Endoscopic and Image Analysis of the Airway in Patients with Mucopolysaccharidosis Type IVA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Ethical Considerations
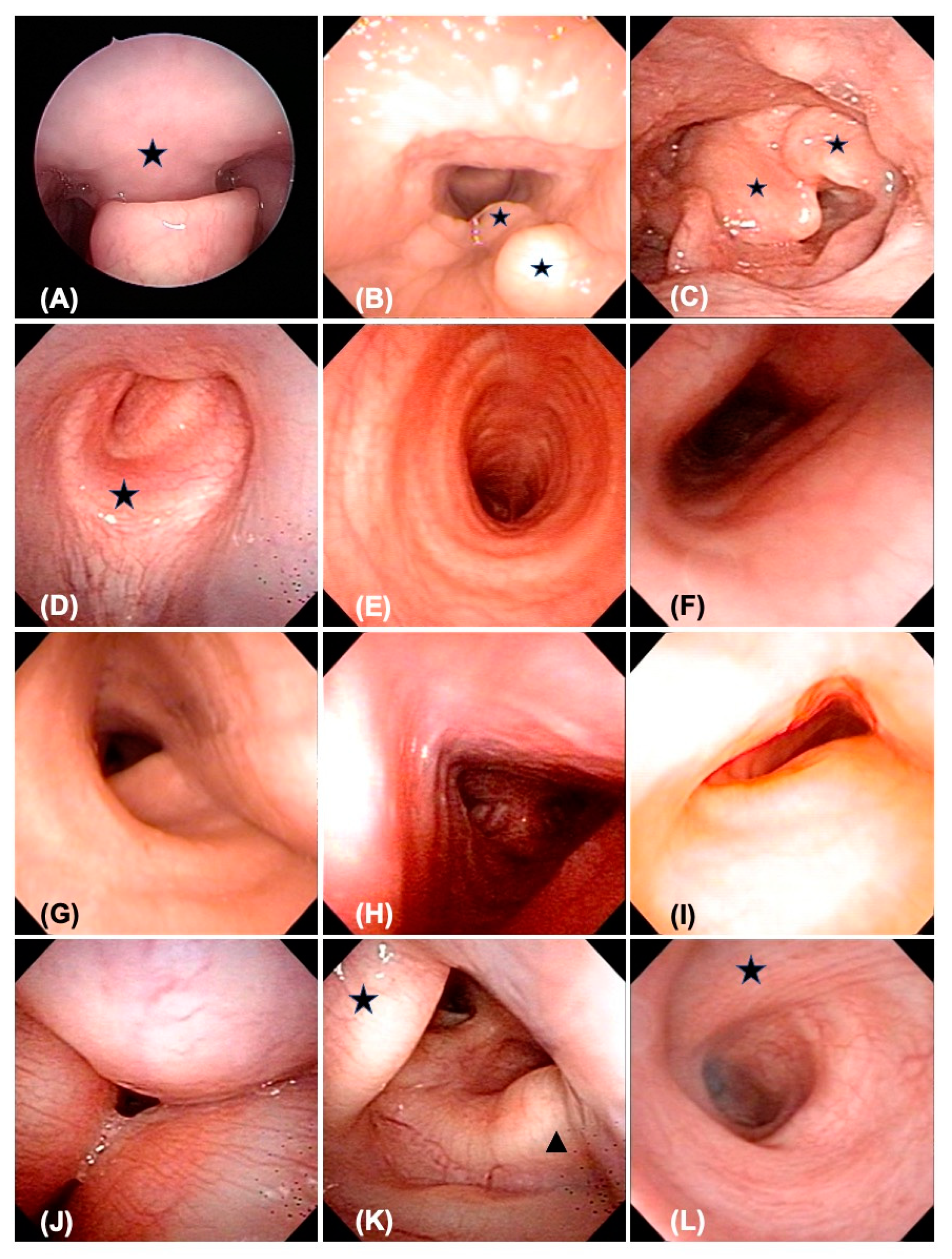
2.3. Fiberoptic Bronchoscopy (FB)
| Parameters | Grading | Measurement | |
|---|---|---|---|
| (a) | |||
| Pharynx | Adenoids [19] | 0 | Post adenoidectomy |
| 1 | Adenoid tissue not in contact with other structures | ||
| 2 | Adenoid tissue in contact with torus tubaris | ||
| 3 | Adenoid tissue in contact with torus tubaris, vomer | ||
| 4 | Adenoid tissue in contact with torus tubaris, vomer, soft palate | ||
| Tonsils [18] | 0 | No tonsils seen in pillars or post tonsillectomy | |
| 1 | Within the pillars | ||
| 2 | Extended to the pillars | ||
| 3 | Extended past the pillars | ||
| 4 | Extended to the midline | ||
| Tongue [18] | I | Full visibility of uvula, tonsils and pillars | |
| IIa | Visibility of most of the uvula but not of the entire tonsils/pillars. | ||
| IIb | Visualization of the entire soft palate to the uvular base. | ||
| III | Partial visibility of the soft palate with distal end absent | ||
| IV | Visibility of only the hard palate | ||
| Prolapsed soft palate (high larynx) | – | Uvula not touching the epiglottis | |
| + | Uvula touching the epiglottis | ||
| Larynx | 20 LM | – | Smooth supraglottic tissue |
| + | Redundant supraglottic tissue, without prolapse or airway compromise | ||
| ++ | Redundant supraglottic tissue, with prolapse and airway compromise | ||
| VC granulation | – | Thin and smooth vocal cord | |
| + | Vocal cord bulging and granulated change | ||
| Cricoid thickness | – | Cricoid cartilage smooth with patent lumen | |
| + | Cricoid cartilage thickening with narrowed lumen | ||
| Trachea | Stenosis [20] | I | 0% to 50% decrease in lumen surface |
| II | 51% to 70% decrease | ||
| III | 71% to 99% decrease | ||
| IV | No evidence of detectable lumen | ||
| Shape | C | C-shaped lumen, normal tracheal morphology | |
| U | U-shaped lumen, mild to moderate lateral collapse of tracheal lumen | ||
| D | D-shaped lumen, mild to moderate anterior–posterior collapse of tracheal lumen | ||
| T | Triangular lumen, moderate to severe tracheal deformity caused by multiple factors | ||
| W | Worm-shaped lumen, severe tracheal deformity caused by multiple factors | ||
| B | Tracheal lumen in the shape of the Mercedes–Benz logo, severe tracheal deformity caused by multiple factors | ||
| Deposit nodule | – | No evidence of GAGs deposition in the tracheal lumen | |
| + | Nodular deposition of GAGs in the tracheal wall | ||
| Kinking | – | No sharp-angled kinking in the tracheal lumen | |
| + | Tracheal torsion and bending with sharp-angled kinking in the lumen | ||
| TM with rigid lumen | – | Tracheoesophageal membrane stays supple | |
| + | Deformed tracheal ring and lumen with circumferential stiffness, involving the tracheoesophageal membrane | ||
| Bronchus | Collapse | – | Bronchial lumen patent |
| + | Bronchial lumen collapsed | ||
| (b) | |||
| Chest | Deformity | – | Normal thoracic cage and sternal curve |
| + | Thoracic cage and sternum deformed, with angle of curved sternum >135 degrees | ||
| ++ | Thoracic cage and sternum deformed, with angle of curved sternum 90~135 degrees | ||
| +++ | Thoracic cage and sternum deformed, with angle of curved sternum <90 degrees | ||
| Sternal angle | Measuring the angle of the deformed sternum by tools of the PACS | ||
| Trachea | Stenosis | – | Normal shape of the trachea, or NW ratio of 76–100% |
| + | Mildly collapsed, NW ratio of 51–75% | ||
| ++ | Moderately collapsed, NW ratio of 26–50% | ||
| +++ | Severely collapsed, NW ratio <25% | ||
| NW ratio | Measuring the ratio of the narrowest and widest cross-sectional area by PACS | ||
| Torsion | – | Straight and smooth trachea | |
| + | Deformed trachea with torsion or bending morphology | ||
| Kinking | – | Straight and smooth trachea | |
| + | Deformed trachea with sharp-angled kinking | ||
| External compression | – | Straight and smooth trachea | |
| + | Deformed trachea caused by innominate artery or aortic arch external compression | ||
| Framework damage | – | Straight and smooth trachea | |
| + | Deformed trachea with framework damage | ||
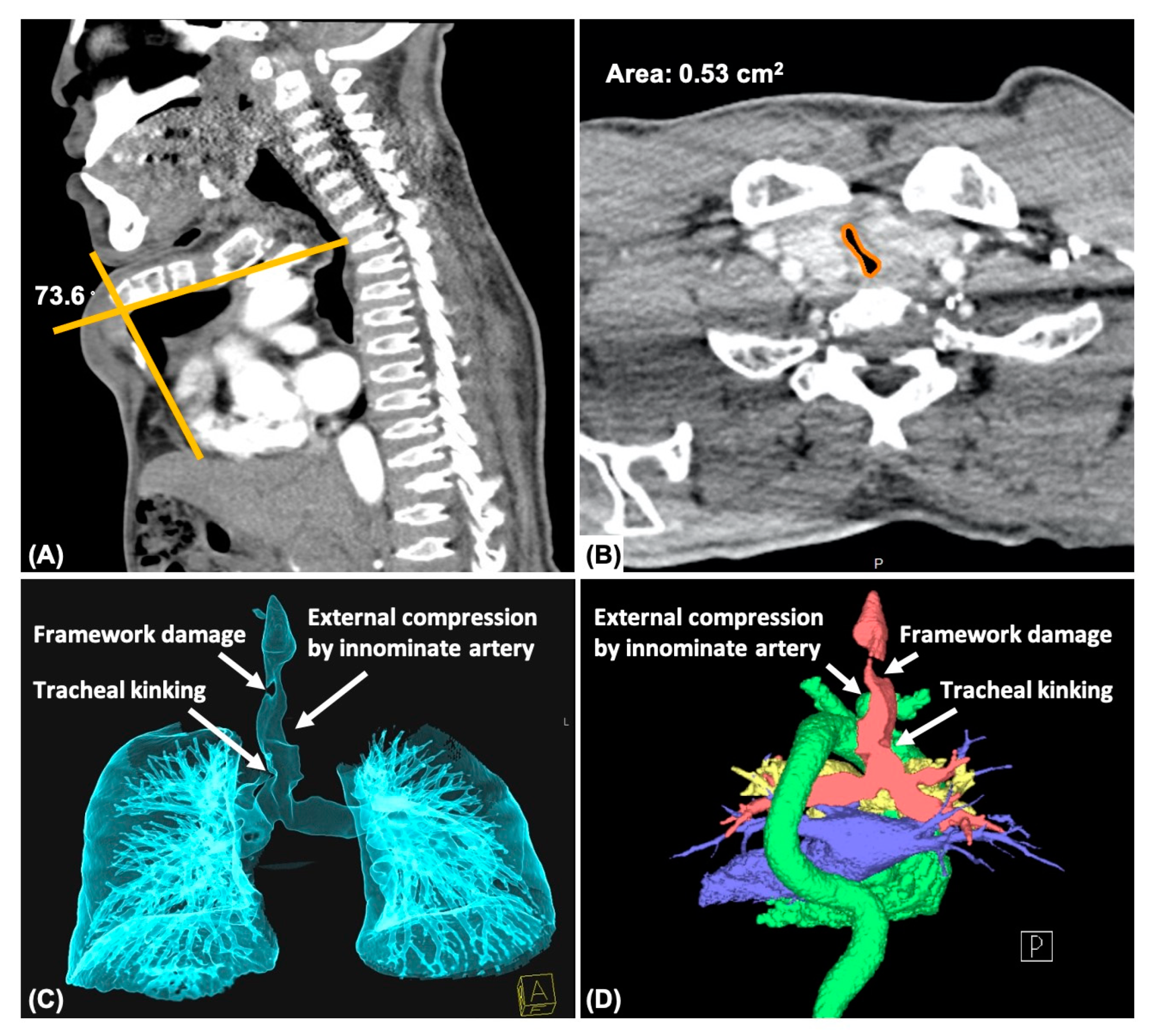
2.4. Computed Tomography (CT)
2.5. Surgical Treatment for the Airway
2.6. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. Fiberoptic Bronchoscopy
3.2.1. Pharynx
3.2.2. Larynx
3.3. Trachea and Bronchi
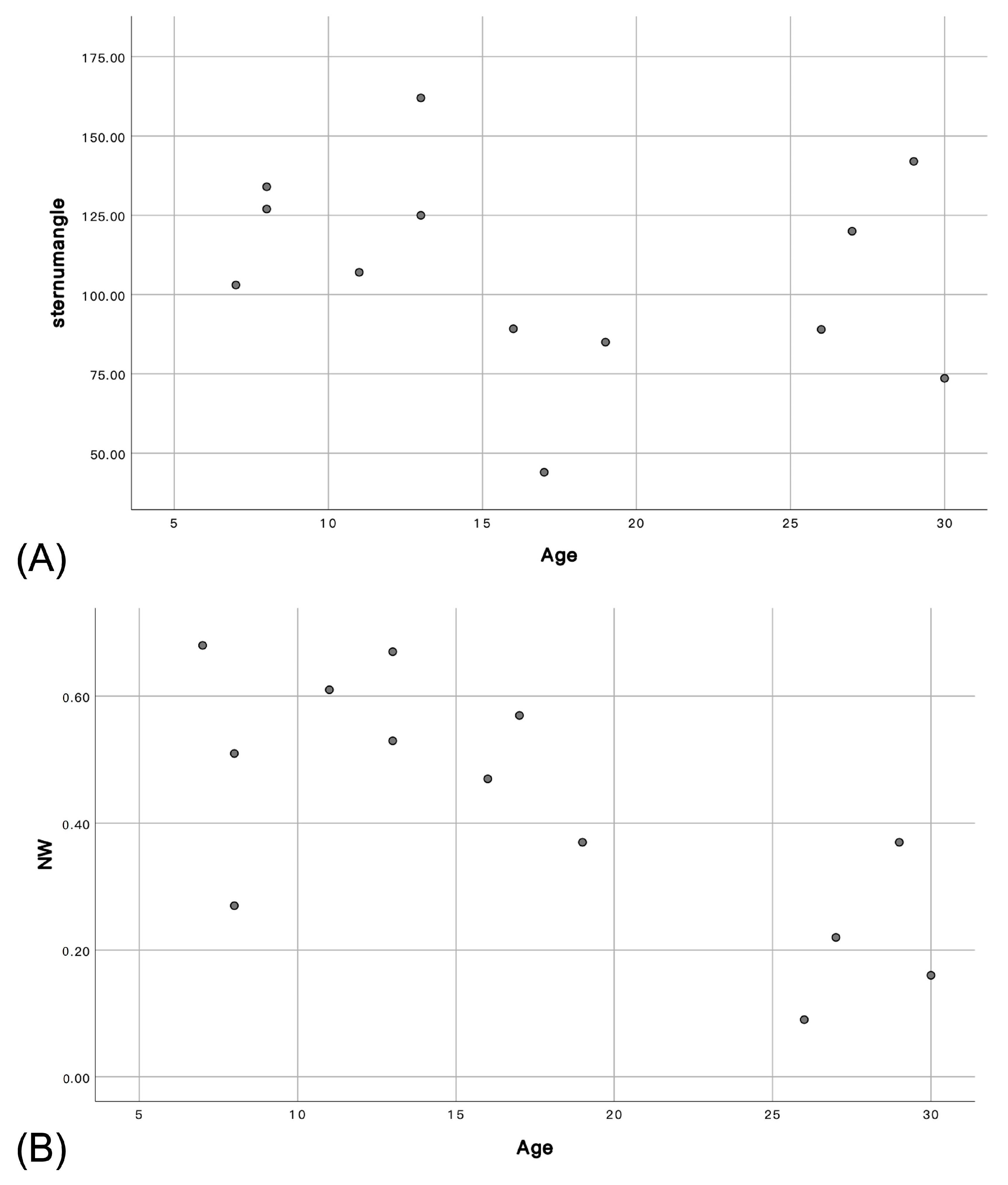
3.4. Computed Tomography
3.4.1. Chest
3.4.2. Trachea
3.5. Surgery with Pathological Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muenzer, J. Mucopolysaccharidoses. Adv. Pediatr. 1986, 33, 269–302. [Google Scholar]
- Peracha, H.; Sawamoto, K.; Averill, L.; Kecskemethy, H.; Theroux, M.; Thacker, M.; Nagao, K.; Pizarro, C.; Mackenzie, W.; Kobayashi, H.; et al. Molecular genetics and metabolism, special edition: Diagnosis, diagnosis and prognosis of Mucopolysaccharidosis IVA. Mol. Genet. Metab. 2018, 125, 18–37. [Google Scholar] [CrossRef]
- Sawamoto, K.; Alvarez Gonzalez, J.V.; Piechnik, M.; Otero, F.J.; Couce, M.L.; Suzuki, Y.; Tomatsu, S. Mucopolysaccharidosis IVA: Diagnosis, Treatment, and Management. Int. J. Mol. Sci. 2020, 21, 1517. [Google Scholar] [CrossRef]
- Simmons, M.A.; Bruce, I.A.; Penney, S.; Wraith, E.; Rothera, M.P. Otorhinolaryngological manifestations of the mucopolysaccharidoses. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 589–595. [Google Scholar] [CrossRef]
- Gonuldas, B.; Yilmaz, T.; Sivri, H.S.; Gucer, K.S.; Kilinc, K.; Genc, G.A.; Kilic, M.; Coskun, T. Mucopolysaccharidosis: Otolaryngologic findings, obstructive sleep apnea and accumulation of glucosaminoglycans in lymphatic tissue of the upper airway. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 944–949. [Google Scholar] [CrossRef]
- Berger, K.I.; Fagondes, S.C.; Giugliani, R.; Hardy, K.A.; Lee, K.S.; McArdle, C.; Scarpa, M.; Tobin, M.J.; Ward, S.A.; Rapoport, D.M. Respiratory and sleep disorders in mucopolysaccharidosis. J. Inherit. Metab. Dis. 2013, 36, 201–210. [Google Scholar] [CrossRef]
- Bianchi, P.M.; Gaini, R.; Vitale, S. ENT and mucopolysaccharidoses. Ital. J. Pediatr. 2018, 44, 127. [Google Scholar] [CrossRef]
- Murgasova, L.; Jurovcik, M.; Jesina, P.; Malinova, V.; Bloomfield, M.; Zeman, J.; Magner, M. Otorhinolaryngological manifestations in 61 patients with mucopolysaccharidosis. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110137. [Google Scholar] [CrossRef]
- Muhlebach, M.S.; Shaffer, C.B.; Georges, L.; Abode, K.; Muenzer, J. Bronchoscopy and airway management in patients with mucopolysaccharidoses (MPS). Pediatr. Pulmonol. 2013, 48, 601–607. [Google Scholar] [CrossRef]
- Yasuda, E.; Fushimi, K.; Suzuki, Y.; Shimizu, K.; Takami, T.; Zustin, J.; Patel, P.; Ruhnke, K.; Shimada, T.; Boyce, B.; et al. Pathogenesis of Morquio A syndrome: An autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013, 109, 301–311. [Google Scholar] [CrossRef]
- Tulebayeva, A.; Sharipova, M.; Boranbayeva, R. Respiratory Dysfunction in Children and Adolescents with Mucopolysaccharidosis Types I, II, IVA, and VI. Diagnostics 2020, 10, 63. [Google Scholar] [CrossRef]
- Tomatsu, S.; Averill, L.W.; Sawamoto, K.; Mackenzie, W.G.; Bober, M.B.; Pizarro, C.; Goff, C.J.; Xie, L.; Orii, T.; Theroux, M. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol. Genet. Metab. 2016, 117, 150–156. [Google Scholar] [CrossRef]
- Hendriksz, C.J.; Harmatz, P.; Beck, M.; Jones, S.; Wood, T.; Lachman, R.; Gravance, C.G.; Orii, T.; Tomatsu, S. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol. Genet. Metab. 2013, 110, 54–64. [Google Scholar] [CrossRef]
- Sawamoto, K.; Suzuki, Y.; Mackenzie, W.G.; Theroux, M.C.; Pizarro, C.; Yabe, H.; Orii, K.E.; Mason, R.W.; Orii, T.; Tomatsu, S. Current therapies for Morquio A syndrome and their clinical outcomes. Expert. Opin. Orphan. Drugs 2016, 4, 941–951. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hsieh, L.C.; Su, C.H.; Lin, H.Y.; Lin, S.P.; Lee, K.S. Airway Management of the Deformed Trachea Using T-Tube Stents in Patients with Mucopolysaccharidosis Type IVA. Ann. Otol. Rhinol. Laryngol. 2022, 131, 562–566. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montano, A.M.; Oikawa, H.; Smith, M.; Barrera, L.; Chinen, Y.; Thacker, M.M.; Mackenzie, W.G.; Suzuki, Y.; Orii, T. Mucopolysaccharidosis type IVA (Morquio A disease): Clinical review and current treatment. Curr. Pharm. Biotechnol. 2011, 12, 931–945. [Google Scholar] [CrossRef]
- Hendriksz, C.J.; Al-Jawad, M.; Berger, K.I.; Hawley, S.M.; Lawrence, R.; Mc Ardle, C.; Summers, C.G.; Wright, E.; Braunlin, E. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J. Inherit. Metab. Dis. 2013, 36, 309–322. [Google Scholar] [CrossRef]
- Friedman, M.; Salapatas, A.M.; Bonzelaar, L.B. Updated Friedman Staging System for Obstructive Sleep Apnea. Adv. Otorhinolaryngol. 2017, 80, 41–48. [Google Scholar] [CrossRef]
- Parikh, S.R.; Coronel, M.; Lee, J.J.; Brown, S.M. Validation of a new grading system for endoscopic examination of adenoid hypertrophy. Otolaryngol. Head Neck Surg. 2006, 135, 684–687. [Google Scholar] [CrossRef]
- Myer, C.M., 3rd; O’Connor, D.M.; Cotton, R.T. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann. Otol. Rhinol. Laryngol. 1994, 103, 319–323. [Google Scholar] [CrossRef]
- Morimoto, N.; Kitamura, M.; Kosuga, M.; Okuyama, T. CT and endoscopic evaluation of larynx and trachea in mucopolysaccharidoses. Mol. Genet. Metab. 2014, 112, 154–159. [Google Scholar] [CrossRef]
- Pires de Mello, P.; Lopes Barth, A.; de Araujo Torres, D.; Pires de Mello Valente, M.; Dain Gandelman Horovitz, D. Laryngeal, Tracheal, and Bronchial Disease in the Mucopolysaccharidoses: Endoscopic Study. Diagnostics 2020, 10, 37. [Google Scholar] [CrossRef]
- Fernandes, S.; Borges, J.; Martins, M. Awake airway endoscopy in mucopolysaccharidosis: A case report. Braz. J. Anesthesiol. 2021, in press. [CrossRef]
- Gadepalli, C.; Stepien, K.M.; Sharma, R.; Jovanovic, A.; Tol, G.; Bentley, A. Airway Abnormalities in Adult Mucopolysaccharidosis and Development of Salford Mucopolysaccharidosis Airway Score. J. Clin. Med. 2021, 10, 3275. [Google Scholar] [CrossRef]
- Akyol, M.U.; Alden, T.D.; Amartino, H.; Ashworth, J.; Belani, K.; Berger, K.I.; Borgo, A.; Braunlin, E.; Eto, Y.; Gold, J.I.; et al. Recommendations for the management of MPS IVA: Systematic evidence- and consensus-based guidance. Orphanet. J. Rare Dis. 2019, 14, 137. [Google Scholar] [CrossRef]
- Shih, S.L.; Lee, Y.J.; Lin, S.P.; Sheu, C.Y.; Blickman, J.G. Airway changes in children with mucopolysaccharidoses. Acta Radiol. 2002, 43, 40–43. [Google Scholar] [CrossRef]
- Rutten, M.; Ciet, P.; van den Biggelaar, R.; Oussoren, E.; Langendonk, J.G.; van der Ploeg, A.T.; Langeveld, M. Severe tracheal and bronchial collapse in adults with type II mucopolysaccharidosis. Orphanet. J. Rare Dis. 2016, 11, 50. [Google Scholar] [CrossRef]
- Averill, L.W.; Kecskemethy, H.H.; Theroux, M.C.; Mackenzie, W.G.; Pizarro, C.; Bober, M.B.; Ditro, C.P.; Tomatsu, S. Tracheal narrowing in children and adults with mucopolysaccharidosis type IVA: Evaluation with computed tomography angiography. Pediatr. Radiol. 2021, 51, 1202–1213. [Google Scholar] [CrossRef]
- Muhlebach, M.S.; Wooten, W.; Muenzer, J. Respiratory manifestations in mucopolysaccharidoses. Paediatr. Respir. Rev. 2011, 12, 133–138. [Google Scholar] [CrossRef]
- McLaughlin, A.M.; Farooq, M.; Donnelly, M.B.; Foley, K. Anaesthetic considerations of adults with Morquio’s syndrome—A case report. BMC Anesthesiol. 2010, 10, 2. [Google Scholar] [CrossRef]
- Theroux, M.C.; Nerker, T.; Ditro, C.; Mackenzie, W.G. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr. Anaesth. 2012, 22, 901–907. [Google Scholar] [CrossRef]
- You, A.H.; Kim, M.K.; Kang, H.Y. Anesthetic management of an adult patient with Morquio A syndrome. J. Clin. Anesth. 2019, 56, 106–107. [Google Scholar] [CrossRef]
- Pizarro, C.; Davies, R.R.; Theroux, M.; Spurrier, E.A.; Averill, L.W.; Tomatsu, S. Surgical Reconstruction for Severe Tracheal Obstruction in Morquio A Syndrome. Ann. Thorac. Surg. 2016, 102, e329–e331. [Google Scholar] [CrossRef]
- Clark, B.M.; Sprung, J.; Weingarten, T.N.; Warner, M.E. Airway management changes in patients with mucopolysaccharidoses: The role of video laryngoscopy. Can. J. Anaesth. 2017, 64, 981–982. [Google Scholar] [CrossRef]
- Antón-Pacheco, J.L.; Cabezalí, D.; Tejedor, R.; López, M.; Luna, C.; Comas, J.V.; de Miguel, E. The role of airway stenting in pediatric tracheobronchial obstruction☆. Eur. J. Cardio-Thorac. Surg. 2008, 33, 1069–1075. [Google Scholar] [CrossRef]
- Davitt, S.M.; Hatrick, A.; Sabharwal, T.; Pearce, A.; Gleeson, M.; Adam, A. Tracheobronchial stent insertions in the management of major airway obstruction in a patient with Hunter syndrome (type-II mucopolysaccharidosis). Eur. Radiol. 2002, 12, 458–462. [Google Scholar] [CrossRef]
- Karl, R.; Carola, S.; Regina, E.; Thomas, N.; Huber, R.M. Tracheobronchial stents in mucopolysaccharidosis. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 187–192. [Google Scholar] [CrossRef]
- Kampmann, C.; Wiethoff, C.M.; Huth, R.G.; Staatz, G.; Mengel, E.; Beck, M.; Gehring, S.; Mewes, T.; Abu-Tair, T. Management of Life-Threatening Tracheal Stenosis and Tracheomalacia in Patients with Mucopolysaccharidoses. JIMD Rep. 2017, 33, 33–39. [Google Scholar] [CrossRef]
- Gocyk, W.; Warmus, J.; Olechnowicz, H.; Bik-Multanowski, M.; Pawlinski, L.; Kiec-Wilk, B. Case report of endoprosthesis -Y implantation in severe respiratory failure in the MPSII patient; comparison with literature data. BMC Pulm. Med. 2020, 20, 99. [Google Scholar] [CrossRef]
- Soni-Jaiswal, A.; Penney, S.E.; Jones, S.A.; Walker, R.; Rothera, M.P.; Bruce, I.A. Montgomery© T-tubes in the management of multilevel airway obstruction in mucopolysaccharidosis. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1763–1768. [Google Scholar] [CrossRef]

| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| Sex | M | F | F | M | F | F | M | M | M | M | M | F | M | M | M | ||
| Age (years) | 7 | 16 | 11 | 19 | 13 | 8 | 8 | 26 | 27 | 30 | 13 | 17 | 29 | 22 | 21 | ||
| ERT | – | – | – | + (3 years) | – | – | + (1 year) | + (5 months) | – | – | + (10 years) | – | – | – | – | ||
| FB | Pharynx | Adenoids | 2 | 2 | 2 | 2 | 2 | 3 | 0 | 1 | 2 | 2 | 4 | 4 | 2 | 1 | 2 |
| Tonsils | 3 | 3 | 0 | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | ||
| Tongue | III | IV | IV | IV | IV | III | IV | IV | IV | IV | IIb | IV | IV | III | IV | ||
| Prolapsed soft palate | – | + | + | + | – | + | + | + | + | + | – | + | + | + | + | ||
| Larynx | 20 LM | – | + | + | ++ | + | ++ | ++ | ++ | ++ | ++ | – | ++ | ++ | + | ++ | |
| VC granulation | – | + | – | – | – | – | + | + | + | + | – | + | + | – | + | ||
| Cricoid thickness | + | + | + | + | – | + | + | – | + | + | + | – | + | ||||
| Trachea | Stenosis | I | II | I | II | I | I | III | III | III | III | I | III | ||||
| Shape (upper/middle/lower) | C/C/C | U/D/T | U/D/D | T/U/T | C/U/C | U/U/C | T/W/U | B/D/T | W/T/T | B/T/T | C/C/C | W/U/U | |||||
| Deposit node | – | + | – | – | – | – | – | – | – | + | – | – | |||||
| Kinking | – | + | – | – | – | – | – | – | – | + | – | – | |||||
| TM with rigid lumen | – | – | – | + | – | – | + | + | + | + | – | – | |||||
| Bronchus | collapse | – | – | – | – | – | – | – | – | – | – | – | – | ||||
| CT | Chest | Deformity | ++ | +++ | ++ | +++ | – | ++ | ++ | +++ | + | +++ | ++ | +++ | + | ||
| Sternum angle (°) | 103 · | 89.2 | 107 | 85 | 162 | 127 | 134 | 89 | 120 | 73.6 | 125 | 44 | 142 | ||||
| Trachea | Collapse | – | ++ | + | ++ | + | + | ++ | +++ | +++ | +++ | – | + | ++ | |||
| NW ratio | 68% | 47% | 61% | 37% | 53% | 51% | 27% | 9% | 22% | 16% | 67% | 57% | 37% | ||||
| Torsion | – | – | + | + | – | – | + | - | + | + | – | + | + | ||||
| Kinking | – | + | – | – | – | – | – | – | – | + | – | + | – | ||||
| External compression | – | + (I+A) | + (I) | + (I) | – | – | + (I+A) | + (I) | + (I) | + (I+A) | – | – | + (I) | ||||
| Framework damage | – | – | + | – | – | – | – | – | – | + | – | – | + | ||||
| Operation | – | T+A+LM | T+A | A | T+A+LM | – | A A T+LM | T-tube+T+A+LM | T-tube | T-tube +LM | – | – | – | – | – | ||
| Age at operation (years) | – | 16 | 9 | 13 | 13 | – | 6 7 8 | 26 | 27 | 31 | – | – | – | – | – | ||
| Pathology (Colloidal iron stain) | – | Not tested | Tonsils (+) Adenoids (+) | Adenoids (–) | Tonsils (–) Adenoids (–) | – | Tonsils (+) Adenoids (–) | Trachea (+) Arytenoid (–) Tonsil (+) | Deep neck soft tissue (–) Trachea (+) | Arytenoid (+) Cricoid (+) | – | – | – | – | – | ||
| Variables | N (%) |
|---|---|
| Fiberoptic-bronchoscopy | |
| Pharynx (N = 15) | |
| Adenoids | |
| 0 | 1 (6.7) |
| 1 | 2 (13.3) |
| 2 | 9 (60.0) |
| 3 | 1 (6.7) |
| 4 | 2 (13.3) |
| Tonsils | |
| 0 | 1 (6.7) |
| 1 | 0 (0.0) |
| 2 | 1 (6.7) |
| 3 | 12 (80.0) |
| 4 | 1 (6.7) |
| Tongue | |
| I | 0 (0.0) |
| IIa | 0 (0.0) |
| IIb | 1 (6.7) |
| III | 3 (20.0) |
| IV | 11 (73.3) |
| Uvula in larynx (+) | 12 (80.0) |
| Larynx (N = 15) | |
| 20 LM | |
| – | 2 (13.3) |
| + | 4 (26.7) |
| ++ | 9 (60.0) |
| VC granulation (+) | 8 (53.3) |
| Cricoid thickness (+) | 10 (76.9) |
| Trachea and Bronchus (N = 12) | |
| Stenosis | |
| I | 5 (41.7) |
| II | 2 (16.7) |
| III | 5 (41.7) |
| IV | 0 (0) |
| Shape a | |
| C | 4 (33.3) |
| U | 7 (58.3) |
| D | 3 (25.0) |
| T | 6 (50.0) |
| W | 3 (25.0) |
| B | 2 (16.7) |
| Deposit nodule (+) | 2 (16.7) |
| Kinking (+) | 2 (16.7) |
| TM with rigid lumen (+) | 5 (41.7) |
| Bronchus collapse (+) | 0 (0.0) |
| Computed tomography | |
| Chest | |
| Deformity | |
| – | 1 (7.7) |
| + | 2 (15.4) |
| ++ | 5 (38.5) |
| +++ | 5 (38.5) |
| Sternal angle (°) | 107.8/107.0 |
| Trachea | |
| Stenosis | |
| – | 2 (15.4) |
| + | 3 (23.1) |
| ++ | 5 (38.5) |
| +++ | 3 (23.1) |
| NW ratio (%) | 42.5/47.0 |
| Torsion (+) | 8 (61.5) |
| Kinking (+) | 3 (23.1) |
| External compression (+) | 8 (61.5) |
| Framework damage (+) | 3 (23.1) |
| Surgery type and Pathology | |
| Surgery type (N = 15) | |
| Tonsillectomy | 5 (33.3) |
| Adenoidectomy | 6 (40.0) |
| Laser supraglottoplasty | 5 (33.3) |
| Tracheostomy with T-tube stenting | 3 (20.0) |
| Pathology (Colloid iron stain) | |
| Tonsil | 3/5 (60.0) |
| Adenoid | 1/6 (16.7) |
| LM | 1/5 (20.0) |
| Neck | 0/3 (0.0) |
| Trachea | 2/3 (66.7) |
| Cricoid | 1/3 (33.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Su, C.-H.; Lin, C.-Y.; Lin, H.-Y.; Lin, S.-P.; Chuang, C.-K.; Lee, K.-S. Endoscopic and Image Analysis of the Airway in Patients with Mucopolysaccharidosis Type IVA. J. Pers. Med. 2023, 13, 494. https://doi.org/10.3390/jpm13030494
Lee Y-H, Su C-H, Lin C-Y, Lin H-Y, Lin S-P, Chuang C-K, Lee K-S. Endoscopic and Image Analysis of the Airway in Patients with Mucopolysaccharidosis Type IVA. Journal of Personalized Medicine. 2023; 13(3):494. https://doi.org/10.3390/jpm13030494
Chicago/Turabian StyleLee, Yi-Hao, Chin-Hui Su, Che-Yi Lin, Hsiang-Yu Lin, Shuan-Pei Lin, Chih-Kuang Chuang, and Kuo-Sheng Lee. 2023. "Endoscopic and Image Analysis of the Airway in Patients with Mucopolysaccharidosis Type IVA" Journal of Personalized Medicine 13, no. 3: 494. https://doi.org/10.3390/jpm13030494
APA StyleLee, Y.-H., Su, C.-H., Lin, C.-Y., Lin, H.-Y., Lin, S.-P., Chuang, C.-K., & Lee, K.-S. (2023). Endoscopic and Image Analysis of the Airway in Patients with Mucopolysaccharidosis Type IVA. Journal of Personalized Medicine, 13(3), 494. https://doi.org/10.3390/jpm13030494








