Abstract
The advent of immunotherapy, especially immune checkpoint inhibitors (ICIs), has revolutionized antitumor therapy. Programmed cell death receptor 1 (PD-1) and programmed cell death ligand 1 (PD-L1) are among the most promising targets for encouraging the immune system to eliminate cancer cells. PD-1/PD-L1 have made clinical remission for numerous solid tumors, including metastatic triple-negative breast cancer (TNBC). In recent years, integrating PD-1/PD-L1 inhibitors into existing treatments in early-stage TNBC has attracted wide attention. Herein, we summarize the clinical benefit of PD-1/PD-L1 inhibitors plus neoadjuvant chemotherapy, adjuvant chemotherapy, and targeted therapy in early-stage TNBC. Possible immunotherapy biomarkers, immune-related adverse events (irAEs), and the key challenges faced in TNBC anti-PD-1/PD-L1 therapy are also concluded. Numerous studies on immunotherapy are ongoing, and PD-1/PD-L1 inhibitors have demonstrated great clinical prospects in early-stage TNBC. To maximize the efficacy of anti-PD-1/PD-L1 therapy, further research into the challenges which still exist is necessary.
1. Introduction
Breast cancer exhibits the top incidence rate of all malignancies, ranking as the chief cause of cancer mortality in women [1]. Among all breast cancers, the subtype triple-negative breast cancer (TNBC) makes up 15 to 20%, which is defined as estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and human epidermal growth factor receptor-2 (HER-2)-negative [2]. TNBC is well characterized by a poorer prognosis, higher possibility for relapse, and earlier age of onset than other subtypes [3,4]. Therefore, it is imperative to explore novel and efficacious remedies for TNBC.
Recent progress in this regard has been achieved in the utilization of immunotherapy aimed at enhancing antitumor immunity to eliminate malignant cells, which has been deemed as a significant breakthrough in revolutionizing antitumor therapy. As a major category of immunotherapy, immune checkpoint inhibitors (ICIs) are now entering extensive clinical practice. Tumor cells can escape immune surveillance by exploiting immune checkpoints, which contribute to activating coinhibitory signaling pathways and immune tolerance [5]. Programmed cell death receptor 1 (PD-1) and programmed cell death ligand 1 (PD-L1) are currently among the most promising ICI targets to be blocked to attack malignant cells through an immune-mediated process [6,7].
PD-1/PD-L1 inhibitors have shown substantial clinical benefits in malignancies that occur in lung, kidney, bladder, and skin [8,9,10,11]. Compared with other subtypes, considerable evidence has shown that immunotherapy may have a better response rate in TNBC. There exist more tumor-infiltrating lymphocytes (TILs), larger numbers of mutations, and relatively higher PD-L1 expression in TNBC [12,13]. Additionally, higher levels of TILs indicate better outcomes in TNBC [14]. ICIs can strengthen the progress of immune clearance, thus making the use of PD-1/PD-L1 inhibitors a possible strategy against TNBC [15].
Monumental progress has been seen in the field of combining ICIs with adjuvant chemotherapy in metastatic TNBC, although there are some limitations in monotherapy with PD-1/PD-L1 inhibitors [16,17]. In 2019, the European Commission and the Food and Drug Administration (FDA) approved atezolizumab combined with nab-paclitaxel in metastatic TNBC with positive PD-L1, which established the initial immunotherapy regimen approved for breast cancer patients [18]. Inspiring results in metastatic TNBC greatly boosted the subsequent investigation into the usage of PD-1/PD-L1 monoclonal antibodies in early-stage TNBC clinical practice, and more encouraging data has recently emerged. Although previous reviews and meta-analyses have provided insights into anti-PD-1/PD-L1 therapy in cancer, the efficacy of PD-1/PD-L1 inhibitors, in early-stage TNBC in particular, has never been systematically reviewed [19,20,21]. In this article, immunotherapies based on PD-1/PD-L1 inhibitors in early-stage TNBC are summarized, including neoadjuvant chemotherapy, adjuvant chemotherapy, and targeted therapy. The latest results from clinical trials are summarized, as well as possible immunotherapy biomarkers, immune-related adverse events (irAEs), and the challenges which are being faced in the field. To succeed in the application of PD-1/PD-L1 inhibitors in early-stage TNBC, efforts in both basic research and clinical development are needed.
2. The Rationale for PD-1/PD-L1 Blockade
PD-1 and PD-L1 are demonstrated as members of the immunoglobulin (Ig) superfamily, and both have been identified as transmembrane proteins. PD-1 can be detected on the activated T-cell membrane surface [22,23]. The presence of PD-L1 in normal tissue is well-documented, acting as the ligand of PD-1. T-cell activity is inhibited by PD-1 and PD-L1 interactions, resulting in immune tolerance. The PD-1/PD-L1 pathway is a crucial element of physiological immune homeostasis [24]. However, abnormally expressed PD-L1 has been detected in multiple kinds of carcinoma, including breast cancer, colorectal cancer, lung cancer, and melanoma [25]. It may have connections with cytokines in the tumor microenvironment (TME), especially interferon-γ (IFN-γ). When undergoing an immune attack, IFN-γ is the prominent soluble cytokine inducing the expression of PD-L1 in tumor cells [26]. As IFN-γ binds to its receptor, the enhanced expression of transcription factors upregulates PD-L1 transcription and translation in cancer cells [27].
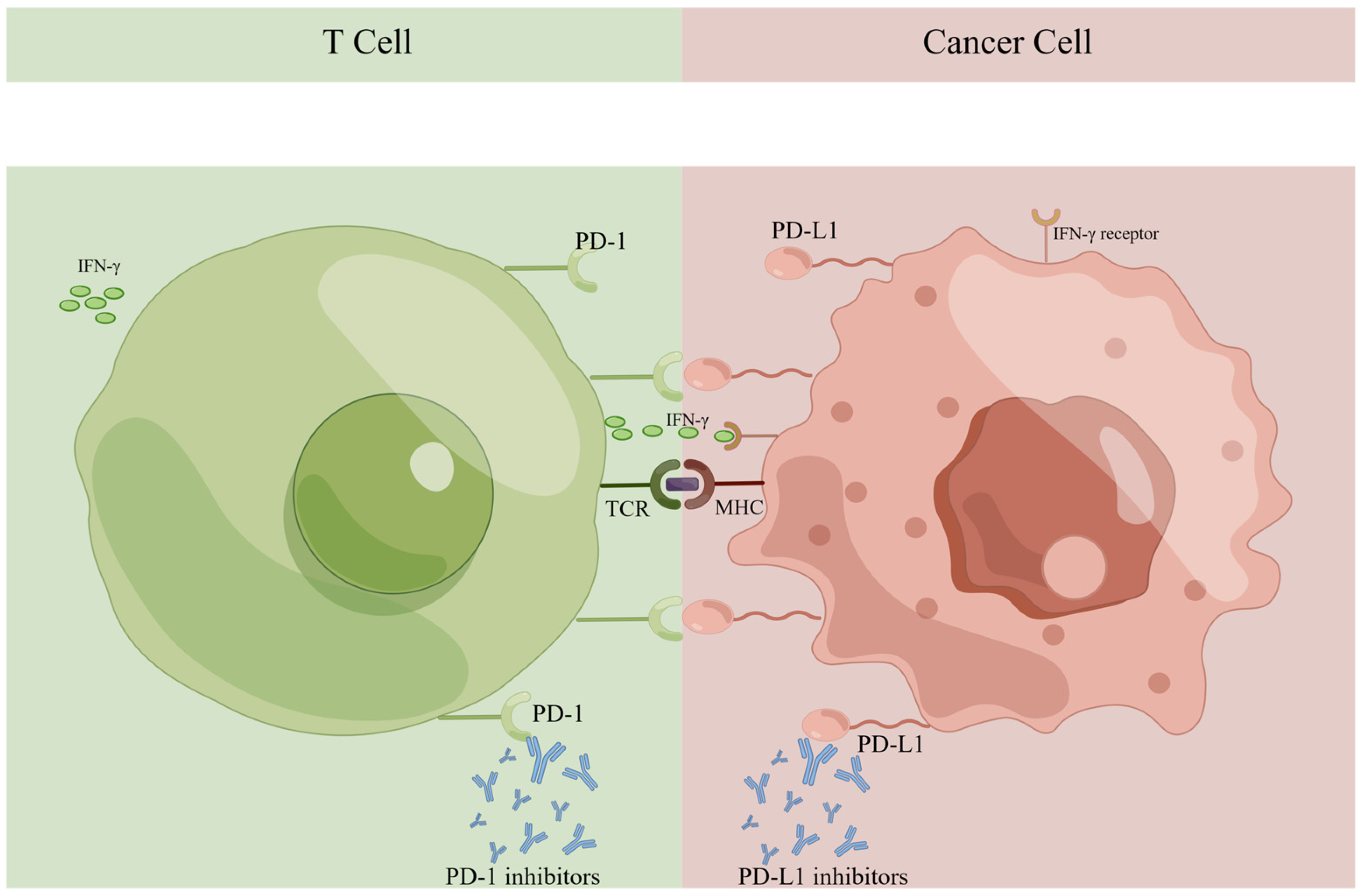
The proliferation of lymphocytes mediated by the T-cell receptor (TCR) is suppressed when PD-1 and PD-L1 are engaged, hence inducing immunosurveillance [28,29]. The higher the expression of PD-L1 in tumors, the more immune-suppressive the TME may become, as shown in Figure 1. Inhibiting PD-1 or PD-L1 can contribute to antitumor immunity and induce carcinoma regression by various pathways, including (1) reinvigorating lymphocyte activity and cytotoxic cytokine release; (2) activating and proliferating CD8+ T-cells specific to tumor antigens; (3) dislodging the apoptosis of lymphocytes induced by PD-1/PD-L1 interaction; and (4) boosting the immune discrimination of tumor cells [30,31].
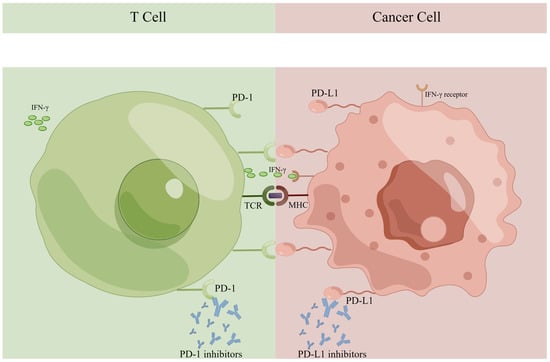
Figure 1.
Immune checkpoints PD-1 and PD-L1 in the tumor microenvironment. PD-1, programmed cell death receptor 1; PD-L1, programmed cell death ligand 1; IFN-γ, interferon-γ; TCR, T-cell receptor; MHC, major histocompatibility complex.
In the new era of personalized medicine, PD-1/PD-L1 inhibitors are attracting ever-increasing attention. PD-1 antibodies (i.e., pembrolizumab and nivolumab) and PD-L1 antibodies (i.e., atezolizumab, durvalumab, and avelumab) have generated effective efficacy in multiple kinds of malignancies [8,9,10,11,18]. Following the permission of atezolizumab in metastatic TNBC therapy, integrating PD-1/PD-L1 inhibitors into early-stage TNBC treatment to improve prognosis has entered the research spotlight. In recent years, a number of clinical trials have been conducted to evaluate the clinical profit of PD-1/PD-L1 inhibitors in early-stage TNBC. The major trend in current trials is to combine anti-PD-1/PD-L1 therapy with neoadjuvant chemotherapy, adjuvant therapy, or targeted therapy.
3. PD-1/PD-L1 Inhibitors Plus Neoadjuvant Chemotherapy
Neoadjuvant therapy is the most recommended strategy for treating high-risk, early-stage TNBC [32,33]. It has also been demonstrated that ICIs plus neoadjuvant chemotherapy may be promising in treating early-stage TNBC, and nine representative studies in this aspect are shown in Table 1. For participants receiving neoadjuvant chemotherapy, not only is the pathological complete response (pCR) rate estimated as an observation endpoint, but some survival and safety indicators are also included.

Table 1.
PD-1/PD-L1 inhibitors plus neoadjuvant chemotherapy trials in early-stage TNBC.
The GeparNuevo study (NCT02685059) is a phase II trial with a placebo control. It consists of 174 primary TNBC patients in clinical stage I, II, or III. The participants were treated with nab-paclitaxel, epirubicin and cyclophosphamide, plus durvalumab or placebo. Although the patients receiving durvalumab witnessed a slight increase in their pCR rate compared with the patients who were receiving the placebo, there was no statistical significance (53.4% versus [vs.] 44.2%, p = 0.287). Favorably, there was a statistically significant tendency for improved three-year survival, including invasive disease-free survival (iDFS), distant disease-free survival (DDFS), overall survival (OS), and no new safety signals occurring. In the durvalumab group and placebo group, iDFS was 85.6% vs. 77.2% (p = 0.036), DDFS was 91.7% vs. 78.4% (p = 0.005), and OS was 95.2% vs. 83.5% (p = 0.006), respectively [34,35,36].
In the phase II clinical trial I-SPY2 (NCT01042379), a subgroup was designed to investigate pembrolizumab with neoadjuvant chemotherapy. In total, 69 subjects with early-stage breast cancer were randomized to different treatment groups of pembrolizumab combined with paclitaxel, adriamycin, and cyclophosphamide, while 181 patients who accepted standard neoadjuvant chemotherapy were taken as the control group. For the pembrolizumab vs. control arm in the total population, the pCR rate was 44% vs. 17%. A pCR rate of 60% was achieved with pembrolizumab compared with 22% with placebo. In those at high risk in early-stage TNBC, pembrolizumab exceeded the estimated pCR rates by more than twice, indicating a promising clinical future for pembrolizumab [37].
The NeoPACT phase II trial (NCT03639948) aimed to investigate neoadjuvant pembrolizumab with carboplatin and docetaxel in 117 TNBC patients. The study measured pCR rates as a primary endpoint, while event-free survival (EFS) rates and residual tumor burden (RCB) were taken as the secondary endpoints. No patient experienced disease progression during the neoadjuvant therapy. The pCR rate of pembrolizumab plus carboplatin and docetaxel neoadjuvant therapy was 58%, which is comparable to the pCR rate seen in neoadjuvant immunotherapy with anthracycline-based chemotherapy. The 2-year EFS rate was 89% for the total population, 98% for the pCR group, and 82% for the non-pCR group. These results support that it is not inferior to integrate pembrolizumab with a non-anthracycline neoadjuvant chemotherapy strategy, which may inspire new clinical trials in early-stage TNBC [38].
The KEYNOTE-173 phase Ib trial (NCT02622074) is a multicohort study which evaluated six regimens of pembrolizumab with chemotherapy as neoadjuvant treatment. In total, 60 early-stage TNBC patients were registered to six cohorts in the trial, and there was a range of pCR rates from 49% to 71%. The overall pCR rate ended at 60%, and a range of 80% to 100% 12-month EFS and OS rates across all cohorts (100% for four cohorts) was observed. The study demonstrated the promising antitumor activity of pembrolizumab plus neoadjuvant chemotherapy, and a positive connection was proven between PD-L1 expression, levels of stromal TILs (sTILs), and pCR rates [39].
On the basis of the KEYNOTE-173 study, the phase III KEYNOTE-522 trial (NCT03036488) was conducted. This is a study of neoadjuvant and adjuvant pembrolizumab with chemotherapy in early-stage TNBC patients. In total 1174 patients who were previously untreated, non-metastatic, and centrally confirmed with TNBC were recruited and randomized to the pembrolizumab group and control group in a 2:1 ratio. The patients in the experiment group were administered pembrolizumab, with paclitaxel and carboplatin, followed by pembrolizumab for four cycles plus anthracyclines as neoadjuvant therapy. Following definitive surgery, nine cycles of pembrolizumab and chemotherapy were administrated as an adjuvant treatment. For patients in the control group, pembrolizumab was replaced by a placebo. The dual primary endpoints included pCR and EFS. In the primary analysis, the pCR rates were 64.8% and 51.2% of the experiment group and the control group (p = 0.00055). The interim analysis also revealed that pembrolizumab with chemotherapy could significantly increase pCR rates regardless of the PD-L1 status expressed by the breast tumor. PD-L1-positive patients had a 14.2% increase in pCR (68.9% vs. 54.9%, 95% confidence interval [CI]: 5.3% to 23.1%), and PD-L1-negative patients had an 18.3% increase (45.3% vs. 30.3%, 95% CI: −3.3% to 36.8%). An analysis of the subgroups demonstrated that pembrolizumab is more likely to benefit patients with a heavier tumor burden, in a late stage of the disease, and with positive lymph nodes. In the fourth interim analysis, the estimated 36-month EFS rate in the experiment group was 84.5%, while it was 76.8% in the control group. Previous studies have confirmed that RCB grades after neoadjuvant chemotherapy could be used as a prognosis biomarker [40,41,42]. The KEYNOTE-522 study analyzed the correlation between RCB grades and EFS, which was reported at the American Society of Clinical Oncology (ASCO) meeting in 2022. The study suggested that the addition of immunotherapy reduced RCB scores and improved EFS. Based on a series of promising results and the relatively mild side effects of pembrolizumab from KEYNOTE-522 and other studies, both the European Medicines Agency (EMA) and the FDA approved joining pembrolizumab and chemotherapy for neoadjuvant therapy and subsequent adjuvant therapy, establishing the first immunotherapy regimen approved in high-risk, early-stage TNBC [36,43].
The NeoTRIPaPDL1 phase III trial (NCT02620280) adopted the approach of integrating atezolizumab with carboplatin and nab-paclitaxel. In total, 280 early-stage TNBC patients were recruited and assigned randomly to undergo chemotherapy with or without atezolizumab as a neoadjuvant therapy. The trial aimed to compare EFS, as well as the rate of pCR. In the intention-to-treat (ITT) population, the pCR rate increased by 2.7% (43.5% vs. 40.8%) in the experiment group, showing no statistical significance (p = 0.066), which doubted immunotherapy in early-stage TNBC patients. The study also defined a group of “immune-rich” patients who had higher PD-L1 expressions and more TILs in the TME. Positively, the 2021 European Society for Medical Oncology (ESMO) congress reported that the pCR rate in “immune-rich” patients increased (87% vs. 72%) with the atezolizumab treatment. A long-term follow-up on EFS is necessary [44].
Another phase III study, IMpassion 031 (NCT03197935), aimed to assess the addition of atezolizumab to neoadjuvant chemotherapy in 333 early-stage TNBC patients. Patients were enrolled and equally randomized to atezolizumab and placebo groups. The primary endpoints were pCR rates among ITT and PD-L1-positive patients, while the secondary endpoints aimed to estimate EFS, DFS, and OS. There was a dramatic improvement in pCR rates in atezolizumab-containing groups, both in the ITT population (58% vs. 41%, p = 0.0044) and in PD-L1-positive participants (69% vs. 49%, p = 0.021). The existing results consolidated that atezolizumab combined with nab-paclitaxel and anthracycline can potentially enhance clinical benefit in neoadjuvant therapy for TNBC [45].
In addition, there are several ongoing trials exploring atezolizumab as a neoadjuvant therapy in early-stage TNBC. Atezolizumab combined with neoadjuvant chemotherapy and subsequent adjuvant atezolizumab for one year is being evaluated in the NSABP B-59 phase III study (NCT03281954). The trial allocated 1550 patients with stage T2 or T3 TNBC and randomized patients to the atezolizumab group or the placebo group. Patients in the atezolizumab group are receiving atezolizumab plus paclitaxel and carboplatin, followed by anthracycline. The placebo is replacing atezolizumab in the control group. EFS will be taken as the primary endpoint, and the secondary endpoints will be OS, the pCR rate, and DDFS [46]. Another ongoing phase II study MIRINAE (NCT03756298) aims to assess the clinical benefit of joining atezolizumab with capecitabine in TNBC patients who have completed neoadjuvant treatment but still present with residual tumors. In this study, 284 patients have been recruited to participate, and the major outcome is five-year disease-free survival (DFS). We are expecting favorable results to emerge from this study [47].
4. PD-1/PD-L1 Inhibitors Plus Adjuvant Chemotherapy
As a PD-1 inhibitor, pembrolizumab in early-stage, high-risk TNBC neoadjuvant therapy has been endorsed by the FDA. However, it remains unclear how immunotherapy could benefit early-stage TNBC patients. Clinical trials integrating PD-1/PD-L1 antibodies with adjuvant therapy are ongoing, as displayed in Table 2.

Table 2.
PD-1/PD-L1 inhibitors plus adjuvant chemotherapy trials in early-stage TNBC.
The IMpassion 030 trial (NCT03498716) is a prospective phase III study which aims to evaluate whether adjuvant atezolizumab in conjunction with anthracycline/paclitaxel chemotherapy is clinically beneficial for operable TNBC patients. The study was designed to enroll 2300 stage II or III TNBC patients. Patients are set to undergo atezolizumab and chemotherapy, or chemotherapy alone. iDFS has been set as the primary endpoint, while OS, DFS, recurrence-free interval (RFI), distant RFI, and adverse events are to be the secondary endpoints. An investigation and extensive analysis are underway to determine the results [48].
In the A-BRAVE trial (NCT02926196), avelumab is being assessed as an adjuvant treatment and post-neoadjuvant treatment. In this phase III study, 474 participants who have non-metastatic primary invasive high-risk TNBC were assigned randomly to the avelumab arm and the observation arm. Patients will be administered 200 mg of avelumab or be observed under the guidelines. DFS in the total population and DFS in PD-L1-positive patients were designed to be primary outcome measurements, while OS and safety profiles will be analyzed as the secondary outcome measurements [49].
Another phase III trial (NCT02954874) is investigating pembrolizumab as an adjuvant treatment for TNBC in the Southwest Oncology Group (SWOG 1418). A total of 1155 patients who have positive lymph nodes or residual tumors after neoadjuvant chemotherapy have been recruited, and all patients underwent their final breast surgery before being registered. In the observation arm, patients will be monitored at standard clinical intervals with no immunotherapy. In the pembrolizumab arm, patients will receive pembrolizumab. The primary endpoints are iDFS, the severity of fatigue, and physical function, and the secondary endpoints are OS, distant DFS, adverse events, etc.
More research into immunotherapy as an adjuvant treatment is required in order to clarify its clinical outlook.
5. PD-1/PD-L1 Inhibitors Plus Targeted Therapy
Following the in-depth understanding of gene mutations and molecular pathways in breast cancer, therapies targeting certain molecules make up a significant part of TNBC treatment. Various kinds of targeted therapies may help to stimulate antitumor immunity, hence making them potential strategies to rise above the innate resistance to PD-1/PD-L1 monoclonal antibodies [50]. Research on combining poly (ADP-ribose) polymerase (PARP) inhibitors, Akt inhibitors, vascular endothelial growth factor receptor (VEGFR) inhibitors, or cell cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors with PD-1/PD-L1 antibodies in early-stage TNBC is already underway, as displayed in Table 3.

Table 3.
PD-1/PD-L1 inhibitors plus targeted therapy trials in early-stage TNBC.
It has been shown in preclinical studies that PARP inhibition promotes neoantigen presentation and activates T-cells. As the proportion of TILs and PD-L1 expression are also upregulated by PARP inhibitors, PARP inhibitors are assumed to treat TNBC with PD-1/PD-L1 inhibitors [51,52]. Accessing the integration of ICIs and PARP inhibitors as a neoadjuvant setting was carried out by a subgroup in the I-SPY2 study (NCT01042379). In total, 372 high-risk patients with HER2-negative breast cancer were investigated, and 163 TNBC patients were included. Twenty-one TNBC patients were randomized to the experimental group, who received durvalumab, the PARP inhibitor olaparib, paclitaxel, and adriamycin/cyclophosphamide. The other 142 TNBC patients in the control group only underwent chemotherapy. Compared with the control group, the pCR rate of the experiment group nearly doubled (37% vs. 20%) in all participants. The extensive analysis of the TNBC subgroup revealed that the pCR rates were 47% vs. 27%, respectively. The results of this study further revealed that patients with TNBC can benefit from the combination of immunosuppressants and PARP inhibitors [53]. A window-of-opportunity clinical trial (NCT03594396) has recruited 54 participants with stage II/III TNBC; olaparib and durvalumab will be administered to patients before commencing standard neoadjuvant chemotherapy. The changes in tumor biology will be compared and analyzed as the primary endpoint, while the pCR rates, response rate, and adverse events are being collected as the secondary endpoints.
Preclinical studies have proven that the PI3K/Akt signaling pathway has a tough relationship with CD8+ T-cell differentiation and memory activity [54,55]. Inhibiting Akt is a promising way to regulate the immunosuppressive TME by reviving memory T-cells [56]. There are reasons to expect that combining PD-1/PD-L1 antibodies and Akt inhibitors may benefit breast cancer patients. The BARBICAN trial (NCT05498896) was organized to evaluate the addition of the Akt inhibitor ipatasertib to chemotherapy and atezolizumab in early-stage TNBC patients with and without PI3CA/Akt1/PTEN genetic mutations. In total, 146 TNBC patients were randomly assigned to the experimental group and control group. The participants received atezolizumab with paclitaxel, doxorubicin, and cyclophosphamide in the control group. In the experimental group, ipatasertib was administered to atezolizumab and chemotherapy. The pCR rates and five-year objective response rates (ORR) were set as the primary and secondary endpoints, respectively. In all patients, the pCR rates were 48.5% for the control group and 49.3% for the experiment group (p = 0.729). Congruously, the benefit of Akt inhibition was observed neither in PD-L1-positive nor PD-L1-negative subgroups [57].
The vascular endothelial growth factor (VEGF) is secreted by immune cells in the TME, including tumor-associated macrophages (TAM). Besides contributing to angiogenesis, the VEGF can act as an immunomodulator to promote local and systemic immunosuppressive TME. VEGFs can result in the suppression of antigen presentation, the stimulation of regulatory T-cells, and the activation of tumor-associated macrophages. Therefore, VEGFs can be considered immune suppressive [58,59]. A preclinical study proved that anti-angiogenic therapy can sensitize TNBC cell lines to PD-1 blockade in vitro [60]. As a novel treatment approach for cancer, combining targeting VEGFR with immunotherapy is set to enter clinical trials. The BRE-03 trial (NCT04427293) is a phase I window-of-opportunity trial which aims to enroll 12 patients with stage I, II, or III TNBC. Lenvatinib, a VEGFR inhibitor, will be administrated for a week, and 200 mg of pembrolizumab will be administered on the first day of the treatment. Infiltration of CD8+ TILs in primary tumors, representing a T-cell inflamed TME, will be measured as the primary outcome. Another phase II trial in China called NeoCAT aims to recruit 58 patients with operable invasive TNBC (T1cN1-2 or T2-4N0-2) and high proportions of TILs (>10% in baseline breast tumor). Eligible patients will receive the PD-1 inhibitor camrelizumab and the VEGFR inhibitor apatinib as neoadjuvant treatments. After eight cycles, the pCR rate will be analyzed as the primary endpoint. Secondary outcome measurements include ORR, breast conservation rate, emergent adverse events, etc.
CDK4/6 are the key factors in controlling the cell cycle, which can block the phosphorylation of retinoblastoma inhibitors and cause cell cycle arrest [61]. Studies have shown that CDK4/6 is correlated to the onset and progression of a variety of malignancies [62]. Inhibitors of CDK4/6 have demonstrated initial success in treating hormone receptor-positive breast cancer and a diversity of malignancies [63,64]. The treatment with CDK4/6 inhibitors activates CD8+ T-cells and heightens the effect of anti-PD-1/PD-L1 agents, indicating the theoretical possibility of the addition of CDK4/6 inhibitors to PD-1/PD-L1 antibodies [65,66]. In a phase II single-arm study (NCT05112536), the CDK4/6 inhibitor trilaciclib combined with chemotherapy and pembrolizumab will be evaluated for its mechanism of action, safety, and efficacy. A total of 24 patients with TNBC will be administered trilaciclib, pembrolizumab, anthracycline, and paclitaxel. After taking a single dose of trilaciclib for seven days as the lead-in, the primary outcome will be the shift in the ratio of CD8+ T-cells to regulatory T-cells (Tregs) in cancer. The pCR rates and emergent adverse events are set as the secondary endpoints.
Despite the fact that anti-PD-1/PD-L1 agents in conjunction with targeted therapy need to be explored further in early-stage TNBC, clinical research on this topic already shows promising prospects.
6. Potential Biomarkers of Immunotherapy Response in TNBC
Despite the progress made in anti-PD-1/PD-L1 treatment, some patients still show no response to immunotherapy regimens. The appropriate biomarkers of immunotherapy response can be a significant basis for initiating ICIs treatment, which has been under exploration in TNBC clinical trials for many years. Emerging potential biomarkers include PD-L1 expression, tumor mutational burden (TMB), TILs, and mismatch repair (MMR) deficiency [67,68].
In previous clinical studies in metastatic TNBC, immunotherapy was more likely to work for PD-L1-positive patients, as discovered by the KEYNOTE-012 study and IMpassion 130 study [18,69]. Contradictorily, it is still possible for PD-L1-negative patients to show sensitivity to ICIs. In the KEYNOTE-522 and the NeoTRIPaPDL1 studies, response to ICIs showed little to no correlation with PD-L1 expression [43,44]. To make matters worse, there is currently no standard assay to detect PD-L1. Commonly used immunohistochemical assays include SP263, DAKO 22C3, 28–8, Ventana SP142, and 73–10 assays. Adopting the different expression-scoring criteria of PD-L1 in different clinical trials leads to different conclusions [70,71]. As a result, it will be suggested that the patient receives atezolizumab plus nab-paclitaxel therapy if the patient is PD-L1 positive in the SP142 assay (score ≥ 1%). However, if one is PD-L1 positive in the 22C3 test (combined positive score [CPS] ≥ 10), it would be suggested that the patient receives pembrolizumab therapy [72,73,74]. Owing to this, the function of PD-L1 expression as an immunotherapy biomarker in TNBC is still under debate. Moreover, a few PD-L1-negative patients do indeed benefit from PD-1/PD-L1 blockade. Because of this finding, there is a need to screen more biomarkers.
In tumors, TMB denotes how many somatic mutations there are in gene-coding regions. Elevated TMB contributes to more neoantigens and triggers an intrinsic immune response as it is recognized by the immune system. In solid tumors, high TMB refers to > 10 mutations per megabase of DNA (mut/Mb), and it is connected with sensitivity to anti-PD-1/L1 agents across multiple malignancies [75,76]. Higher TMB also correlates with longer progression-free survival (PFS) in metastatic TNBC patients treated with ICIs [77]. The prospect of using TMB as an immunotherapeutic efficacy biomarker is promising.
Higher TILs levels have also shown an association with improved responses to ICIs. In the KEYNOTE-119 trial in metastatic TNBC, patients who had high TILs ended with an improved pembrolizumab response and survival [78]. Similarly, researchers found that TNBC patients in the early stage who had high CD8+ T-cell density and a high expression of immune-related genes concluded their treatment with higher pCR rates of durvalumab in the neoadjuvant setting [14,79]. PD-L1-positive patients showed longer PFS or OS if their tumors were infiltrated with high TILs in the IMpassion 130 trial [80]. The conjunction of multiple indicators is important, as this trial confirmed.
The MMR system makes up a crucial part in sustaining the steadiness of the genome, which is divided into two categories: deficiency and proficiency. A deficiency of MMR leads to many somatic mutations occurring in simple repetitive sequences, resulting in microsatellite instability (MSI) [81]. It is more likely for MSI tumors to benefit from anti-PD-1/PD-L1 therapy [82]. For inoperable or metastatic solid carcinoma patients with elevated MSI, pembrolizumab was authorized by the FDA in May 2017. Nonetheless, dMMR in breast cancer was detected at only less than 2%, and the value of dMMR for PD-1/PD-L1 blockade therapy in TNBC still requires further investigation [83]. Taken together, immunotherapy biomarkers indicating prognosis in TNBC require further exploration, and the combination of different markers is a possible approach which may be successful.
7. IrAEs
IrAEs may exist in multiple organs in patients receiving anti-PD-1/PD-L1 treatment. The pathophysiology and molecular mechanism of irAEs are not yet completely understood. It was theoretically proven that irAEs may be caused by complex interactions between multiple factors including T-cells, antibodies, and cytokines that are autoreactive [84]. For instance, there is T-cell infiltration in both tumor and normal tissue. Activated T-cell upregulates the release of inflammatory cytokines, and the antigens shared by cancer and normal tissue can contribute to the progress of irAEs [85].
IrAEs vary from the targets of immunotherapy [86]. An onset of irAEs can arise at any time after initiating immunotherapy, in the range of weeks to months [87]. For patients who receive PD-1/PD-L1 antibodies, the observed adverse effects are involved mainly in the endocrine system, the digestive system, and the respiratory system. In addition, dermatologic toxicity and rare immune-related adverse events have also been reported [88].
The most common endocrine toxicities resulting from PD-1/PD-L1 inhibitors are hypothyroidism, hyperthyroidism, thyroiditis, hypophysitis, primary adrenal insufficiency, and insulin-dependent diabetes mellitus [89]. Hyperthyroidism occurs more frequently, which is often unabiding and may occur at the head of hypothyroidism. In TNBC studies using ICIs, the reported incidence of hypothyroidism ranged from 4% to 18.0%, whereas hyperthyroidism ranged from 1% to 4% [90,91]. An insufficiency in the adrenal gland can either be primary or secondary, and secondary adrenal insufficiency is known as hypophysitis. In both the ISPY-2 trial and the KEYNOTE-522 trial, there were participants who experienced adrenal insufficiency. Diabetes mellitus occurs at a relatively low frequency, at 1–2% across ICIs regimens [92]. In most endocrine toxicity cases, immunotherapy can be continued and high-dose corticoids are rarely needed. Meanwhile, a lifelong replacement is usually required for persisting endocrine deficiency [93].
Dermatologic toxicity is another irAE in TNBC immunotherapy, which has diverse clinical manifestations. Maculopapular rashes are believed to be the most frequently occurring. Dermatologic toxicity is rarely severe and usually does not discontinue ICIs therapy [94]. Hepatitis often presents as abnormal transaminases, occurring in 1–6.3% of TNBC participants during ICIs therapy [95]. The discontinuation of PD-1/PD-L1 antibodies ought to be taken into account when alanine transaminase or aspartate aminotransferase reaches four times more than the upper limit of what is deemed normal [93,96]. Gastrointestinal toxicity is often presented with diarrhea and colitis. In patients administrated with PD-1/PD-L1 inhibitors, diarrhea is more common than colitis [97]. Pneumonitis is a fatal pulmonary toxicity, with diverse clinical features and imaging findings. Pneumonitis can happen later than other irAEs after months of therapy initiation. There is a 1% to 4% incidence of immune-related pneumonitis among breast cancer patients [91,98]. Other rare irAEs have been reported, including toxicity towards the nervous system, heart, kidney, oculus, and hematopoiesis [99].
The clinical features of irAEs exhibit diversification among individuals and multiple systems are involved. Despite the fact that most irAEs can be treated by temporarily suppressing the immune system with corticoids or other immunosuppressant agents, severe irAEs lead to irreversible tissue damage, the discontinuation of treatment, or even death [100]. To manage irAEs, a deeper understanding is needed to optimize curative effects and prognosis.
8. Challenges in TNBC Immunotherapy
Up until now, PD-1/PD-L1 inhibitors have been suggested as a second-line therapy for PD-L1-positive TNBC patients. To further promote the progress of anti-PD-1/PD-L1 therapy in the quest for an improved prognosis, we should be acutely aware of the challenges that we face.
The mechanisms of primary and secondary immune escape to ICIs remain unclear. Some PD-L1-positive patients failed to respond to PD-1/PD-L1 inhibitors, referring to a primary immune escape. After responding to ICIs for years, patients may suffer from secondary immune escape, which results in tumor regrowth [101]. There is currently no complete understanding of the molecular drivers involved in the immune escape, however, they are considered to be crucial parts of resistance to immunotherapy. Multiple studies have outlined a variety of pathways that may lead to primary immune escape, including the transforming growth factor-β (TGFβ) signaling pathway, the Wnt-β-catenin signaling pathway, and the VEGF/VEGFR axis [102,103]. Ongoing clinical trials intend to combine targeted therapy with anti-PD-1/PD-L1 agents to overcome immune escape to ICIs, which may stimulate the immunogenicity of tumors.
In order to be an indication for a targeted population, biomarkers require further optimization. A number of trials have been conducted to analyze biomarkers that can be utilized to maximize personal benefits. However, immunotherapy biomarkers can perform differently under different circumstances. Although the 22C3 test of PD-L1 expression has been included in the National Comprehensive Cancer Network (NCCN) guidelines, some PD-L1-negative patients still respond to PD-1/PD-L1 inhibitors [104,105]. Moreover, the molecular subtypes of TNBC are under debate, indicating the heterogeneity of TNBC [106]. Composite biomarkers serving as the indications for anti-PD-1/PD-L1 therapy in TNBC patients require further research.
The pattern of combined regimens is another challenge. The response rate of immunotherapy monotherapy is somewhat low, which leads to the demand for combined regimens. Ongoing clinical trials are attempting to combine immunotherapy with existing antitumor therapies, including chemotherapy, targeted therapy, etc. In order to find the most effective remedy, combinations of various agents and immunotherapy are being explored. The NeoPACT trial proved the non-inferiority of non-anthracycline chemotherapy compared to anthracycline chemotherapy in combination with PD-L1 inhibitors [38]. Numerous studies on combining targeted therapy with immunotherapy are currently in progress. To optimize regimens, cumulative evidence is vital.
Improved endpoints are required to better evaluate clinical efficacy. Due to the fact that patients undergoing immunotherapy can exhibit flexible responses, clinical trials have been conducted with endpoints that are not well-matched for measuring curative effects in some cases. For instance, it is possible that patients may experience inflammation-induced tumor growth before they witness a reduction in tumor volume, which is called pseudoprogression [107]. The immune response evaluation criteria in solid tumors (iRECIST) was proposed in 2017, followed by the raise of immune-modified RECIST and immune-modified PFS in 2018 [108,109]. Unfortunately, the criteria are subject to limitations due to the variety of ICIs responses and the difficulty of distinguishing irAEs from tumor progression. An improved evaluation of the endpoints and the time points of follow-up require further discussion.
9. Conclusions
TNBC is deemed as the most challenging subtype of breast cancer, and inhibitors of PD-1/PD-L1 can favor TNBC patient outcomes by remodeling the TME and improving anti-tumor immunity. The KEYNOTE-522 trial established the first anti-PD-1/PD-L1 regimen permitted by the FDA in early-stage TNBC, which shed light on the utilization of PD-1/PD-L1 antibodies in the neoadjuvant setting. In addition, integrating anti-PD-1/PD-L1 regents into adjuvant chemotherapy and targeted therapy is showing promising therapeutic potential. As for the side effects, irAEs in early-stage TNBC immunotherapy should be monitored judiciously. Meanwhile, future work focusing on the challenges in the field is necessary to improve immunotherapy. Altogether, combining solid preclinical evidence and sufficient translational studies with convincing clinical trials is a firm foundation for accelerating the clinical development of PD-1/PD-L1 inhibitors in early-stage TNBC.
Author Contributions
Writing—original draft preparation, T.Y. and W.L.; writing—review and editing, T.Y., W.L. and J.Z.; visualization, J.Z.; supervision, T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [PubMed]
- Borri, F.; Granaglia, A. Pathology of triple negative breast cancer. Semin. Cancer Biol. 2021, 72, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buque, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Rey-Cardenas, M.; Guerrero-Ramos, F.; Gomez, D.L.L.A.; Carretero-Gonzalez, A.; Bote, H.; Herrera-Juarez, M.; Carril-Ajuria, L.; Martin-Soberon, M.; Sepulveda, J.M.; Billalabeitia, E.G.; et al. Recent advances in neoadjuvant immunotherapy for urothelial bladder cancer: What to expect in the near future. Cancer Treat. Rev. 2021, 93, 102142. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Huang, A.C.; Zappasodi, R. A decade of checkpoint blockade immunotherapy in melanoma: Understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef]
- Sendur, M. Adjuvant immunotherapy for renal cell carcinoma. Lancet Oncol. 2022, 23, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- Romero, D. Benefit in patients with PD-L1-positive TNBC. Nat. Rev. Clin. Oncol. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Johnson, B.R.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Savas, P.; Loi, S. Expanding the Role for Immunotherapy in Triple-Negative Breast Cancer. Cancer Cell 2020, 37, 623–624. [Google Scholar] [CrossRef]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Latif, F.; Bint, A.J.H.; Malik, H.; Sadaf, H.; Sarfraz, A.; Sarfraz, Z.; Cherrez-Ojeda, I. Atezolizumab and pembrolizumab in triple-negative breast cancer: A meta-analysis. Expert Rev. Anticancer Ther. 2022, 22, 229–235. [Google Scholar] [CrossRef]
- Noguchi, E.; Shien, T.; Iwata, H. Current status of PD-1/PD-L1 blockade immunotherapy in breast cancer. Jpn. J. Clin. Oncol. 2021, 51, 321–332. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Zhou, S.; Jiang, H.; Zhu, K.; Wang, R. Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: A meta-analysis. Int. Immunopharmacol. 2020, 80, 106214. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhao, B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: Meta-analysis. Br. Med. J. 2018, 362, k3529. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Xiong, G.; Cao, Z.; Yang, G.; Zheng, S.; Song, X.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017, 407, 57–65. [Google Scholar] [CrossRef]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Wang, B.; Yang, J.; Wang, Y.; Luo, F.; Xu, J.; Zhao, C.; Liu, R.; Chu, Y. The IFN-gamma/PD-L1 axis between T cells and tumor microenvironment: Hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflamm. 2018, 15, 290. [Google Scholar] [CrossRef]
- Verma, V.; Shrimali, R.K.; Ahmad, S.; Dai, W.; Wang, H.; Lu, S.; Nandre, R.; Gaur, P.; Lopez, J.; Sade-Feldman, M.; et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1(+) CD38(hi) cells and anti-PD-1 resistance. Nat. Immunol. 2019, 20, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin. Immunol. 2021, 226, 108707. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Schneeweiss, A.; Huober, J.; Braun, M.; Rey, J.; Blohmer, J.U.; Furlanetto, J.; Zahm, D.M.; Hanusch, C.; Thomalla, J.; et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann. Oncol. 2022, 33, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Cusmai, A.; Acquafredda, S.; Giovannelli, F.; Rinaldi, L.; Misino, A.; Palmiotti, G. KEYNOTE-522, IMpassion031 and GeparNUEVO: Changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 2022, 18, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Sharma, P.; Stecklein, S.R.; Yoder, R.; Staley, J.M.; Schwensen, K.; O'Dea, A.; Nye, L.E.; Elia, M.; Satelli, D.; Crane, G.; et al. Clinical and biomarker results of neoadjuvant phase II study of pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer (TNBC) (NeoPACT). J. Clin. Oncol. 2022, 40, 513. [Google Scholar] [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.H.; Munoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Symmans, W.F.; Yau, C.; Chen, Y.Y.; Balassanian, R.; Klein, M.E.; Pusztai, L.; Nanda, R.; Parker, B.A.; Datnow, B.; Krings, G.; et al. Assessment of Residual Cancer Burden and Event-Free Survival in Neoadjuvant Treatment for High-risk Breast Cancer: An Analysis of Data From the I-SPY2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1654–1663. [Google Scholar] [CrossRef]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated with Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.S.; Lae, M.; Reyal, F.; Sonke, G.S.; et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: A multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.A.; Mcarthur, H.L.; Kuemmel, S.; Bergh, J.; Park, Y.H.; Hui, R.N.; et al. Event-free survival by residual cancer burden after neoadjuvant pembrolizumab plus chemotherapy versus placebo plus chemotherapy for early TNBC: Exploratory analysis from KEYNOTE-522. J. Clin. Oncol. 2022, 40, 503. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann. Oncol. 2022, 33, 534–543. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Loibl, S.; Jackisch, C.; Rastogi, P.; Seiler, S.; Lucas, P.C.; Denkert, C.; Costantino, J.; Nekljudova, V.; Wolmark, N.; Geyer, C. GeparDouze/NSABP B-59: A randomized double-blind phase III clinical trial of neoadjuvant chemotherapy with atezolizumab or placebo in patients with triple negative breast cancer (TNBC) followed by adjuvant atezolizumab or placebo. Ann. Oncol. 2019, 30, iii38. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, G.M.; Kim, J.H.; Kim, H.; Park, K.H.; Park, Y.H.; Baek, S.K.; Sim, S.H.; Ahn, H.K.; Lee, G.W.; et al. Randomized, phase II trial to evaluate the efficacy and safety of atezolizumab plus capecitabine adjuvant therapy compared to capecitabine monotherapy for triple receptor-negative breast cancer (TNBC) with residual invasive cancer after neoadjuvant chemotherapy (MIRINAE trial, KCSG-BR18-21). J. Clin. Oncol. 2020, 38, TPS597. [Google Scholar]
- Ignatiadis, M.; Mcarthur, H.L.; Bailey, A.; Martinez, J.; de Azambuja, E.; Metzger, O.; Lai, C.; Franzoi, M.A.; Goulioti, T.; Daly, F.; et al. ALEXANDRA/IMpassion030: A phase III study of standard adjuvant chemotherapy with or without atezolizumab in early stage triple negative breast cancer. Ann. Oncol. 2019, 30, 97. [Google Scholar] [CrossRef]
- Conte, P.F.; Dieci, M.V.; Bisagni, G.; De Laurentiis, M.; Tondini, C.A.; Schmid, P.; De Salvo, G.L.; Moratello, G.; Guarneri, V. Phase III randomized study of adjuvant treatment with the ANTI-PD-L1 antibody avelumab for high-risk triple negative breast cancer patients: The A-BRAVE trial. J. Clin. Oncol. 2020, 38, TPS598. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Mardis, E.R. Neoantigens and genome instability: Impact on immunogenomic phenotypes and immunotherapy response. Genome Med. 2019, 11, 71. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021, 39, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Meng, L.H. Emerging roles of class I PI3K inhibitors in modulating tumor microenvironment and immunity. Acta Pharmacol. Sin. 2020, 41, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, A.N.; Finlay, D.; Preston, G.; Sinclair, L.V.; Waugh, C.M.; Tamas, P.; Feijoo, C.; Okkenhaug, K.; Cantrell, D.A. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 2011, 34, 224–236. [Google Scholar] [CrossRef]
- Crompton, J.G.; Sukumar, M.; Roychoudhuri, R.; Clever, D.; Gros, A.; Eil, R.L.; Tran, E.; Hanada, K.; Yu, Z.; Palmer, D.C.; et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015, 75, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Bofill, J.S.; Bermejo, B.; Phillips, M.; Wheatley, D.; Neus, F.; Schem, C.; Stradella, A.; Mele, M.; Salgado, A.C.; et al. BARBICAN: A randomized, phase II study to determine the contribution of ipatasertib to neoadjuvant chemotherapy plus atezolizumab in women with triple-negative breast cancer. Ann. Oncol. 2021, 32, S411–S412. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; Li, X.; et al. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int. J. Biol. Sci. 2022, 18, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Bagley, S.J.; Wen, P.Y.; Lim, M.; Platten, M.; Colman, H.; Ashley, D.M.; Wick, W.; Chang, S.M.; Galanis, E.; et al. Systematic review of combinations of targeted or immunotherapy in advanced solid tumors. J. Immunother. Cancer 2021, 9, e002459. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Jia, W.; Deng, H.; Li, G.; Deng, W.; Chen, J.; Kim, B.; Jiang, W.; Liu, Q.; et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020, 26, 1712–1724. [Google Scholar] [CrossRef]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef]
- Fassl, A.; Geng, Y.; Sicinski, P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science 2022, 375, c1495. [Google Scholar] [CrossRef]
- O'Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef]
- Ingham, M.; Schwartz, G.K. Cell-Cycle Therapeutics Come of Age. J. Clin. Oncol. 2017, 35, 2949–2959. [Google Scholar] [CrossRef]
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef]
- Goel, S.; Decristo, M.J.; Watt, A.C.; Brinjones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 2018, 17, 129. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Buttner, R.; Gosney, J.R.; Skov, B.G.; Adam, J.; Motoi, N.; Bloom, K.J.; Dietel, M.; Longshore, J.W.; Lopez-Rios, F.; Penault-Llorca, F.; et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 3867–3876. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Chen, Z.; Zheng, Y. Combined immunotherapy for metastatic triple-negative breast cancer based on PD-1/PD-L1 immune checkpoint blocking. Int. Immunopharmacol. 2022, 113, 109444. [Google Scholar] [CrossRef]
- Dodson, A.; Parry, S.; Lissenberg-Witte, B.; Haragan, A.; Allen, D.; O'Grady, A.; Mcclean, E.; Hughes, J.; Miller, K.; Thunnissen, E. External quality assessment demonstrates that PD-L1 22C3 and SP263 assays are systematically different. J. Pathol. Clin. Res. 2020, 6, 138–145. [Google Scholar] [CrossRef]
- Rugo, H.S.; Loi, S.; Adams, S.; von Moos, R.; Schmid, P.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Winer, E.P.; et al. Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): Post-hoc analysis of IMpassion130. Ann. Oncol. 2019, 30, v858–v859. [Google Scholar] [CrossRef]
- Sompuram, S.R.; Torlakovic, E.E.; ‘t Hart, N.A.; Vani, K.; Bogen, S.A. Quantitative comparison of PD-L1 IHC assays against NIST standard reference material 1934. Mod. Pathol. 2022, 35, 326–332. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Keenan, T.E.; Pernas, S.; Exman, P.; Jain, E.; Garrido-Castro, A.C.; Hughes, M.; Bychkovsky, B.; Umeton, R.; Files, J.L.; et al. Tumor Mutational Burden and PTEN Alterations as Molecular Correlates of Response to PD-1/L1 Blockade in Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 2565–2572. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Munoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Criscitiello, C.; Esposito, A.; Trapani, D.; Curigliano, G. Prognostic and predictive value of tumor infiltrating lymphocytes in early breast cancer. Cancer Treat. Rev. 2016, 50, 205–207. [Google Scholar] [CrossRef]
- Emens, L.A.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Dieras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Nguyen-Duc, A.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. JNCI-J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Elhalawani, H.; Fouad, M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: A meta-analysis. Future Oncol. 2016, 12, 413–425. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.R.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; Leboeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Gumusay, O.; Callan, J.; Rugo, H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022, 192, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw, W.G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients with Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef] [PubMed]
- de Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Thomas, M.; Dougan, M. Diagnosis and Management of Hepatitis in Patients on Checkpoint Blockade. Oncologist 2018, 23, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A. New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. J. Natl. Compr. Cancer Netw. 2018, 16, 594–596. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A. A review of cancer immunotherapy toxicity. Ca-Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. Jama Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Mcdermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Giannakis, M.; Wells, D.K.; Hamada, T.; Mu, X.J.; Quist, M.; Nowak, J.A.; Nishihara, R.; Qian, Z.R.; Inamura, K.; et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018, 8, 730–749. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.; Murugesan, K.; Elvin, J.A.; Huang, R.; Kurzrock, R. PD-L1 gene amplification and focality: Relationship with protein expression. J. Immunother. Cancer 2023, 11, e006311. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Bou, Z.M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou, Z.M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers 2022, 14, 1253. [Google Scholar] [CrossRef]
- Hodi, F.S.; Hwu, W.J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C.; et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients with Advanced Melanoma Treated with Pembrolizumab. J. Clin. Oncol. 2016, 34, 1510–1517. [Google Scholar] [CrossRef]
- Hodi, F.S.; Ballinger, M.; Lyons, B.; Soria, J.C.; Nishino, M.; Tabernero, J.; Powles, T.; Smith, D.; Hoos, A.; Mckenna, C.; et al. Immune-Modified Response Evaluation Criteria in Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J. Clin. Oncol. 2018, 36, 850–858. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litiere, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).