White Matter Hyperintensities in Young Patients from a Neurological Outpatient Clinic: Prevalence, Risk Factors, and Correlation with Enlarged Perivascular Spaces
Abstract
1. Introduction
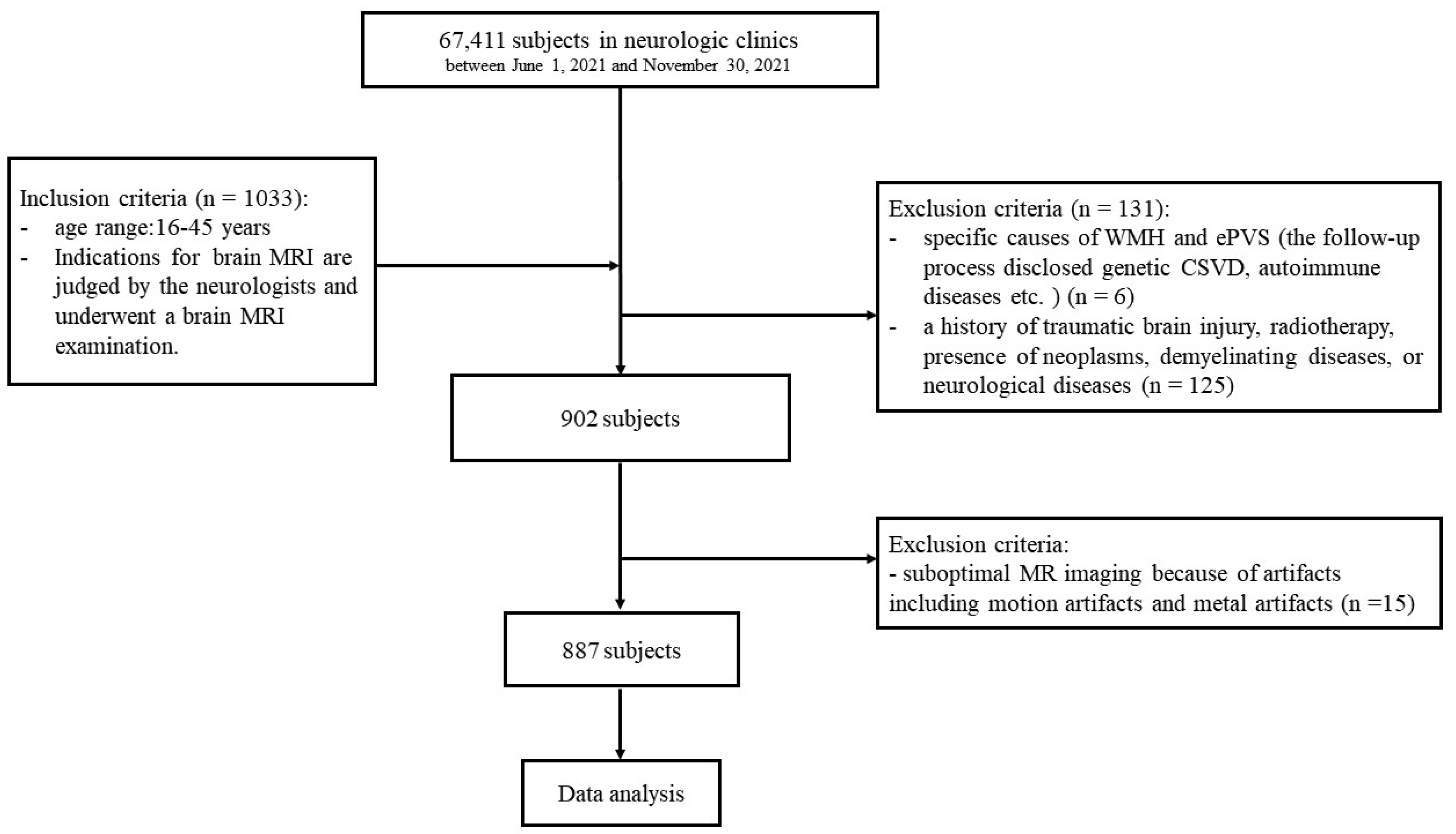
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
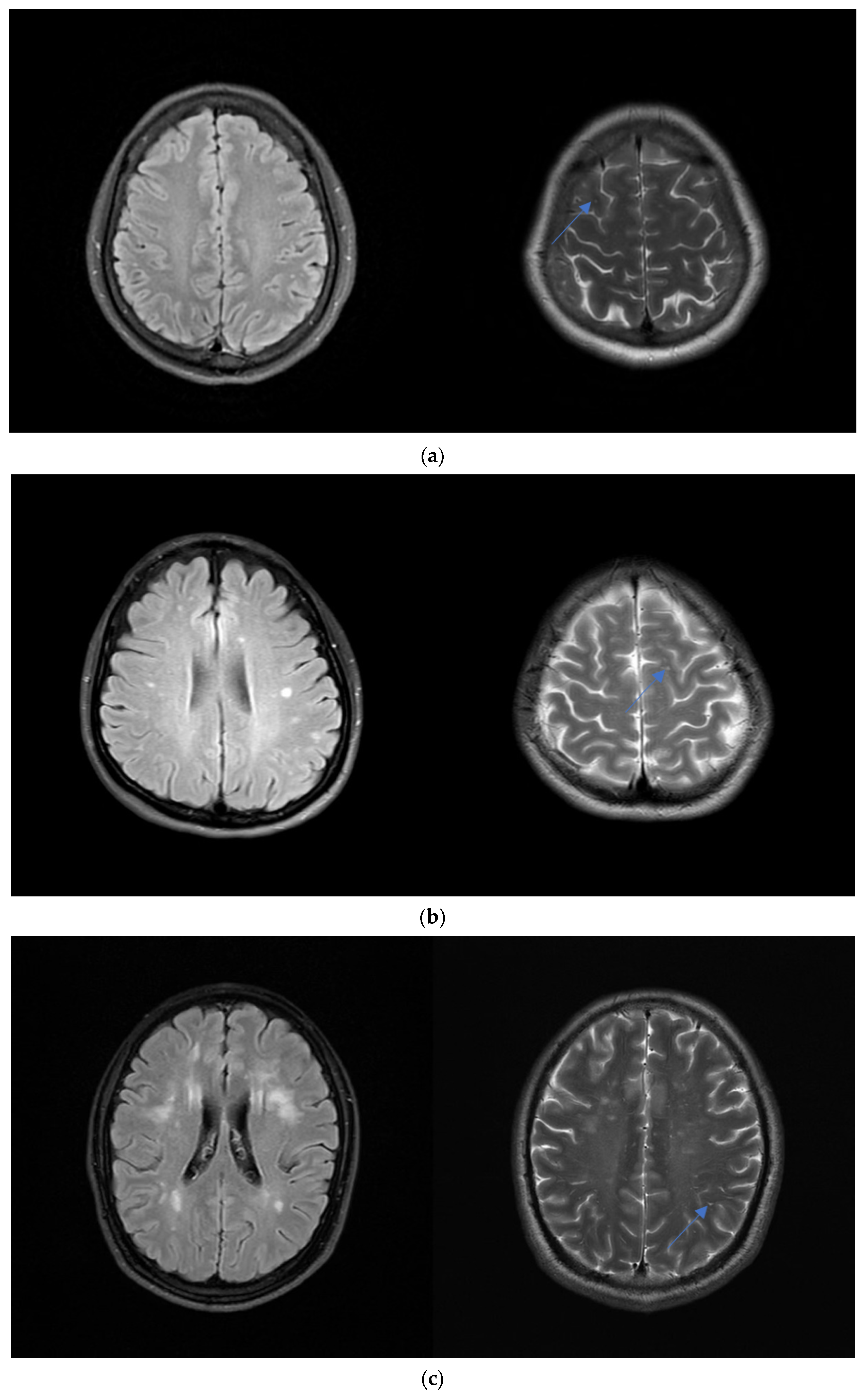
2.3. Brain MRI
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Participants
3.2. Distribution and Severity of WMH
3.3. Risk Factors of WMH
3.4. Correlation between WMH and ePVS
4. Discussion
4.1. Effects of Demographic Factors on WMH
4.2. Influence of Clinical Symptoms on WMH
4.3. Correlation between WMH and ePVS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyquist, P.A.; Bilgel, M.; Gottesman, R.; Yanek, L.R.; Moy, T.F.; Becker, L.C.; Cuzzocreo, J.L.; Prince, J.; Wasserman, B.A.; Yousem, D.M.; et al. Age Differences in Periventricular and Deep White Matter Lesions. Neurobiol. Aging 2015, 36, 1653–1658. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, R.; Jiaerken, Y.; Wang, S.; Yu, W.; Hong, H.; Lian, C.; Li, K.; Zeng, Q.; Luo, X.; et al. Deep White Matter Hyperintensity Is Associated with the Dilation of Perivascular Space. J. Cereb. Blood Flow Metab. 2021, 41, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Markus, H.S. The Clinical Importance of White Matter Hyperintensities on Brain Magnetic Resonance Imaging: Systematic Review and Meta-Analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef]
- Guevarra, A.C.; Ng, S.C.; Saffari, S.E.; Wong, B.Y.X.; Chander, R.J.; Ng, K.P.; Kandiah, N. Age Moderates Associations of Hypertension, White Matter Hyperintensities, and Cognition. J. Alzheimer’s Dis. 2020, 75, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Laveskog, A.; Wang, R.; Bronge, L.; Wahlund, L.-O.; Qiu, C. Perivascular Spaces in Old Age: Assessment, Distribution, and Correlation with White Matter Hyperintensities. AJNR Am. J. Neuroradiol. 2018, 39, 70–76. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, J.G.; Van den Hof, M.; Boyd, A.C.; Caan, M.W.A.; Wit, F.W.N.M.; Reiss, P.; Pajkrt, D. Differences in Location of Cerebral White Matter Hyperintensities in Children and Adults Living with a Treated HIV Infection: A Retrospective Cohort Comparison. PLoS ONE 2020, 15, e0241438. [Google Scholar] [CrossRef]
- Ter Telgte, A.; van Leijsen, E.M.C.; Wiegertjes, K.; Klijn, C.J.M.; Tuladhar, A.M.; de Leeuw, F.-E. Cerebral Small Vessel Disease: From a Focal to a Global Perspective. Nat. Rev. Neurol. 2018, 14, 387–398. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Yang, S.; Qin, W.; Yang, L.; Fan, H.; Li, Y.; Yin, J.; Hu, W. The Relationship between Ambulatory Blood Pressure Variability and Enlarged Perivascular Spaces: A Cross-Sectional Study. BMJ Open 2017, 7, e015719. [Google Scholar] [CrossRef]
- Seixas, A.A.; Turner, A.D.; Bubu, O.M.; Jean-Louis, G.; de Leon, M.J.; Osorio, R.S.; Glodzik, L. Obesity and Race May Explain Differential Burden of White Matter Hyperintensity Load. Clin. Interv. Aging 2021, 16, 1563–1571. [Google Scholar] [CrossRef]
- Mineura, K.; Sasajima, H.; Kikuchi, K.; Kowada, M.; Tomura, N.; Monma, K.; Segawa, Y. White Matter Hyperintensity in Neurologically Asymptomatic Subjects. Acta Neurol. Scand. 1995, 92, 151–156. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Cobbe, S.M.; Bollen, E.L.; Buckley, B.M.; Ford, I.; Jukema, J.W.; Hyland, M.; Gaw, A.; et al. The Design of a Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am. J. Cardiol. 1999, 84, 1192–1197. [Google Scholar] [CrossRef]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Wang, B.; Chu, Y.-H.; Jiang, Y.; Cui, M.; Wang, H.; Chen, X. MRI-Based Investigation of Association Between Cerebrovascular Structural Alteration and White Matter Hyperintensity Induced by High Blood Pressure. J. Magn. Reson. Imaging 2021, 54, 1516–1526. [Google Scholar] [CrossRef]
- Pantoni, L.; Garcia, J.H. Pathogenesis of Leukoaraiosis: A Review. Stroke 1997, 28, 652–659. [Google Scholar] [CrossRef]
- Kurth, T.; Mohamed, S.; Maillard, P.; Zhu, Y.-C.; Chabriat, H.; Mazoyer, B.; Bousser, M.-G.; Dufouil, C.; Tzourio, C. Headache, Migraine, and Structural Brain Lesions and Function: Population Based Epidemiology of Vascular Ageing-MRI Study. BMJ 2011, 342, c7357. [Google Scholar] [CrossRef]
- Lu, S.; Song, L.; Wang, D.; Zhang, X.; Lv, X.; Yin, H.; Gao, Y.; Liu, X.; Tang, J. White Matter Hyperintensity and Cognitive Impairments in Chronic Insomniacs. Neuroreport 2019, 30, 612–618. [Google Scholar] [CrossRef]
- Yaffe, K.; Nasrallah, I.; Hoang, T.D.; Lauderdale, D.S.; Knutson, K.L.; Carnethon, M.R.; Launer, L.J.; Lewis, C.E.; Sidney, S. Sleep Duration and White Matter Quality in Middle-Aged Adults. Sleep 2016, 39, 1743–1747. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Arenaza-Urquijo, E.M.; Knopman, D.S.; Schwarz, C.G.; Brown, R.D.; Rabinstein, A.A.; Gunter, J.L.; Senjem, M.L.; Przybelski, S.A.; Lesnick, T.; et al. White Matter Hyperintensities: Relationship to Amyloid and Tau Burden. Brain 2019, 142, 2483–2491. [Google Scholar] [CrossRef]
- Potter, G.M.; Chappell, F.M.; Morris, Z.; Wardlaw, J.M. Cerebral Perivascular Spaces Visible on Magnetic Resonance Imaging: Development of a Qualitative Rating Scale and Its Observer Reliability. Cereb. Dis. 2015, 39, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.J.; Khandwala, V.; Wang, D.; Maloney, T.; Sucharew, H.; Horn, P.; Haverbusch, M.; Alwell, K.; Gangatirkar, S.; Mahammedi, A.; et al. Automated Grading of Enlarged Perivascular Spaces in Clinical Imaging Data of an Acute Stroke Cohort Using an Interpretable, 3D Deep Learning Framework. Sci. Rep. 2022, 12, 788. [Google Scholar] [CrossRef] [PubMed]
| 16–25 Years (n = 159) | 26–35 Years (n = 260) | 36–45 Years (n = 468) | ||
|---|---|---|---|---|
| Sex | Male, n (%) | 52 (32.7%) | 116 (44.6%) | 221 (47.2%) |
| Vascular risk factors | Diabetes, n (%) | 1 (0.6%) | 7 (2.6%) | 16 (3.4%) |
| Hypertension, n (%) | 4 (2.5%) | 20 (7.6%) | 48 (12.3%) | |
| Hyperlipidemia, n (%) | 2 (1.2%) | 6 (2.3%) | 17 (3.6%) | |
| Symptoms | Absent, n (%) | 6 (3.7%) | 19 (7.3%) | 28 (5.9%) |
| Headache, n (%) | 54 (33.9%) | 54 (20.7%) | 119 (25.4%) | |
| Dizziness, n (%) | 19 (11.9%) | 54 (20.7%) | 104 (22.2%) | |
| Vertigo, n (%) | 6 (3.7%) | 10 (3.8%) | 19 (4.0%) | |
| Syncope, n (%) | 5 (3.1%) | 7 (2.6%) | 5 (1.0%) | |
| Light-headedness, n (%) | 3 (1.8%) | 9 (3.4%) | 15 (3.2%) | |
| Somatic symptoms, n (%) | 10 (6.2%) | 30 (11.5%) | 30 (6.4%) | |
| Hearing disturbances, n (%) | 2 (1.2%) | 9 (3.4%) | 20 (4.2%) | |
| Visual disturbances, n (%) | 3 (1.8%) | 8 (3.0%) | 10 (2.1%) | |
| Convulsions, n (%) | 5 (3.1%) | 21 (8.0%) | 20 (4.2%) | |
| Tremors, n (%) | 3 (1.8%) | 6 (2.3%) | 12 (2.5%) | |
| Sleeping disturbances, n (%) | 5 (3.1%) | 13 (5.0%) | 35 (7.4%) | |
| Unspecified, n (%) | 38 (23.8%) | 20 (7.6%) | 51 (10.8%) |
| 16–25 Years (n = 159) | 26–35 Years (n = 260) | 36–45 Years (n = 468) | ||
|---|---|---|---|---|
| DWMH | 0, n (%) | 124 (77.9%) | 169 (65.0%) | 278 (59.4%) |
| 1, n (%) | 32 (20.1%) | 74 (28.4%) | 153 (32.6%) | |
| 2, n (%) | 3 (1.8%) | 16 (6.1%) | 34 (7.2%) | |
| 3, n (%) | 0 (0.0%) | 1 (0.3%) | 3 (0.6%) | |
| PWMH | 0, n (%) | 158 (99.3%) | 242 (93.0%) | 423 (90.3%) |
| 1, n (%) | 1 (0.6%) | 9 (3.4%) | 30 (6.4%) | |
| 2, n (%) | 0 (0.6%) | 6 (2.3%) | 10 (2.1%) | |
| 3, n (%) | 0 (0.0%) | 3 (1.1%) | 5 (1.0%) | |
| CSO-ePVS score | 0, n (%) | 21 (13.2%) | 0 (0.0%) | 0 (0.0%) |
| 1, n (%) | 128 (80.5%) | 254 (97.7%) | 70 (14.9%) | |
| 2, n (%) | 8 (5.0%) | 4 (1.5%) | 340 (72.6%) | |
| 3, n (%) | 2 (1.2%) | 1 (0.4%) | 55 (11.7%) | |
| 4, n (%) | 0 (0.0%) | 1 (0.4%) | 3 (0.6%) | |
| BG-ePVS score | 1, n (%) | 154 (96.8%) | 253 (97.3%) | 282 (60.2%) |
| 2, n (%) | 3 (3.1%) | 3 (1.2%) | 147 (37.1%) | |
| 3, n (%) | 2 (1.3%) | 2 (0.8%) | 8 (1.7%) | |
| 4, n (%) | 0 (0.0%) | 2 (0.8%) | 4 (0.9%) |
| DWMH | p-Value | PWMH | p-Value | |||
|---|---|---|---|---|---|---|
| Absent (n = 571) | Present (n = 316) | Absent (n = 823) | Present (n = 64) | |||
| Age (years) | <0.001 | 0.001 | ||||
| 16–25, n (%) | 124 (21.7) | 35 (11.1) | 158 (19.2) | 1 (1.6) | ||
| 26–35, n (%) | 169 (29.6) | 91 (28.8) | 242 (29.4) | 18 (28.1) | ||
| 36–45, n (%) | 278 (48.7) | 190 (60.1) | 423 (51.4) | 45 (70.3) | ||
| Male, n (%) | 250 (43.8) | 139 (44.0) | 0.953 | 350 (42.5) | 39 (60.9) | 0.004 |
| Diabetes, n (%) | 15 (2.6) | 9 (2.8) | 0.846 | 21 (2.6) | 3 (4.7) | 0.539 |
| Hypertension, n (%) | 39 (6.8) | 43 (13.6) | 0.001 | 67 (8.1) | 15 (23.4) | <0.001 |
| Hyperlipidemia, n (%) | 16 (2.8) | 9 (2.8) | 0.968 | 21 (2.6) | 4 (6.3) | 0.184 |
| Symptoms | ||||||
| Absent, n (%) | 38 (6.7) | 15 (4.7) | 0.251 | 52 (6.3) | 1 (1.6) | 0.122 |
| Headache, n (%) | 137 (24.0) | 90 (28.5) | 0.142 | 212 (25.8) | 15 (23.4) | 0.682 |
| Dizziness, n (%) | 117 (20.5) | 60 (19.0) | 0.592 | 165 (20.0) | 12 (18.8) | 0.802 |
| Vertigo, n (%) | 24 (4.2) | 11 (3.5) | 0.597 | 31 (3.8) | 4 (6.3) | 0.326 |
| Syncope, n (%) | 7 (1.2) | 10 (3.2) | 0.044 | 17 (2.1) | 0 (0.0) | 0.492 |
| Light-headedness, n (%) | 18 (3.2) | 9 (2.8) | 0.801 | 25 (3.0) | 2 (1.9) | 0.999 |
| Somatic symptoms, n (%) | 47 (8.2) | 23 (7.3) | 0.614 | 65 (7.9) | 5 (7.8) | 0.981 |
| Hearing disturbance, n (%) | 20 (3.5) | 11 (3.5) | 0.987 | 30 (3.6) | 1 (1.6) | 0.603 |
| Visual disturbance, n (%) | 13 (2.3) | 8 (2.5) | 0.811 | 19 (2.3) | 2 (3.1) | 0.999 |
| Convulsion, n (%) | 33 (5.8) | 13 (4.1) | 0.284 | 41 (5.0) | 5 (7.8) | 0.489 |
| Tremor, n (%) | 14 (2.5) | 7 (2.2) | 0.824 | 18 (2.2) | 3 (4.7) | 0.401 |
| Sleep disturbance, n (%) | 24 (4.2) | 29 (9.2) | 0.003 | 41 (5.0) | 12 (18.8) | <0.001 |
| Unspecified, n (%) | 79 (13.8) | 30 (9.5) | 0.059 | 107 (13.0) | 2 (3.1) | 0.020 |
| OR (95% CI) | p | |
|---|---|---|
| DWMH | ||
| Age (years) | ||
| 16–25 | 1 (referent) | |
| 26–35 | 1.913 (1.195–3.061) | 0.007 |
| 36–45 | 2.336 (1.514–3.605) | <0.001 |
| Hypertension | 1.915 (1.201–3.053) | 0.006 |
| Headache | 1.450 (1.035–2.030) | 0.031 |
| Syncope | 3.647 (1.329–10.004) | 0.012 |
| Sleep disturbance | 2.404 (1.344–4.299) | 0.003 |
| Unspecified | 0.885 (0.549–1.427) | 0.617 |
| PWMH | ||
| Age | ||
| 16–25 | 1 (referent) | |
| 26–35 | 9.852 (1.293–75.097) | 0.027 |
| 36–45 | 12.393 (1.679–91.490) | 0.014 |
| Female | 0.555 (0.324–0.950) | 0.032 |
| Hypertension | 2.645 (1.370–5.107) | 0.004 |
| Hyperlipidemia | 1.865 (0.591–5.883) | 0.288 |
| Absent | 0.263 (0.035–1.970) | 0.194 |
| Sleep disturbance | 3.860 (1.855–8.032) | <0.001 |
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| CSO-ePVS | 1.828 | 1.120–2.983 | 0.016 | 1.766 | 1.079–2.892 | 0.024 | 1.828 | 1.098–3.045 | 0.020 |
| BG-ePVS | 1.667 | 1.163–2.391 | 0.005 | 1.570 | 1.088–2.267 | 0.016 | 1.540 | 1.059–2.239 | 0.024 |
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| CSO-ePVS | 2.551 | 0.910–7.153 | 0.075 | 2.074 | 0.794–5.421 | 0.137 | 2.067 | 0.787–5.431 | 0.141 |
| BG-ePVS | 3.465 | 1.861–6.452 | <0.001 | 3.007 | 1.616–5.595 | 0.001 | 3.427 | 1.802–6.520 | <0.001 |
| DWMH | Gamma Coefficient | p | PWMH | Gamma Coefficient | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||||
| CSO-ePVS | 0.263 | <0.001 | ||||||||||
| Low | 333 (70.4) | 117 (24.7) | 23 (4.9) | 0 (0.0) | ||||||||
| High | 238 (57.5) | 142 (34.3) | 30 (7.2) | 4 (1.0) | ||||||||
| BG-ePVS | 0.289 | <0.001 | 0.579 | <0.001 | ||||||||
| Low | 467 (67.8) | 183 (26.6) | 37 (5.4) | 2 (0.3) | 657 (95.4) | 18 (2.6) | 9 (1.3) | 5 (0.7) | ||||
| High | 60 (52.5) | 46 (38.4) | 11 (8.1) | 1 (1.0) | 166 (83.8) | 22 (11.1) | 7 (3.5) | 3 (1.5) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Q.; Wang, M.; Zhang, D.; Wei, X.; Li, W. White Matter Hyperintensities in Young Patients from a Neurological Outpatient Clinic: Prevalence, Risk Factors, and Correlation with Enlarged Perivascular Spaces. J. Pers. Med. 2023, 13, 525. https://doi.org/10.3390/jpm13030525
Zou Q, Wang M, Zhang D, Wei X, Li W. White Matter Hyperintensities in Young Patients from a Neurological Outpatient Clinic: Prevalence, Risk Factors, and Correlation with Enlarged Perivascular Spaces. Journal of Personalized Medicine. 2023; 13(3):525. https://doi.org/10.3390/jpm13030525
Chicago/Turabian StyleZou, Qiaoqiao, Mingliang Wang, Danni Zhang, Xiaoer Wei, and Wenbin Li. 2023. "White Matter Hyperintensities in Young Patients from a Neurological Outpatient Clinic: Prevalence, Risk Factors, and Correlation with Enlarged Perivascular Spaces" Journal of Personalized Medicine 13, no. 3: 525. https://doi.org/10.3390/jpm13030525
APA StyleZou, Q., Wang, M., Zhang, D., Wei, X., & Li, W. (2023). White Matter Hyperintensities in Young Patients from a Neurological Outpatient Clinic: Prevalence, Risk Factors, and Correlation with Enlarged Perivascular Spaces. Journal of Personalized Medicine, 13(3), 525. https://doi.org/10.3390/jpm13030525









