Effect of Hydrogen on AM Pyroptosis Induced by Severe Burns in Rats
Abstract
1. Background
2. Materials and Methods
2.1. Animals and Experimental Groups
2.2. Burn Model
2.3. Sample Collection
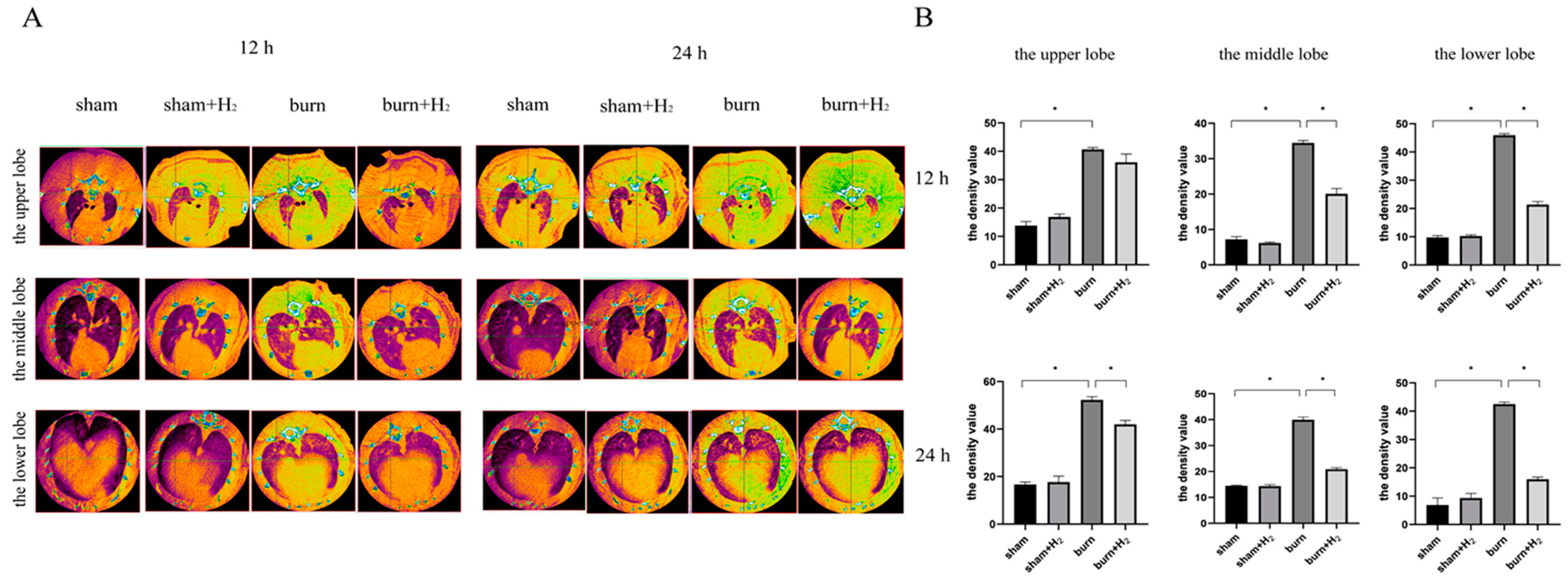
2.4. CT Scan and Density Analysis of the Lung Tissue
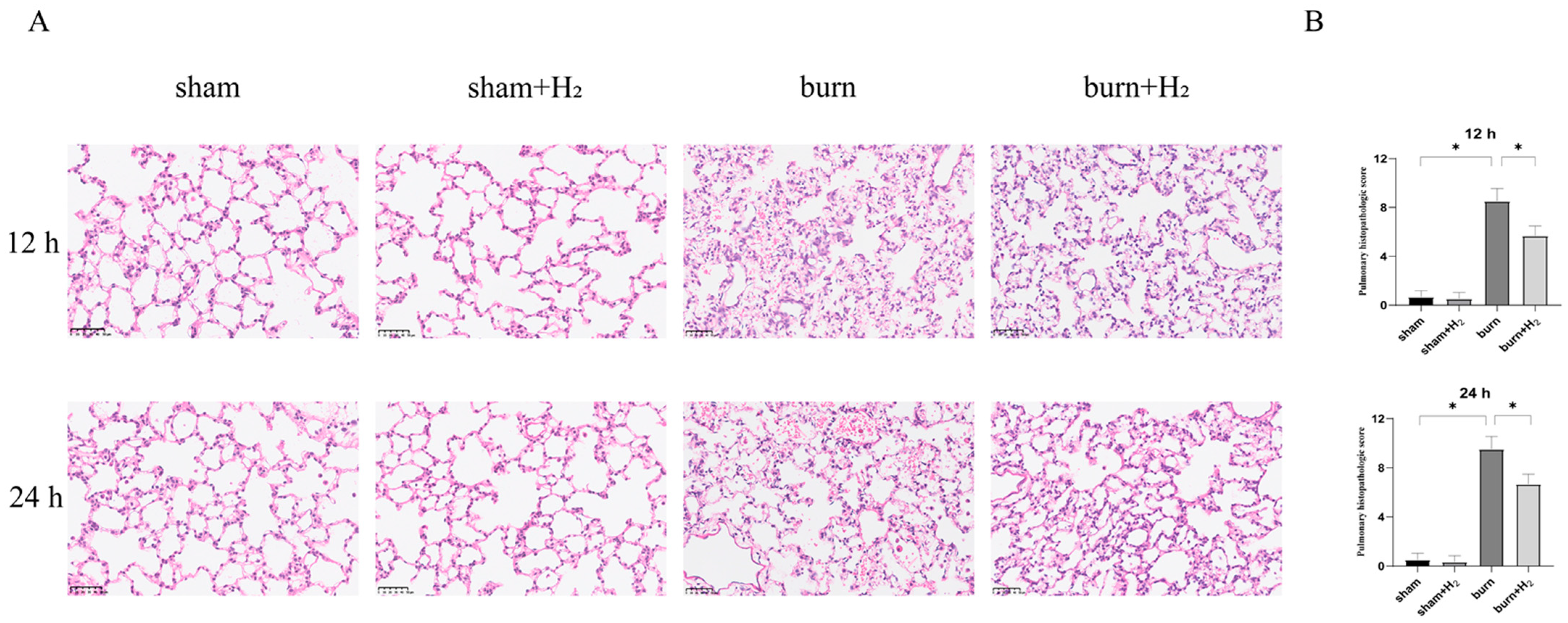
2.5. HE Staining
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
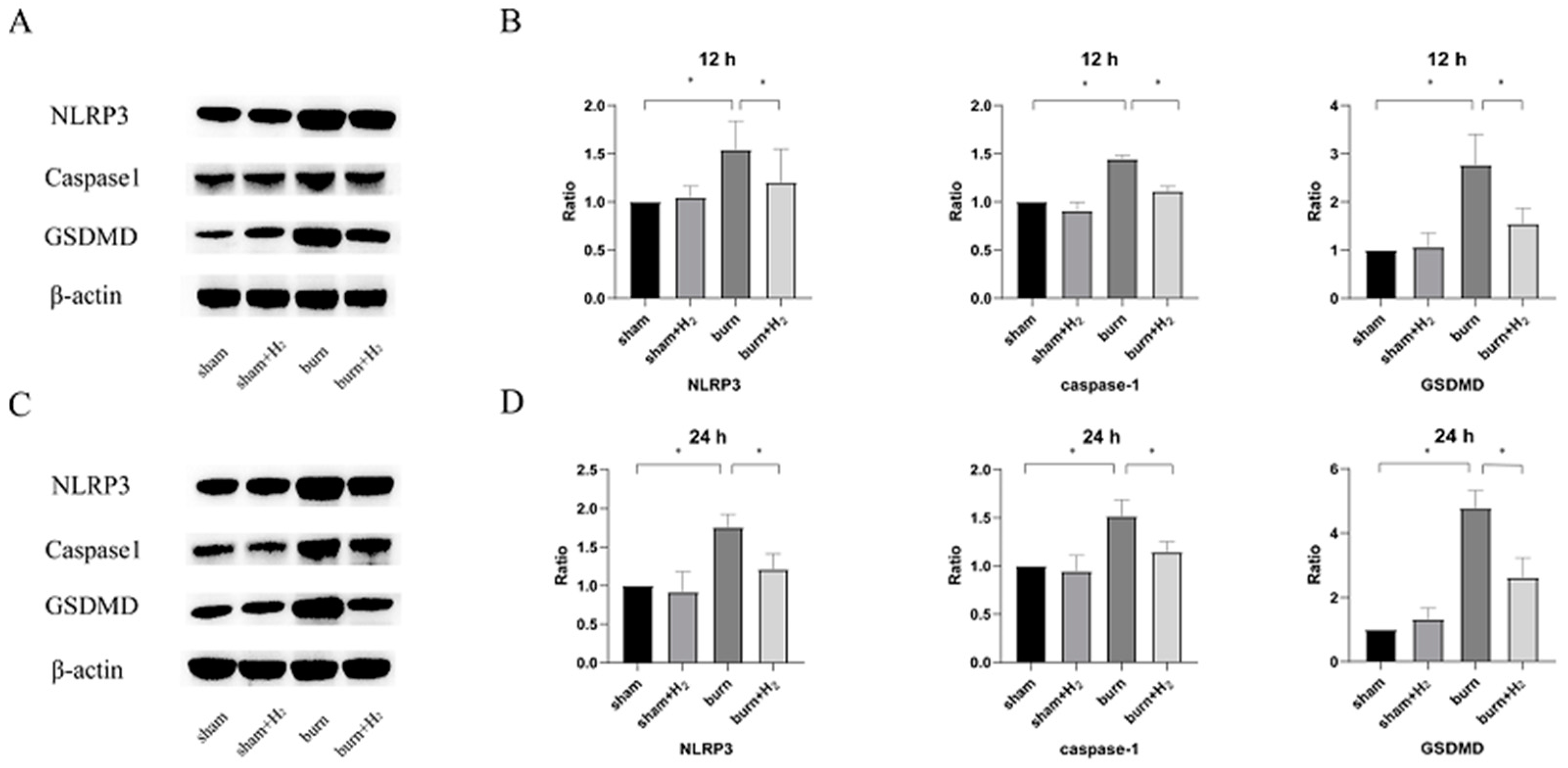
2.7. Western Blot Assay
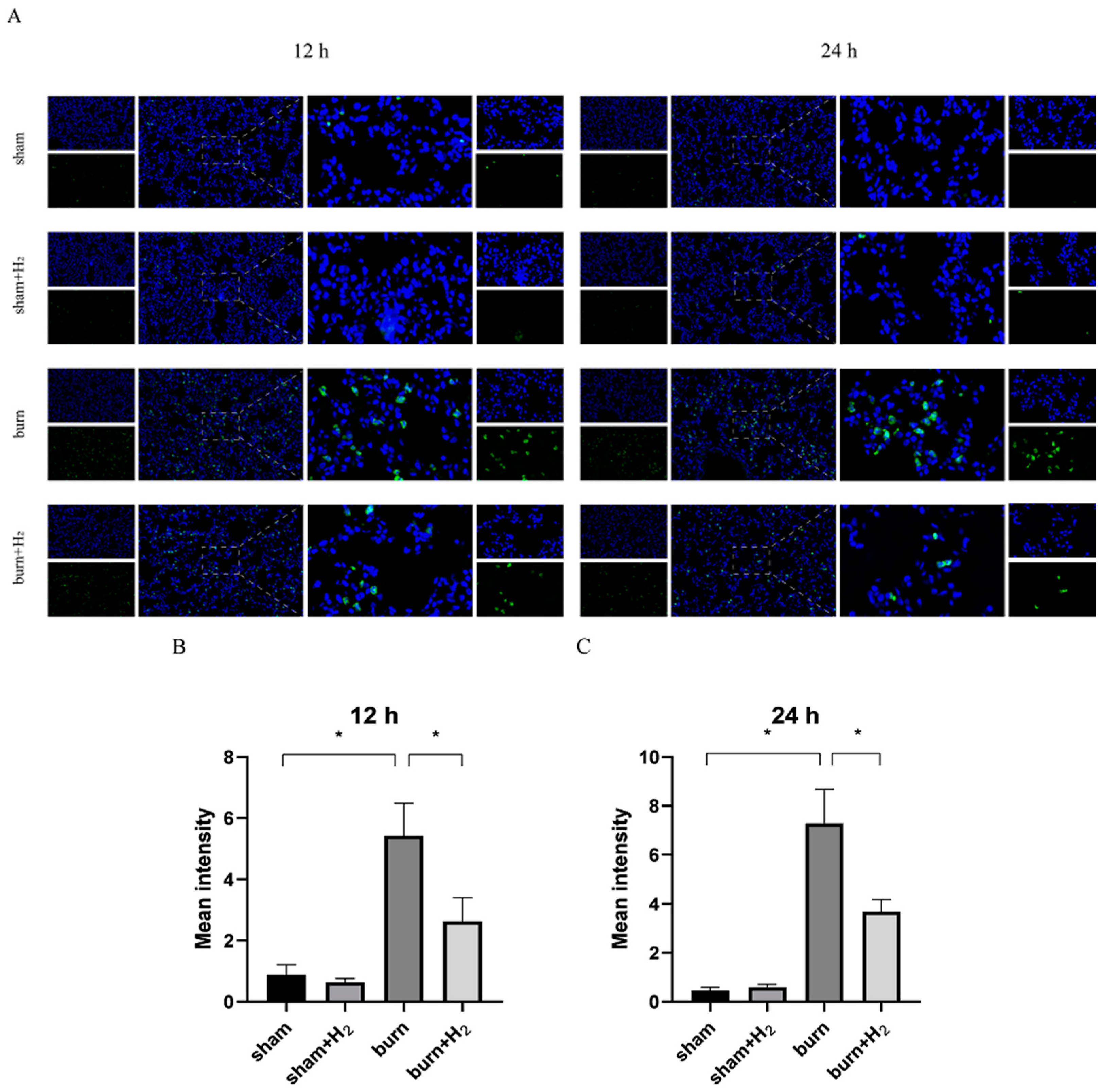
2.8. Immunofluorescence Staining
2.9. Transmission Electron Microscopy
2.10. Statistical Analysis
3. Results
3.1. Hydrogen Partially Reversed the Increase in Lung Tissue Density and Reduced Pulmonary Inflammation
3.2. Hydrogen Mitigates Histological Damage to the Lung after a Burn
3.3. Hydrogen Attenuates Burn-Induced Changes in the Expression of NLRP3 and Caspase-1
3.4. Hydrogen Reduces Inflammation Caused by Burns
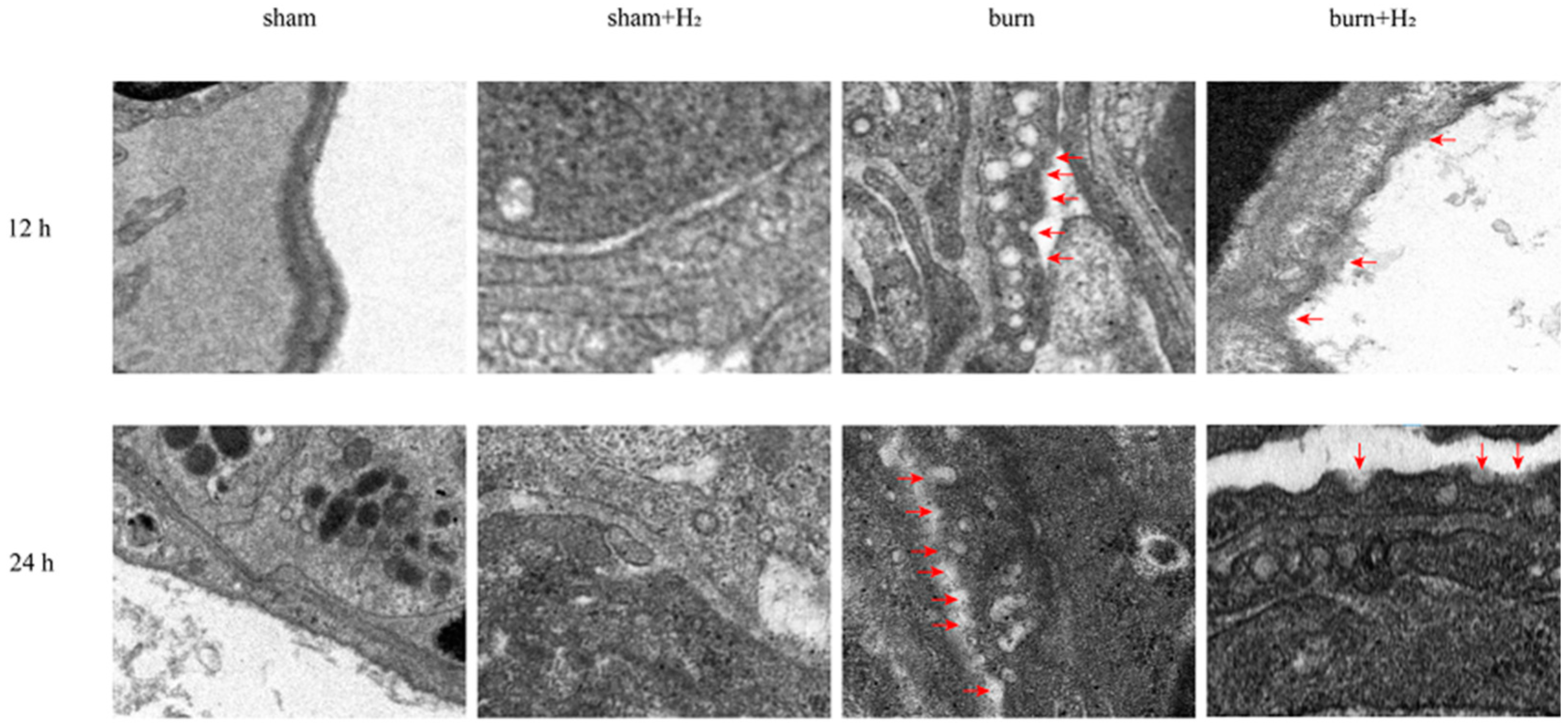
3.5. Hydrogen Reduced the Expression of GSDMD and AM Pyroptosis Caused by the Burn
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boldeanu, L.; Boldeanu, M.V.; Bogdan, M.; Meca, A.D.; Coman, C.G.; Buca, B.R.; Tartau, C.G.; Tartau, L.M. Immunological approaches and therapy in burns (Review). Exp. Ther. Med. 2020, 20, 2361–2367. [Google Scholar] [CrossRef]
- Lutmer, J.; Watkins, D.; Chen, C.-L.; Velten, M.; Besner, G. Heparin-binding epidermal growth factor–like growth factor attenuates acute lung injury and multiorgan dysfunction after scald burn. J. Surg. Res. 2013, 185, 329–337. [Google Scholar] [CrossRef]
- Huang, X.; Xiu, H.; Zhang, S.; Zhang, G. The Role of Macrophages in the Pathogenesis of ALI/ARDS. Mediat. Inflamm. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Niesler, U.; Palmer, A.; Radermacher, P.; Huber-Lang, M.S. Role of Alveolar Macrophages in the Inflammatory Response After Trauma. Shock 2014, 42, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Fan, J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018, 19, 50. [Google Scholar] [CrossRef]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- He, X.; Qian, Y.; Li, Z.; Fan, E.K.; Li, Y.; Wu, L.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. TLR4-Upregulated IL-1β and IL-1RI Promote Alveolar Macrophage Pyroptosis and Lung Inflammation through an Autocrine Mechanism. Sci. Rep. 2016, 6, 31663. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, Y.; Zhang, J. Recent Advances in Studies of Molecular Hydrogen against Sepsis. Int. J. Biol. Sci. 2019, 15, 1261–1275. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Liu, W. Combined early fluid resuscitation and hydrogen inhalation attenuates lung and intestine injury. World J. Gastroenterol. 2013, 19, 492. [Google Scholar] [CrossRef]
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Maekawa, Y.; Kawamura, A.; Abe, T.; Ohta, S.; Fukuda, K.; et al. H2 Gas Improves Functional Outcome After Cardiac Arrest to an Extent Comparable to Therapeutic Hypothermia in a Rat Model. J. Am. Heart Assoc. 2012, 1, e003459. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, G.; Liu, S.-Y.; Li, J.-B.; Wang, J.-F.; Bo, L.-L.; Qian, L.-R.; Sun, X.-J.; Deng, X.-M. Hydrogen-Rich Saline Protects Against Renal Ischemia/Reperfusion Injury in Rats. J. Surg. Res. 2011, 167, e339–e344. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Tan, J.-W.; Huang, L.-J.; Jia, L.; Liu, Y.-Q.; Zhao, Y.-Q.; Wang, K.; Dong, J.-H. Inhalation of hydrogen gas reduces liver injury during major hepatotectomy in swine. World J. Gastroenterol. 2012, 18, 5197–5204. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Zhao, W.; Liu, T. The protective effects of hydrogen on HO-1 expression in the brain after focal cerebral ischemia reperfusion in rats. Turk. J. Med. Sci. 2016, 46, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Gloor, B.; Blinman, T.A.; Rigberg, D.A.; Todd, K.E.; Lane, J.S.; Hines, O.J.; Reber, H.A. Kupffer Cell Blockade Reduces Hepatic and Systemic Cytokine Levels and Lung Injury in Hemorrhagic Pancreatitis in Rats. Pancreas 2000, 21, 414–420. [Google Scholar] [CrossRef]
- Sio, S.W.; Ang, S.F.; Lu, J.; Moochhala, S.; Bhatia, M. Substance P Upregulates Cyclooxygenase-2 and Prostaglandin E Metabolite by Activating ERK1/2 and NF-κB in a Mouse Model of Burn-Induced Remote Acute Lung Injury. J. Immunol. 2010, 185, 6265–6276. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Tong, J.; Zhang, Y.; Yu, P.; Liu, J.; Mei, X.; Meng, J. Protective Effect of Hydrogen Gas on Mouse Hind Limb Ischemia-Reperfusion Injury. J. Surg. Res. 2021, 266, 148–159. [Google Scholar] [CrossRef]
- Qin, C.; Bian, Y.X.; Feng, T.T.; Zhang, J.H.; Yu, Y.H. Effects of hydrogen on the lung damage of mice at early stage of severe burn. Chin. J. Burns 2017, 33, 682–687. [Google Scholar]
- Chai, J.-K.; Cai, J.-H.; Deng, H.-P.; Zou, X.-F.; Liu, W.; Hu, Q.-G.; Shen, C.-A.; Yin, H.-N.; Zhang, X.-B.; Chi, Y.-F.; et al. Role of neutrophil elastase in lung injury induced by burn–blast combined injury in rats. Burns 2013, 39, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhang, D.-H.; Hu, Q.; Liu, L.-Y.; Yu, Y.-H.; Chai, J.-K. Usage of density analysis based on micro-CT for studying lung injury associated with burn–blast combined injury. Burns 2018, 44, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cai, W.; Yang, X.; Jia, Y.; Zheng, Z.; Wang, H.; Li, J.; Li, Y.; Gao, J.; Fan, L.; et al. ROS-Mediated NLRP3 Inflammasome Activity Is Essential for Burn-Induced Acute Lung Injury. Mediat. Inflamm. 2015, 2015, 720457. [Google Scholar] [CrossRef]
- Vinaik, R.; Abdullahi, A.; Barayan, D.; Jeschke, M.G. NLRP3 inflammasome activity is required for wound healing after burns. Transl. Res. 2020, 217, 47–60. [Google Scholar] [CrossRef]
- Vinaik, R.; Barayan, D.; Abdullahi, A.; Jeschke, M.G. Browning and Beiging of Adipose Tissue: Its Role in the Regulation of Energy Homeostasis and as a Potential Target for Alleviating Metabolic Diseases NLRP3 Inflammasome Mediates White Adipose Tissue Browning after Burn. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E751–E759. [Google Scholar] [CrossRef]
- Stanojcic, M.; Vinaik, R.; Abdullahi, A.; Chen, P.; Jeschke, M.G. NLRP3 knockout enhances immune infiltration and inflammatory responses and improves survival in a burn sepsis model. Immunology 2022, 165, 195–205. [Google Scholar] [CrossRef]
- Kang, J.Y.; Xu, M.M.; Sun, Y.; Ding, Z.X.; Wei, Y.Y.; Zhang, D.W.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- Kong, X.; Gao, M.; Liu, Y.; Zhang, P.; Li, M.; Ma, P.; Shang, P.; Wang, W.; Liu, H.; Zhang, Q.; et al. GSDMD-miR-223-NLRP3 axis involved in B(a)P-induced inflammatory injury of alveolar epithelial cells. Ecotoxicol. Environ. Saf. 2022, 232, 113286. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Luo, Y.; Li, J.; Shang, L.; Zhou, F.; Yang, S. Honokiol alleviates LPS-induced acute lung injury by inhibiting NLRP3 inflammasome-mediated pyroptosis via Nrf2 activation in vitro and in vivo. Chin. Med. 2021, 16, 127. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, N.; Lin, H.; Zhang, L.; Jiang, Y.; Zhao, Y.; Han, Q.; Wang, X.; Yu, Y.; Qin, C. Effect of Hydrogen on AM Pyroptosis Induced by Severe Burns in Rats. J. Pers. Med. 2023, 13, 377. https://doi.org/10.3390/jpm13030377
Luo N, Lin H, Zhang L, Jiang Y, Zhao Y, Han Q, Wang X, Yu Y, Qin C. Effect of Hydrogen on AM Pyroptosis Induced by Severe Burns in Rats. Journal of Personalized Medicine. 2023; 13(3):377. https://doi.org/10.3390/jpm13030377
Chicago/Turabian StyleLuo, Ning, Hua Lin, Linlin Zhang, Yi Jiang, Yue Zhao, Qingqing Han, Xin Wang, Yonghao Yu, and Chao Qin. 2023. "Effect of Hydrogen on AM Pyroptosis Induced by Severe Burns in Rats" Journal of Personalized Medicine 13, no. 3: 377. https://doi.org/10.3390/jpm13030377
APA StyleLuo, N., Lin, H., Zhang, L., Jiang, Y., Zhao, Y., Han, Q., Wang, X., Yu, Y., & Qin, C. (2023). Effect of Hydrogen on AM Pyroptosis Induced by Severe Burns in Rats. Journal of Personalized Medicine, 13(3), 377. https://doi.org/10.3390/jpm13030377





