.7%) | 20.1 [18.6, 21.5] | |||||
| History of colon disease | 0.56059 | 0.57315 | 1.07 [0.84, 1.35] | ||||
| Yes | 1/19 (5.3%) | 18.6 [12.2, 25] | |||||
| No [Ref] | 29/418 (6.9%) | 20.1 [18.6, 21.6] | |||||
| Davies Score | 0.00000 | 0.00000 (*) | |||||
| 1 [Ref] | 8/20 (40.0%) | 41.1 [35.5, 46.7] | |||||
| 2 | 18/169 (10.7%) | 28.4 [26, 30.8] | 0.01631 | 2.05 [1.14, 3.70] | |||
| 3 | 4/184 (2.2%) | 14.6 [13.1, 16.2] | 0.00000 | 5.68 [3.14, 10.3] | |||
| 4 | 0/64 (0.0%) | 7.28 [6.63, 7.94] | 0.00000 | 27.1 [14.1, 51.9] | |||
| Charlson Score | 0.00000 | 0.00000 | 3.44 [2.79, 4.25] | ||||
| ≤4 [Ref] | 25/244 (10.2%) | 26.6 [24.6, 28.6] | |||||
| >4 | 5/193 (2.6%) | 11.62 [10.3, 13] | |||||
| Age adjusted Charlson score | 0.00000 | 0.00000 | 2.76 [2.23, 3.42] | ||||
| ≤6 [Ref] | 24/161 (14.9%) | 29.4 [26.9, 32] | |||||
| >6 | 6/276 (2.2%) | 14.5 [13.2, 15.9] | |||||
| RCRI | 0.00000 | 0.00000 (*) | |||||
| 0 [Ref] | 11/28 (39.3%) | 50.2 [46.3, 54.1] | |||||
| 1 | 17/154 (11.0%) | 29.4 [27.1, 31.8] | 0.00001 | 3.12 [1.87, 5.22] | 0.12970 | 1.52 [0.89, 2.62] | |
| 2 | 2/247 (0.8%) | 11.2 [10.6, 11.8] | 0.00000 | 21.8 [12.5, 37.9] | 0.00000 | 6.49 [3.53, 12.0] | |
| 3 | 0/8 (0.0%) | 8.88 [0.4, 17.4] | 0.00000 | 18.3 [7.76, 43.2] | 0.00022 | 6.51 [2.41, 17.6] | |
| BMI < 18.5 kg/m2 | 0.00000 | 0.00000 | 1.39 [1.24, 1.56] | ||||
| yes | 4/107 (3.7%) | 13.8 [11.6, 15.9] | |||||
| No [Ref] | 26/313 (7.9%) | 22.1 [204, 23.8] | |||||
| Anemia | 0.00000 | 0.00001 | 1.27 [1.14, 1.41] | ||||
| Yes | 21/296 (7.1%) | 17.4 [15.9, 19] | |||||
| No [Ref] | 9/141 (6.4%) | 25.4 [22.6, 28.2] | |||||
| WBC-aN | 0.19796 | 0.21356 | 1.09 [0.94, 1.28] | ||||
| Yes | 5/55 (9.1%) | 17.5 [13.9, 21.1] | |||||
| No [Ref] | 25/382 {6.5%) | 20.4 [18.8, 21.9] | |||||
| Platelets-aN | 0.39752 | 0.41584 | 1.18 [0.79, 1.77] | ||||
| Yes | 1/7 (0.0%) | 13.1 [9.9, 16.4] | |||||
| No [Ref] | 29/430 (2.9%) | 201 [18.6, 21.5] | |||||
| NLR | 0.00000 | 0.00000 | 6.85 [5.21, 8.99] | ||||
| ≤2.61 [Ref] | 28/152 (18.4%) | 34.51 [31.9, 37.2] | |||||
| >2.61 | 2/285 (0.7%) | 12.37 [11.6, 13.1] | |||||
| PLR | 0.00000 | 0.00000 | 10.4 [7.24, 15.0] | ||||
| ≤140 [Ref] | 19/104 (18.3%) | 40.2 [37.4, 43.0] | |||||
| >140 | 11/333 (3.3%) | 13.5 [12.7, 14.3] | |||||
| LMR | 0.00000 | 0.00000 | 17.5 [11.1, 27.8] | 0.00000 | 13.1 [7.69, 22.4] | ||
| >2.96 [Ref] | 11/79 (13.9%) | 44.6 [42.2, 47] | |||||
| ≤2.96 | 19/358 (5.3%) | 14.1 [13.3, 14.9] | |||||
| PNI | 0.01325 | 0.01727 | 1.52 [1.07, 2.16] | ||||
| >34.9 [Ref] | 15/386 (3.9%) | 20.5 [19, 21.9] | |||||
| ≤34.9 | 15/51 (29.4%) | 13.1 [10.5, 15.7] | |||||
| Diagnosis | 0.10014 | 0.11830 (*) | |||||
| H [Ref] | 5/46 (10.9%) | 22. 0 [17.0, 27] | |||||
| O | 22/349 (6.3%) | 20.3 [18.7, 21. 9] | 0.39284 | 1.15 [0.83, 1.60] | 0.00000 | 2.13 [1.46, 3.11] | |
| P | 3/42 (7.1%) | 16.0 [11.8, 20.1] | 0.04828 | 1.56 [1.00, 2.43] | 0.00000 | 3.59 [2.20, 5.87] | |
| Tumor site | 0.00000 | 0.00000 (*) | |||||
| S [Ref] | 17/133 (12.8%) | 25.1 [22.4, 27.9] | |||||
| A | 3/59 (5.1%) | 23.6 [19.6, 27.6] | 0.37414 | 1.15 [0.84, 1.59] | |||
| T | 4/31 (12.9%) | 21.0 [15.4, 26.5] | 0.23380 | 1.29 [0.84, 1.96] | |||
| D | 3/64 (3.1%) | 15.9 [13.2, 18.6] | 0.00020 | 1.80 [1.32, 2.46] | |||
| R | 4/150 (2.7%) | 15.4 [13.2, 17.6] | 0.00000 | 1.90 [1.48, 2.43] | |||
| Macroscopic invasion | 0.00000 | 0.00000 | 2.15 [1.90, 2.42] | 0.00000 | 2.60 [2.02, 3.34] | ||
| Yes | 0/121 (0.0%) | 9.36 [8.58, 10.2] | |||||
| No [Ref] | 30/316 (9.5%) | 24.1 [22.3, 25.9] | |||||
| Synchronous metastasis | 0.00000 | 0.00000 | 2.16 [1.88, 2.49] | ||||
| Yes | 1/78 (1.3%) | 9 [7.7, 10.3] | |||||
| No [Ref] | 29/359 (8.1%) | 22.4 [20.8, 24] | |||||
| Carcinomatosis | 0.00000 | 0.00000 | 2.73 [2.06, 3.62] | ||||
| Yes | 0/14 (0.0%) | 6.5 [5.34, 7.66] | |||||
| No [Ref] | 30/423 (7.1%) | 20.5 [19, 21.9] | |||||
| Surgery—tumor resection | 0.00000 | 0.00000 | 2.49 [2.04, 3.05] | 0.00000 | 2.46 [1.94, 3.11] | ||
| No | 5/205 (2.4%) | 13.3 [11.6, 15.0] | |||||
| Yes [Ref] | 25/232 (10.8%) | 25.9 [23.9, 27.8] | |||||
| Lymph node dissection | 0.00000 | 0.00000 | 1.90 [1.47, 2.46] | ||||
| Yes [Ref] | 9/82 (11.0%) | 29.8 [26.6, 33.0] | |||||
| No | 21/355 (5.9%) | 17.8 [16.2, 19.3] | |||||
| Stage (pTNM) | 0.00000 | 0.00000 (*) | |||||
| 2 [Ref] | 16/113 (14.2%) | 40.3 [38.0, 42.5] | |||||
| 3 | 12/234 (5.1%) | 13.9 [13.1, 14.6] | 0.00000 | 22.4 [13.8, 36.4] | |||
| 4 | 1/90 (2.2%) | 8.52 [7.82, 9.22] | 0.00000 | 70.7 [41.3, 121] | |||
| Grading | 0.00454 | 0.00719 (*) | |||||
| 1 [Ref] | 8/118 (6.8%) | 21.3 [18.4, 24.2] | |||||
| 2 | 21/280 (7.5%) | 20.3 [18.6, 22.2] | 0.54297 | 1.07 [0.85, 1.34] | |||
| 3 | 1/39 (2.6%) | 13.6 [10.5, 16.8] | 0.00231 | 1.78 [1.23, 2.59] | |||
| Staged surgery | 0.00000 | 0.00000 | 0.25 [0.20, 0.32] | ||||
| Yes | 11/83 (13.3%) | 37.6 [34.4, 40.7] | |||||
| No [Ref] | 15/271 (5.5%) | 11.5 [10.8, 12.2] | |||||
| Stoma reversal | 0.00000 | 0.00000 | 0.20 [0.14, 0.29] | ||||
| Yes | 11/60 (18.3%) | 39.2 [36.0, 42.5] | |||||
| No [Ref] | 15/244 (6.1%) | 11.8 [11.1, 12.6] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, G.B.; Firescu, D.; Mihailov, R.; Constantin, I.; Ștefanopol, I.A.; Iordan, D.A.; Ștefănescu, B.I.; Bîrlă, R.; Panaitescu, E. A Novel Clinical Nomogram for Predicting Overall Survival in Patients with Emergency Surgery for Colorectal Cancer. J. Pers. Med. 2023, 13, 575. https://doi.org/10.3390/jpm13040575
Constantin GB, Firescu D, Mihailov R, Constantin I, Ștefanopol IA, Iordan DA, Ștefănescu BI, Bîrlă R, Panaitescu E. A Novel Clinical Nomogram for Predicting Overall Survival in Patients with Emergency Surgery for Colorectal Cancer. Journal of Personalized Medicine. 2023; 13(4):575. https://doi.org/10.3390/jpm13040575
Chicago/Turabian StyleConstantin, Georgiana Bianca, Dorel Firescu, Raul Mihailov, Iulian Constantin, Ioana Anca Ștefanopol, Daniel Andrei Iordan, Bogdan Ioan Ștefănescu, Rodica Bîrlă, and Eugenia Panaitescu. 2023. "A Novel Clinical Nomogram for Predicting Overall Survival in Patients with Emergency Surgery for Colorectal Cancer" Journal of Personalized Medicine 13, no. 4: 575. https://doi.org/10.3390/jpm13040575
APA StyleConstantin, G. B., Firescu, D., Mihailov, R., Constantin, I., Ștefanopol, I. A., Iordan, D. A., Ștefănescu, B. I., Bîrlă, R., & Panaitescu, E. (2023). A Novel Clinical Nomogram for Predicting Overall Survival in Patients with Emergency Surgery for Colorectal Cancer. Journal of Personalized Medicine, 13(4), 575. https://doi.org/10.3390/jpm13040575








