1. Introduction
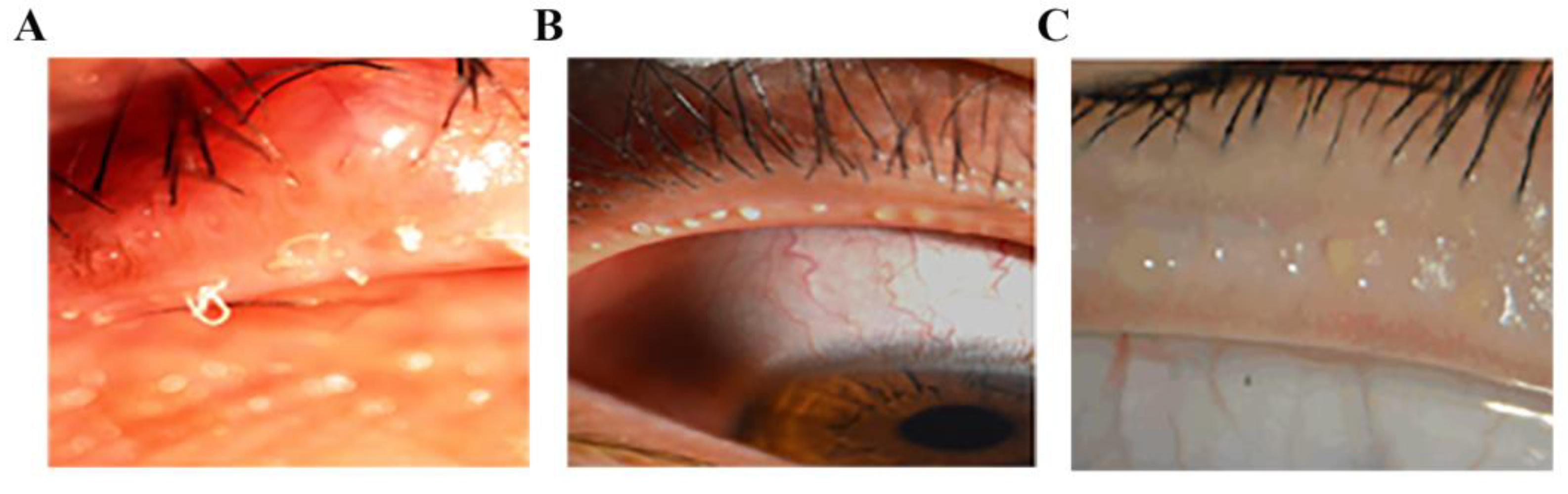
Meibomian gland dysfunction (MGD) is a chronic, non-specific inflammation of the meibomian glands, characterized by the obstruction of meibomian ducts or the abnormal secretion of meibomian glands. In MGD, glandular lipid secretion is damaged and the ocular surface cannot maintain the stability of tear film. Rapid evaporation of tear film and increased osmotic pressure of tear film are the main causes of hyper-evaporative dry eye (
Figure 1) [
1].
MGD causes changes in tear film composition and affects ocular surface health, which is the main cause of evaporative dry eye. The main manifestations are increased tear evaporation, gradual hyperosmolar, inflammation, damage to ocular surface epithelial cells [
2], severe damage to the cornea, and even blindness. The survey portrayed that the incidence of MGD in the Asian population is very high (49.2−69.3%), and is one of the most common forms of eye disease in Asia [
3]. MGD is currently incurable and requires chronic disease treatment. The cost of MGD treatment in the United States and Japan has reached USD 55 billion per year [
4]. The control of inflammation plays a crucial role in the treatment of MGD.
Environmental pollution, smoking, blepharitis, lack of sleep and other factors can lead to MGD [
5]. Its main pathological changes are excessive keratinization of the glandular ducts and an increased viscosity of eyelid fat, which cause blockage of the MG opening, the abnormal differentiation of MG epithelial cells, increased inflammatory mediators, and the infiltration of neutrophils, which in turn cause the blockage of MG glandular ducts [
6]. Studies have shown that the occurrence and development of MGD is accompanied by the infiltration of macrophages [
7]. Macrophages belong to leukocytes, which are phagocytic cells specialized by the innate immune system. Together with neutrophils, they become the first responders to infection, participating in the recognition, phagocytosis and degradation of cell debris and pathogens, maintaining tissue homeostasis and tissue repair and remodeling [
8]. Previous studies on the pathogenesis of MGD have mainly focused on the response of MG to inflammatory stimuli, the anti-inflammatory effect of meiborate, the “flora imbalance” of the eyelid margin, and the infiltration of central granulocytes. Although macrophages have been proven to infiltrate MGD tissues, no relative study has been reported about the specific function and mechanism of different activated macrophages in the immune response of MGD progression [
9,
10,
11].
TIPE2 is a cytoplasmic protein that exists in myeloid, lymphoid and liver cells, and is expressed differently at different stages of cell development. With the deepening of research, TIPE2 is found to be a reversed coordinator of immunity that is involved in the stable immune state, expressed in both immune and non-immune tissues, and is an ideal biomarker [
12]. In the study of ophthalmic diseases, TIPE2 acts as an essential character in the inflammation of choroid. Suo et al. [
13] observed the expression of TIPE2 in the cytoplasm and nucleus of retinal pigment epithelial cells under normal and inflammatory conditions. With the development of inflammatory response, the cell activity decreased and the expression of TIPE2 decreased. It is clear that the immune function of macrophages is concerned with the standard of TIPE2. After LPS was used to entice mouse bone-marrow-derived macrophages to M1 macrophages, the expression level of TIPE2 was observed to decrease. After IL-4 was induced to M2 macrophages, the standard of TIPE2 was observed to increase [
14]. However, the role of TIPE2 in the polarization of macrophages and the immune response of MGD progression is still unknown. In this study, an ApoE
-/- MGD mouse model and MGD cadaveric MG tissue were used to explore the characters of macrophages (M1/M2) and the distribution of TIPE2 in the progression of MGD, verifying the regulation of TIPE2 in macrophage polarization around MG in the immune response in order to provide new anti-inflammatory targets for MGD treatment.
2. Materials and Methods
In this study, an MGD model of ApoE-/- mice was used which showed MGD symptoms at 5 months of age. Based on this, a sampling time point for the MGD model of ApoE-/- mice was developed. Normal mice of the same age were used as a control group. MG tissues and secretions were collected from the ApoE-/- MGD mouse model (1, 3, 5, and 7 months old). MG tissues in each group were divided into three parts, which were prepared into frozen sections and mRNA was extracted for detection. All studies were conducted in line with the declaration of the Association for Visual and Ophthalmic Research on the use of animals in ophthalmology and optical experiments, and were approved by the Animal Ethics Committee of Shanghai Jiao Tong University.
None of the 4 MGD cadaveric donors had known systemic diseases. Four donors were divided into two groups: two with mild MGD and two with severe MGD. The donor’s medical history and eye history were identified and collected before the tarsus was removed from the upper eyelid of the fresh corpse. MG tissues and secretions from each group of donors were collected. The MG tissues of each group were divided into three parts and prepared into frozen sections for detection. The removal of donor cornea and tarsal plate was performed by ophthalmologists according to the National Industry Standards for Ophthalmic Bank Management in China. The use of human tissue in the study complied with the provisions of the Helsinki Declaration.
The meibomian gland tissues were collected and embedded in optimal cutting temperature (OCT) compound or paraffin. Then, the tissues were cut into sagittal sections (5 μm thick) and stored at −80 °C (frozen sections) or room temperature (paraffin sections).
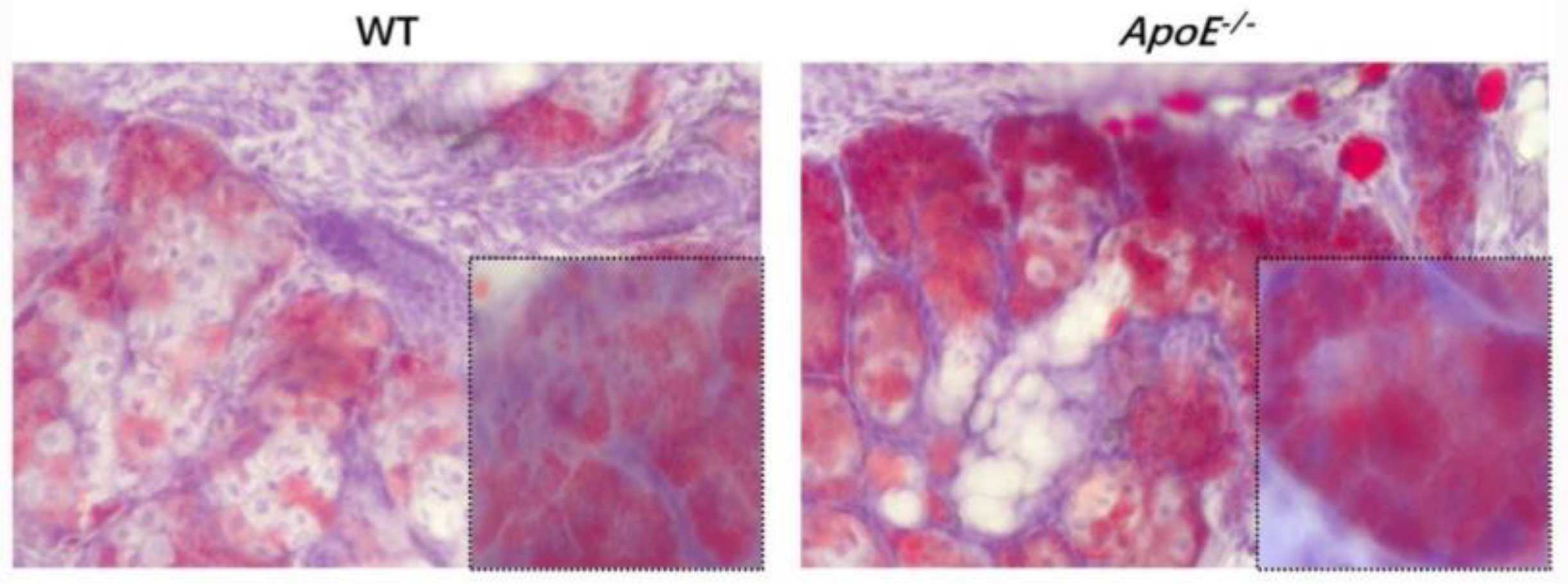
Mice frozen MG portions were fastened in 4% paraformaldehyde for 10 min, rinsed in phosphate-buffered saline (PBS) for 5 min, and dyed in prepared oil red O solution for 10 min. After washing with PBS for 5 min, the portions were counterstained with hematoxylin and installed in 90% glycerol. The MG eyelid fat and inflammatory cell infiltration of the two groups of mice were observed to determine whether the modeling was successful.
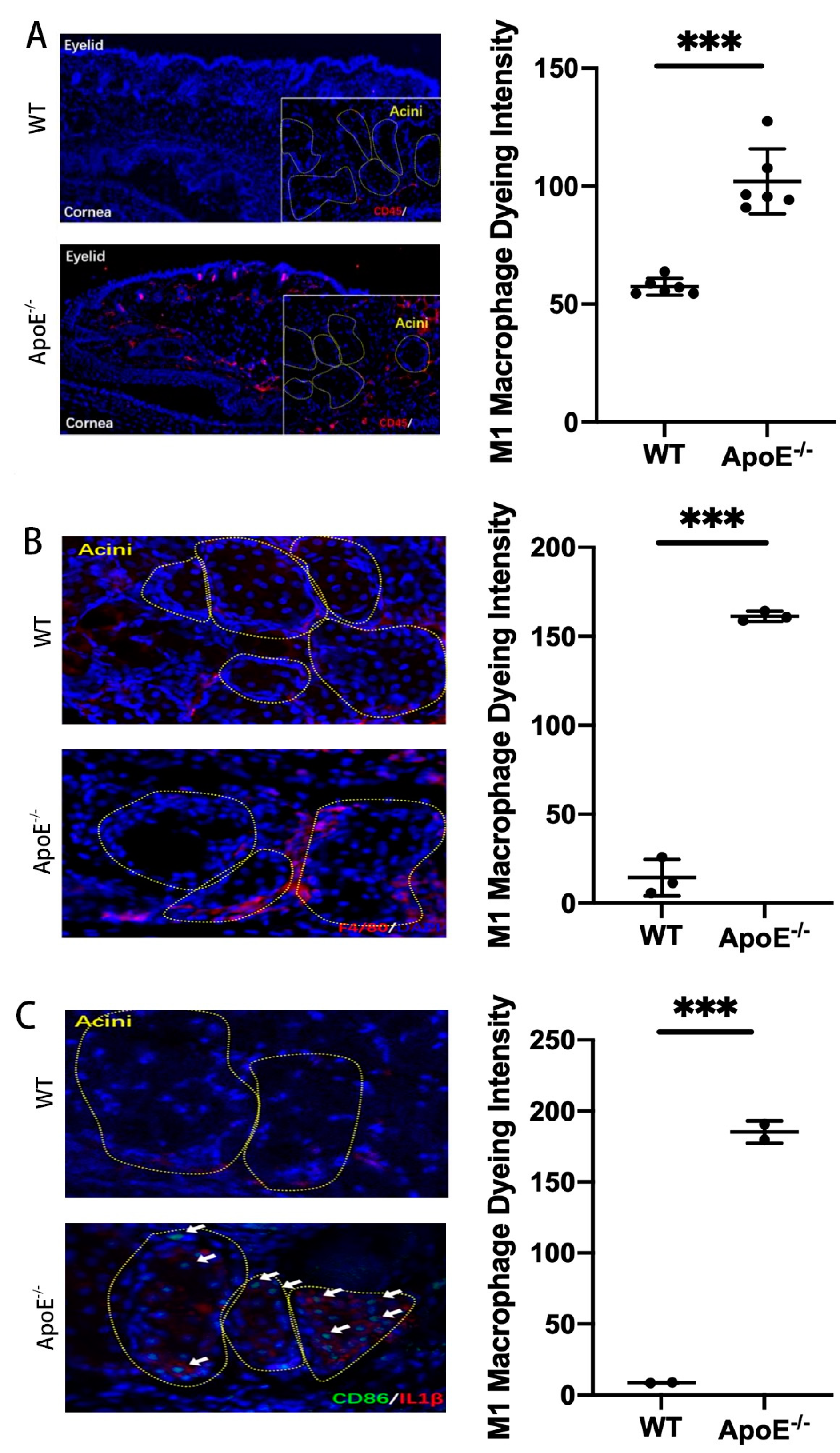
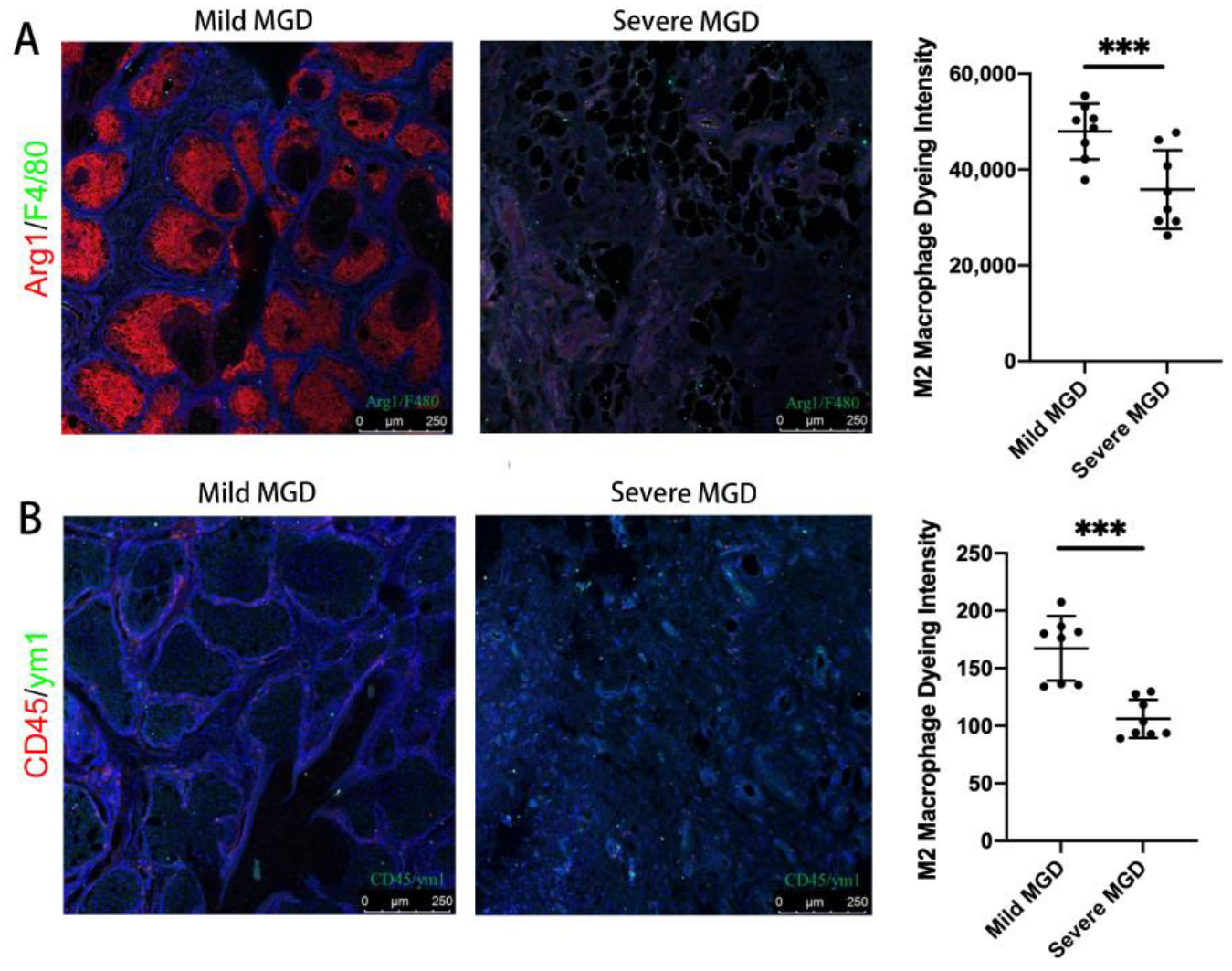
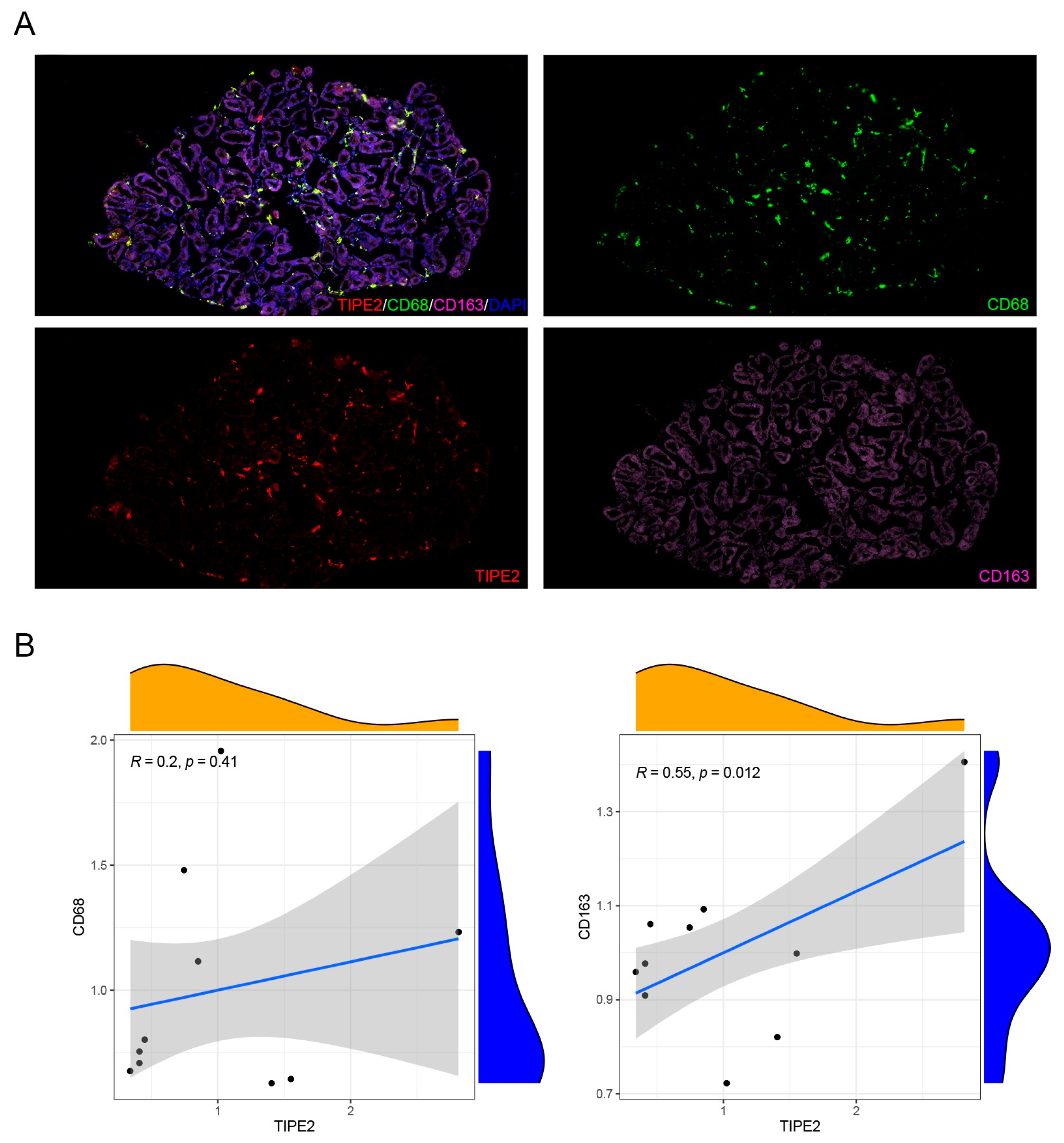
Mice inflammatory cells, M1 macrophage markers (CD45, F4/80, CD86, CD68) and M2 macrophage markers (CD163), and human M1 macrophage markers (CD45, F4/80) and M2 macrophage markers (ARG1, YM1) were detected using an immunofluorescence technique. Thus, the dominant macrophage type in this process could be determined. Frozen sections of MGD mice and human model MG tissues were scanned using a PANNORAMIC (3DHISTECH, Hungary) panoramic section scanner and observed with CaseViewer software (2.4 version, 3DHISTECH, Hungary). Halo analysis software (v3.0.311.314, Indica labs, USA) was utilized to quantify the amount and area of positive cells in the target area of MGD mice slices. Thus, the expression changes in TIPE2 in different types of macrophages in MGD meibomian glands were qualitatively and quantitatively observed.
RAW 246.7 cell line is a mature immortalized mouse macrophage cell line. The culture system used DMEM medium, 10% fetal bovine serum and 1% double antibody, and the liquid was changed every two days. When the cells grew to 70–80%, they were passaged at 1:3, and P3 cells were used for subsequent experiments.
IL-4 and LPS were used in four concentration gradients of 0 ng/mL (control), 10 ng/mL, 20 ng/mL and 40 ng/mL, and four time gradients of 6 h, 12 h, 24 h and 48 h were chosen, with three replicate samples in each group.
Then, 200 µL chloroform was added to a 1.5 mL Eppendorf tube, mixed upside-down, left indoors for 2 min (min), and then centrifuged at 14,000 r/min at 4 °C for 15 min. Then, 500 µL of the upper water phase was absorbed into the new Eppendorf tube, and 600 µL of isopropyl alcohol was added, mixed, and placed at −80 °C for alcohol precipitation for 15 min. The samples were centrifuged at 4 °C and 12,000 r/min for 10 min, and the supernatant was discarded with the pipette. Then, 500 uL 75% ethanol with diethypyrocarbonate (DEPC) was added to each tube, washed fully with vortex, and centrifuged at 12,000 r/min at 4 °C for 5 min. After centrifugation at 12,000 r/min for 5 min, the supernatant was abandoned and the residual liquid was dried by instantaneous centrifugation. Subsequently, it was placed in a fume hood for drying for 10 min until the precipitate became transparent. The precipitate was dissolved in 20 µL DEPC and reserved in a refrigerator at −80 °C for subsequent steps. The extracted mRNA concentration was determined using a spectrophotometer and diluted to a range of 400–600 ng/mL with DEPC. Reverse transcription was undertaken according to the 20 µL system. Firstly, the required premix solution was calculated according to the number of samples, and then the sample was added. Finally, the temperature settings of 25 °C 10 min, 42 °C 60 min, and 70 °C 10 min were used for reverse transcription. The transcribed samples were stored at −20 °C.
MG tissues were isolated with skin, muscle and removed conjunctiva. The total RNA of the MG tissue was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and genomic DNA was removed using DNaseI (TaKara, Shiga, Japan). An ND-2000 spectrophotometer (NanoDrop Technologies, Seattle, WA, USA) was used for RNA quantification. The RNA samples with an eligible value of OD260/280 (1.8–2.2) and OD260/230 (≥2.0) were used for further experimentation. qRT PCR was conducted according to a 10 uL system. First, the premix was configured, and three duplicate samples were added to each sample. Each sample measured ACTIN and TIPE2 genes, and was finally placed in the electrolyte analyzer (Roche, Basel, Switzerland) for qRT-PCR. The thermal analysis conditions used were 95 °C 10 min, 95 °C denaturation 10 s, 60 °C annealing extension 30 s, and 40 cycles. Finally, the data was analyzed using Roche software.
Student’s unpaired t-test was conducted on the density of inflammatory cells around MG in normal mice and MGD mice using GraphPad Prism software (San Diego, CA, USA). Student’s unpaired t-test was performed on the expression of TIPE2 mRNA in M1 and M2 macrophages around MG tissues of IL-4- and LPS-induced MGD mice. A p-value less than 0.05 signified the difference was of statistical significance.
4. Discussion
In this research, we created an MGD mouse model and confirmed the inflammatory impact of M1 macrophages on MGD, the anti-inflammatory impact of M2 macrophages on MGD, and the fact that TIPE2 has a tendency towards the M2-directed polarization of MG macrophages.
Studies have shown that inflammatory factors such as IL-6, matrix metalloproteinase 9, interferon-γ and TNF-α are involved in the pathological process of MGD [
15]. The secretion of inflammatory factors may activate the nuclear factor kappa-B (NF-κB) signaling pathway and the mitogen-activated protein kinase signaling pathway. These pathological mechanisms have been confirmed in studies such as those investigating the link between MGD and high-fat diets [
16]. In the study of cardiovascular plaque stability in mice fed with a high-fat diet, it was found that TIPE2 can reduce macrophage apoptosis by reducing toll-like receptor 4 and the myeloid differentiation primary response gene (88), thus promoting plaque stability [
17]. The observations of the ApoE
-/- MGD mouse model portray that a large quantity of inflammatory cells infiltrated around the MG acinar, and these inflammatory cells were mainly M1 macrophages. At the same time, we carried out tissue section staining of human meibomian glands, and confirmed that there was also macrophage infiltration in the meibomian gland of MGD patients. The severe group was dominated by M1 macrophages, and the mild group was dominated by M2 macrophages. Macrophages belong to leukocytes, which are phagocytic cells specialized by the innate immune system. Together with neutrophils, they become the first responders to infection, participating in the recognition, phagocytosis and degradation of cell debris and pathogens, as well as maintaining tissue homeostasis and tissue repair and remodeling [
18]. Polarization is a simplified description of macrophage heterogeneity and plasticity, and is a continuous state involving multiple factors [
19]. Macrophage polarization can be divided into classical activated M1 macrophages with pro-inflammatory effects and selective activated M2 macrophages with anti-inflammatory effects according to function and cell phenotype [
20]. Polarization reversibility has key therapeutic value in diseases caused by M1/M2 imbalance. The signaling pathways for macrophage polarization to M1 include LPS/toll-like receptor 4 and the NF-κB/phosphatidylinositol 3 kinase pathways. M1 macrophages generally secrete pro-inflammatory factors through interferon-γ and LPS activation [
21]. The signaling pathways of macrophage polarization to M2 include signal transducer and activator of transcription 6 and peroxisome proliferator-activated receptor γ. M2 macrophages can be activated by T helper 2 cytokines to secrete anti-inflammatory factors, which play a role in inhibiting inflammatory response and tissue repair [
22]. Signaling pathway peroxisome proliferator-activated receptor γ specifically guides the lipogenesis of meibomian gland epithelial cells and inhibits the development of MGD [
23]. M1 macrophages, characterized by CD68, CD86, and major compatibility complex II phenotype, play a pro-inflammatory role through the high expression of IL-12, -6, -1β, and other cytokines. M2 macrophages are characterized by CD68, CD209 and Ym1/2, and highly express cytokines and inflammatory medium such as IL-10 and transforming growth factor-β, which play anti-inflammatory and tissue repair roles [
24]. Macrophages are dynamically converted under different microenvironment signals, thereby regulating the immune inflammatory response and affecting the outcome of disease. We observed different types of macrophages in the MG acinar of humans and MGD mice, and M1 type accounted for the majority, which was consistent with the pro-inflammatory effect of the M1 type mentioned above.
The study of the correlation between TIPE2 and immune function began in myeloid cells. Many studies have confirmed that TIPE2 is related to inflammation, and has the function of negatively regulating immune response and prompting apoptosis, which acts as an essential characteristic in maintaining immune homeostasis [
25,
26]. TIPE2 can negatively regulate the mammalian target of rapamycin complex 1 to regulate macrophage polarization by interfering with the excitation of NF-κB cell receptor-associated protease mammalian target of rapamycin complex 1, thereby participating in the inflammatory response [
14]. Wynn et al. reported that TIPE2 promoted the differentiation of macrophages into the M2 type and was necessary for the immunosuppressive function of CD4+ and CD25+ Treg [
27]. In the mice scald model, it was found that in the early stage of inflammatory response, the up-regulation of TIPE2 expression may lessen the intensity of the inflammatory response and avoid the damage caused by an excessive inflammatory response. In the TIPE2
-/- mice stroke model, it was discovered that TIPE2 deficiency increased the amount of neutrophils and macrophages, and elevated the scope of cerebral ischemic injury [
28]. TIPE2 can treat atherosclerosis by inhibiting the NF-κB signaling pathway to decrease the inflammatory response of macrophages [
29]. The over-expression of TIPE2 significantly lowered the proliferation of macrophages and inhibited the secretion of pro-inflammatory factors, becoming a new anti-inflammatory therapy to alleviate muscle weakness in patients with muscular dystrophy [
30]. TIPE2 regulates macrophage M2 polarization and the low expression of pro-inflammatory factors by activating signal transducer and activator of transcription 3 and NF-κB signaling pathways to treat rheumatoid arthritis [
26]. In this study, LPS was used to induce mouse bone-marrow-derived macrophages to M1 macrophages, and the expression level of TIPE2 was observed to decrease. After the induction of M2 macrophages with IL-4, the expression level of TIPE2 was observed to increase, indicating that TIPE2 can regulate macrophage polarization and effectively control the inflammatory response of MGD.
The incidence of MGD has increased year after year because of the ill habits surrounding eye care and diet, as well as air pollution and many other complex factors. MGD is characterized by its chronic and incurable nature, which has a serious impact on quality of life and creates socioeconomic pressures. Therefore, it is necessary to find new treatment strategies for MGD. This study observed the distribution of macrophages in the MG acinar of human and mouse MGD, as well as the characteristics of M2 macrophages in MGD regulated by TIPE2. However, there are still some limitations to this experiment. We have failed to demonstrate the complete mechanism by which TIPE2 regulates M2 macrophages in MGD, but based on all the data we have collected we believe it can work. Thus, there is room for the further study of this mechanism. In future studies, we will further explore the specific mechanism of TIPE2 in macrophage polarization.