Association of Obstructive Sleep Apnea and Atrial Fibrillation in Acute Ischemic Stroke: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Source
2.2. Statistical Analysis
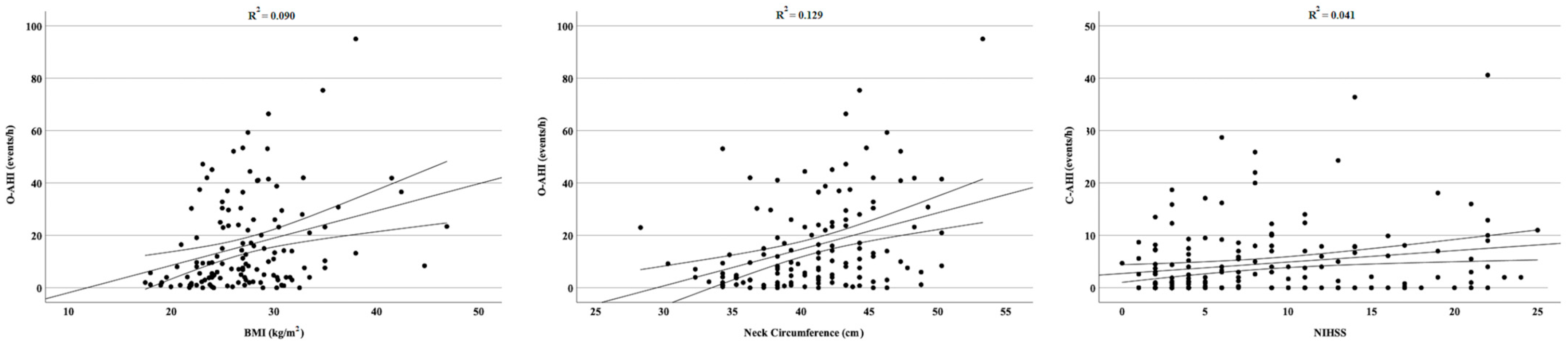
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quan, S.F.; Howard, B.V.; Iber, C.; Kiley, J.P.; Nieto, F.J.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Robbins, J.; Samet, J.M.; et al. The Sleep Heart Health Study: Design, rationale, and methods. Sleep 1997, 20, 1077–1085. [Google Scholar] [PubMed]
- Zhang, L.; Hou, Y.; Po, S.S. Obstructive Sleep Apnoea and Atrial Fibrillation. Arrhythmia Electrophysiol. Rev. 2015, 4, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Marulanda-Londono, E.; Chaturvedi, S. The Interplay between Obstructive Sleep Apnea and Atrial Fibrillation. Front. Neurol. 2017, 8, 668. [Google Scholar] [CrossRef] [PubMed]
- Kanagala, R.; Murali, N.S.; Friedman, P.A.; Ammash, N.M.; Gersh, B.J.; Ballman, K.V.; Shamsuzzaman, A.S.; Somers, V.K. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003, 107, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Dyken, M.E.; Im, K.B. Obstructive sleep apnea and stroke. Chest 2009, 136, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Alio, J. Cardioembolic stroke: Clinical features, specific cardiac disorders and prognosis. Curr. Cardiol. Rev. 2010, 6, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Lipford, M.C.; Flemming, K.D.; Calvin, A.D.; Mandrekar, J.; Brown, R.D., Jr.; Somers, V.K.; Caples, S.M. Associations between Cardioembolic Stroke and Obstructive Sleep Apnea. Sleep 2015, 38, 1699–1705. [Google Scholar] [CrossRef]
- Schutz, S.G.; Lisabeth, L.D.; Shafie-Khorassani, F.; Case, E.; Sanchez, B.N.; Chervin, R.D.; Brown, D.L. Clinical phenotypes of obstructive sleep apnea after ischemic stroke: A cluster analysis. Sleep Med. 2019, 60, 178–181. [Google Scholar] [CrossRef]
- Chen, C.Y.; Ho, C.H.; Chen, C.L.; Yu, C.C. Nocturnal Desaturation is Associated with Atrial Fibrillation in Patients With Ischemic Stroke and Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 729–735. [Google Scholar] [CrossRef]
- Munoz, R.; Duran-Cantolla, J.; Martinez-Vila, E.; Gallego, J.; Rubio, R.; Aizpuru, F.; De La Torre, G. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 2006, 37, 2317–2321. [Google Scholar] [CrossRef]
- Johnson, K.G.; Johnson, D.C. Frequency of sleep apnea in stroke and TIA patients: A meta-analysis. J. Clin. Sleep Med. 2010, 6, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Wolf, P.A.; Kelly-Hayes, M.; Beiser, A.S.; Kase, C.S.; Benjamin, E.J.; D’Agostino, R.B. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996, 27, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Otite, F.O.; Khandelwal, P.; Chaturvedi, S.; Romano, J.G.; Sacco, R.L.; Malik, A.M. Increasing atrial fibrillation prevalence in acute ischemic stroke and TIA. Neurology 2016, 87, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Gordon, C.; Wu, D.; Huang, H.C.; Yuliana, L.T.; Susatia, B.; Marta, O.F.D.; Chiu, H.Y. Dynamic Prevalence of Sleep Disorders Following Stroke or Transient Ischemic Attack: Systematic Review and Meta-Analysis. Stroke 2021, 52, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Parra, O.; Arboix, A.; Bechich, S.; Garcia-Eroles, L.; Montserrat, J.M.; Lopez, J.A.; Ballester, E.; Guerra, J.M.; Sopena, J.J. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am. J. Respir. Crit. Care Med. 2000, 161, 375–380. [Google Scholar] [CrossRef]
- Riglietti, A.; Fanfulla, F.; Pagani, M.; Lucini, D.; Malacarne, M.; Manconi, M.; Ferretti, G.; Esposito, F.; Cereda, C.W.; Pons, M. Obstructive and Central Sleep Apnea in First Ever Ischemic Stroke are Associated with Different Time Course and Autonomic Activation. Nat. Sci. Sleep 2021, 13, 1167–1178. [Google Scholar] [CrossRef]
- Brunetti, V.; Rollo, E.; Broccolini, A.; Frisullo, G.; Scala, I.; Della Marca, G. Sleep and Stroke: Opening Our Eyes to Current Knowledge of a Key Relationship. Curr. Neurol. Neurosci. Rep. 2022, 22, 767–779. [Google Scholar] [CrossRef]
- Mansukhani, M.P.; Calvin, A.D.; Kolla, B.P.; Brown, R.D., Jr.; Lipford, M.C.; Somers, V.K.; Caples, S.M. The association between atrial fibrillation and stroke in patients with obstructive sleep apnea: A population-based case-control study. Sleep Med. 2013, 14, 243–246. [Google Scholar] [CrossRef]
- Dalmar, A.; Singh, M.; Heis, Z.; Cumpian, T.L.; Ceretto, C.; Mortada, M.E.; Bhatia, A.; Niazi, I.; Chua, T.Y.; Sra, J.; et al. Risk of Atrial Fibrillation and Stroke After Bariatric Surgery in Patients with Morbid Obesity With or Without Obstructive Sleep Apnea. Stroke 2021, 52, 2266–2274. [Google Scholar] [CrossRef]
- Song, T.J.; Park, J.H.; Choi, K.H.; Chang, Y.; Moon, J.; Kim, J.H.; Choi, Y.; Kim, Y.J.; Lee, H.W. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017, 30, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.; Milanova, M.; Gugger, M. Sleep-disordered breathing and acute ischemic stroke: Diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke 2006, 37, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Yenokyan, G.; Gottlieb, D.J.; Shahar, E.; O’Connor, G.T.; Resnick, H.E.; Diener-West, M.; Sanders, M.H.; Wolf, P.A.; Geraghty, E.M.; et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2010, 182, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, F.; North, R.; Pieper, K.; Fonarow, G.C.; Kowey, P.R.; Gersh, B.J.; Mahaffey, K.W.; Pokorney, S.; Steinberg, B.A.; Naccarrelli, G.; et al. Risk of major cardiovascular and neurologic events with obstructive sleep apnea among patients with atrial fibrillation. Am. Heart J. 2020, 223, 65–71. [Google Scholar] [CrossRef]
- Yaranov, D.M.; Smyrlis, A.; Usatii, N.; Butler, A.; Petrini, J.R.; Mendez, J.; Warshofsky, M.K. Effect of obstructive sleep apnea on frequency of stroke in patients with atrial fibrillation. Am. J. Cardiol. 2015, 115, 461–465. [Google Scholar] [CrossRef]
- Pengo, M.F.; Faini, A.; Grote, L.; Ludka, O.; Joppa, P.; Pataka, A.; Dogas, Z.; Mihaicuta, S.; Hein, H.; Anttalainen, U.; et al. Impact of Sleep Apnea on Cardioembolic Risk in Patients with Atrial Fibrillation: Data from the ESADA Cohort. Stroke 2021, 52, 712–715. [Google Scholar] [CrossRef]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef]
- Brunetti, V.; Vollono, C.; Testani, E.; Pilato, F.; Della Marca, G. Autonomic Nervous System Modifications During Wakefulness and Sleep in a Cohort of Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1455–1462. [Google Scholar] [CrossRef]
- Robba, C.; Bonatti, G.; Battaglini, D.; Rocco, P.R.M.; Pelosi, P. Mechanical ventilation in patients with acute ischaemic stroke: From pathophysiology to clinical practice. Crit. Care 2019, 23, 388. [Google Scholar] [CrossRef]
- Baillieul, S.; Dekkers, M.; Brill, A.K.; Schmidt, M.H.; Detante, O.; Pepin, J.L.; Tamisier, R.; Bassetti, C.L.A. Sleep apnoea and ischaemic stroke: Current knowledge and future directions. Lancet Neurol. 2022, 21, 78–88. [Google Scholar] [CrossRef]
- Abboud, H.; Berroir, S.; Labreuche, J.; Orjuela, K.; Amarenco, P.; Investigators, G. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann. Neurol. 2006, 59, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Chen, L.S.; Fishbein, M.C.; Lin, S.F.; Nattel, S. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ. Res. 2014, 114, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Leiria, T.L.; Glavinovic, T.; Armour, J.A.; Cardinal, R.; de Lima, G.G.; Kus, T. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Auton. Neurosci. 2011, 161, 68–74. [Google Scholar] [CrossRef]
- Orlandi, G.; Fanucchi, S.; Strata, G.; Pataleo, L.; Landucci Pellegrini, L.; Prontera, C.; Martini, A.; Murri, L. Transient autonomic nervous system dysfunction during hyperacute stroke. Acta Neurol. Scand. 2000, 102, 317–321. [Google Scholar] [CrossRef]
- Tobaldini, E.; Proserpio, P.; Oppo, V.; Figorilli, M.; Fiorelli, E.M.; Manconi, M.; Agostoni, E.C.; Nobili, L.; Montano, N.; Horvath, T.; et al. Cardiac autonomic dynamics during sleep are lost in patients with TIA and stroke. J. Sleep Res. 2020, 29, e12878. [Google Scholar] [CrossRef]
- Alexiev, F.; Brill, A.K.; Ott, S.R.; Duss, S.; Schmidt, M.; Bassetti, C.L. Sleep-disordered breathing and stroke: Chicken or egg? J. Thorac. Dis. 2018, 10, S4244–S4252. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.R.; Fanfulla, F.; Miano, S.; Horvath, T.; Seiler, A.; Bernasconi, C.; Cereda, C.W.; Brill, A.K.; Young, P.; Nobili, L.; et al. SAS Care 1: Sleep-disordered breathing in acute stroke an transient ischaemic attack—Prevalence, evolution and association with functional outcome at 3 months, a prospective observational polysomnography study. ERJ Open Res. 2020, 6, 00334-2019. [Google Scholar] [CrossRef] [PubMed]
- Huhtakangas, J.K.; Saaresranta, T.; Bloigu, R.; Huhtakangas, J. The Evolution of Sleep Apnea Six Months After Acute Ischemic Stroke and Thrombolysis. J. Clin. Sleep Med. 2018, 14, 2005–2011. [Google Scholar] [CrossRef]
- Seiler, A.; Camilo, M.; Korostovtseva, L.; Haynes, A.G.; Brill, A.K.; Horvath, T.; Egger, M.; Bassetti, C.L. Prevalence of sleep-disordered breathing after stroke and TIA: A meta-analysis. Neurology 2019, 92, e648–e654. [Google Scholar] [CrossRef]
- Dancey, D.R.; Hanly, P.J.; Soong, C.; Lee, B.; Shepard, J., Jr.; Hoffstein, V. Gender differences in sleep apnea: The role of neck circumference. Chest 2003, 123, 1544–1550. [Google Scholar] [CrossRef]
- Sacchetti, M.L.; Della Marca, G. Are stroke cases affected by sleep disordered breathings all the same? Med. Hypotheses 2014, 83, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, A.; Brunetti, V.; Broccolini, A.; Caliandro, P.; Frisullo, G.; Morosetti, R.; Pilato, F.; Profice, P.; Giannantoni, N.M.; Sacchetti, M.L.; et al. Dysphagia and Obstructive Sleep Apnea in Acute, First-Ever, Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, M.; Ottenstein, L.; Atanelov, L.; Christian, A.B. Dysphagia after Stroke: An Overview. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Estai, M.; Walsh, J.; Maddison, K.; Shepherd, K.; Hillman, D.; McArdle, N.; Baker, V.; King, S.; Al-Obaidi, Z.; Bamagoos, A.; et al. Sleep-disordered breathing in patients with stroke-induced dysphagia. J. Sleep Res. 2021, 30, e13179. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, X.; Wang, T.; Zhang, L.; Hu, C.; Jia, R.; Zhang, L.; Li, X.; Yan, L.; Zhang, Y.; et al. Effects of Comprehensive Swallowing Intervention on Obstructive Sleep Apnea and Dysphagia After Stroke: A Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2022, 31, 106521. [Google Scholar] [CrossRef]

| Demographic Features | |
| Age, years, mean (SD) | 67.3 (11.6) |
| Gender, male, no. (%) | 95 (54.6) |
| BMI, mean (SD) | 27.0 (5.8) |
| Neck circumference, cm, mean (SD) | 40.7 (4.6) |
| Respiratory Indices | |
| O-AHI, mean (SD) | 17.9 (19.0) |
| C-AHI, mean (SD) | 4.4 (6.7) |
| ODI, mean (SD) | 20.7 (20.5) |
| Clinical Features | |
| OSA, no. (%) | 89 (51.2) |
| Atrial fibrillation, no. (%) | 55 (31.6) |
| Hypertension, no. (%) | 126 (72.4) |
| Diabetes, no. (%) | 53 (30.5) |
| Dyslipidemia, no. (%) | 76 (43.7) |
| Reperfusion therapy, no. (%) | 38 (21.8) |
| NIHSS, mean (SD) | 8.8 (6.8) |
| Dysphagia, no. (%) | 92 (52.9) |
| Pneumonia, no. (%) | 10 (5.7) |
| Death, no. (%) | 2 (1.1) |
| Wake-up stroke, no. (%) | 50 (28.7) |
| OCSP | |
| TACI, no. (%) | 42 (24.1) |
| PACI, no. (%) | 81 (46.6) |
| LACI, no. (%) | 20 (11.5) |
| POCI, no. (%) | 31 (17.8) |
| TOAST | |
| Cardioembolism, no. (%) | 66 (37.9) |
| Large Artery Atherosclerosis, no. (%) | 28 (16.1) |
| Small Vessel Occlusion, no. (%) | 25 (14.4) |
| Other Determined Origin, no. (%) | 4 (2.3) |
| Stroke of Undetermined Etiology, no. (%) | 51 (29.3) |
| OSA+ (n = 89) | OSA− (n = 85) | Mann–Whitney U | χ2 | p | |
|---|---|---|---|---|---|
| Demographic features | |||||
| Age, years, mean (SD) | 68.6 (11.0) | 66.0 (12.2) | 4229.5 | 0.178 | |
| Gender, male, no. (%) | 49 (55.1) | 46 (54.1) | 0.015 | 0.901 | |
| BMI, mean (SD) | 28.4 (6.6) | 25.9 (4.7) | 2792.5 | 0.001 | |
| Neck circumference, cm, mean (SD) | 42.3 (4.3) | 39.4 (4.4) | 2921.0 | <0.001 | |
| C-AHI, mean (SD) | 5.6 (7.2) | 3.2 (6.1) | 4250.0 | 0.028 | |
| O-AHI, mean (SD) | 31.8 (17.7) | 3.7 (2.9) | |||
| ODI, mean (SD) | 34.7 (19.8) | 6.6 (7.2) | |||
| Clinical features | |||||
| Atrial fibrillation, no. (%) | 33 (37.1) | 22 (25.9) | 2.521 | 0.112 | |
| Hypertension, no. (%) | 76 (85.4) | 50 (58.8) | 17.866 | <0.001 | |
| Diabetes, no. (%) | 33 (37.1) | 20 (23.5) | 3.778 | 0.052 | |
| Dyslipidemia, no. (%) | 40 (44.9) | 36 (42.4) | 0.040 | 0.842 | |
| Reperfusion therapy, no. (%) | 22 (24.7) | 16 (18.8) | 0.262 | 0.609 | |
| NIHSS, mean (SD) | 9.3 (6.8) | 8.3 (6.9) | 4128.5 | 0.296 | |
| Dysphagia, no. (%) | 59 (66.3) | 33 (38.8) | 13.165 | <0.001 | |
| Pneumonia, no. (%) | 7 (7.9) | 3 (3.5) | 0.963 | 0.327 | |
| Death, no. (%) | 2 (2.2) | 0 (0.0) | 1.701 | 0.192 | |
| Wake-up stroke, no. (%) | 24 (27.0) | 26 (30.6) | 0.279 | 0.359 | |
| OCSP | 1.944 | 0.584 | |||
| TACI, no. (%) | 25 (28.1) | 17 (20.0) | |||
| PACI, no. (%) | 39 (43.8) | 41 (48.2) | |||
| LACI, no. (%) | 9 (10.1) | 12 (14.1) | |||
| POCI, no. (%) | 16 (18.0) | 15 (16.9) | |||
| TOAST | 3.468 | 0.483 | |||
| Cardioembolism, no. (%) | 37 (56.1) | 29 (43.9) | |||
| Large Artery Atherosclerosis, no. (%) | 14 (50.0) | 14 (50.0) | |||
| Small Vessel Occlusion, no. (%) | 15 (60.0) | 10 (40.0) | |||
| Other Determined Origin, no. (%) | 2 (50.0) | 2 (50.0) | |||
| Stroke of Undetermined Etiology, no. (%) | 21 (41.2) | 30 (58.8) |
| Odds Ratio | 95% Confidence Interval | p | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.011 | 0.960 | 1.064 | 0.684 |
| Gender (Male) | 0.443 | 0.151 | 1.298 | 0.138 |
| BMI | 1.182 | 1.032 | 1.353 | 0.016 |
| Neck Circumference | 1.116 | 0.965 | 1.290 | 0.138 |
| Wake-up stroke | 0.744 | 0.266 | 2.083 | 0.574 |
| NIHSS | 0.941 | 0.856 | 1.034 | 0.208 |
| Dysphagia | 6.945 | 1.964 | 24.552 | 0.003 |
| Hypertension | 5.349 | 1.465 | 19.534 | 0.011 |
| Diabetes | 1.437 | 0.493 | 4.186 | 0.506 |
| Atrial Fibrillation | 0.861 | 0.279 | 2.657 | 0.795 |
| C-AHI | 1.037 | 0.959 | 1.120 | 0.364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunetti, V.; Testani, E.; Losurdo, A.; Vollono, C.; Broccolini, A.; Di Iorio, R.; Frisullo, G.; Pilato, F.; Profice, P.; Marotta, J.; et al. Association of Obstructive Sleep Apnea and Atrial Fibrillation in Acute Ischemic Stroke: A Cross-Sectional Study. J. Pers. Med. 2023, 13, 527. https://doi.org/10.3390/jpm13030527
Brunetti V, Testani E, Losurdo A, Vollono C, Broccolini A, Di Iorio R, Frisullo G, Pilato F, Profice P, Marotta J, et al. Association of Obstructive Sleep Apnea and Atrial Fibrillation in Acute Ischemic Stroke: A Cross-Sectional Study. Journal of Personalized Medicine. 2023; 13(3):527. https://doi.org/10.3390/jpm13030527
Chicago/Turabian StyleBrunetti, Valerio, Elisa Testani, Anna Losurdo, Catello Vollono, Aldobrando Broccolini, Riccardo Di Iorio, Giovanni Frisullo, Fabio Pilato, Paolo Profice, Jessica Marotta, and et al. 2023. "Association of Obstructive Sleep Apnea and Atrial Fibrillation in Acute Ischemic Stroke: A Cross-Sectional Study" Journal of Personalized Medicine 13, no. 3: 527. https://doi.org/10.3390/jpm13030527
APA StyleBrunetti, V., Testani, E., Losurdo, A., Vollono, C., Broccolini, A., Di Iorio, R., Frisullo, G., Pilato, F., Profice, P., Marotta, J., Rollo, E., Scala, I., Calabresi, P., & Della Marca, G. (2023). Association of Obstructive Sleep Apnea and Atrial Fibrillation in Acute Ischemic Stroke: A Cross-Sectional Study. Journal of Personalized Medicine, 13(3), 527. https://doi.org/10.3390/jpm13030527








