Abstract
Pulmonary vascular remodeling is the critical structural alteration and pathological feature in pulmonary hypertension (PH) and involves changes in the intima, media and adventitia. Pulmonary vascular remodeling consists of the proliferation and phenotypic transformation of pulmonary artery endothelial cells (PAECs) and pulmonary artery smooth muscle cells (PASMCs) of the middle membranous pulmonary artery, as well as complex interactions involving external layer pulmonary artery fibroblasts (PAFs) and extracellular matrix (ECM). Inflammatory mechanisms, apoptosis and other factors in the vascular wall are influenced by different mechanisms that likely act in concert to drive disease progression. This article reviews these pathological changes and highlights some pathogenetic mechanisms involved in the remodeling process.
1. Introduction
Pulmonary hypertension (PH) is a life-threatening disorder characterized by elevated pressure in the pulmonary arteries due to increased pulmonary vascular resistance [1]. PH is currently defined by a mean pulmonary artery pressure (mPAP) greater than 20 mm Hg on supine right heart catheterization at rest [2]. This definition differs from the previous threshold of 25 mm Hg or greater in recognition that patients with mPAP of 21 mm Hg to 24 mm Hg are at increased risk of mortality and hospitalization compared with those with an mPAP of 20 mm Hg or lower [3]. As a severe cardiopulmonary disease, PH is characterized by vascular remodeling and occlusion of small pre-capillary pulmonary arteries. Alterations in pulmonary vascular structure and function can lead to resistance to blood flow and the possible development of right-sided heart failure, leading to severe morbidity and mortality. There are several types of PH, which can be familial or secondary to an underlying disease [4]. Regardless of the etiology, the exact pathophysiological mechanisms leading to PH development and progression are primarily unidentified [5].
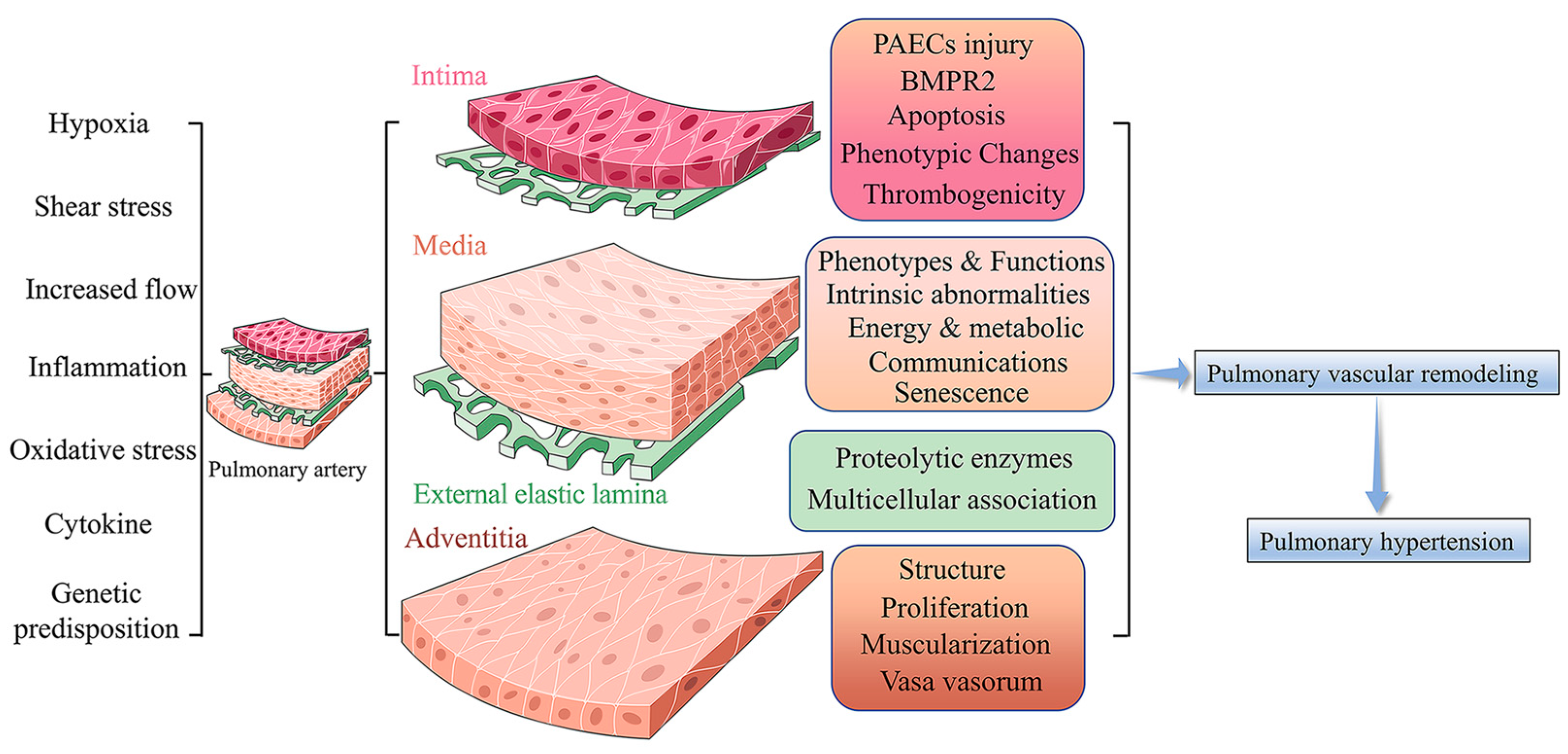
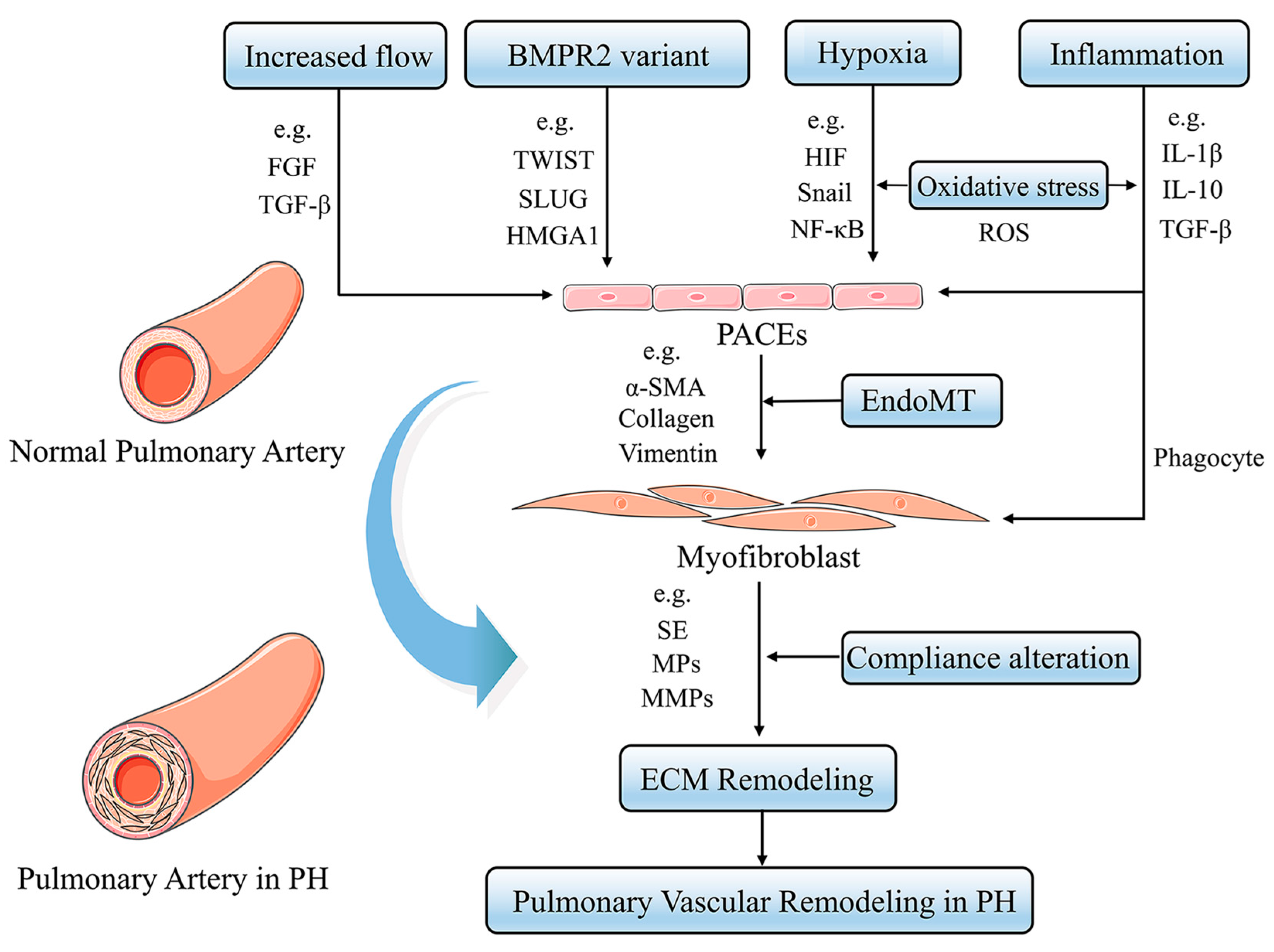
PH is a panvasculopathy, meaning all layers of the vascular wall are involved, which is also reflective of gene-environment interactions and has essential genetic and epigenetic mechanisms. The mechanism of PH is complex, and pulmonary vascular remodeling is the key pathological feature of PH. Pulmonary vascular remodeling involves the proliferation and phenotypic transformation of pulmonary artery endothelial cells (PAECs) and pulmonary artery smooth muscle cells (PASMCs) of the middle membranous pulmonary artery, as well as complex interactions involving external layer pulmonary artery fibroblasts (PAFs) and extracellular matrix (ECM). Inflammatory mechanisms, apoptosis and other factors in the vascular wall are influenced by different mechanisms that likely act in concert to drive disease progression (Figure 1) [6]. Here, we highlight fundamental changes in the pathobiology of PH vascular remodeling and discuss potential therapeutic interventions that may prevent disease progression and even reverse PH vascular remodeling.
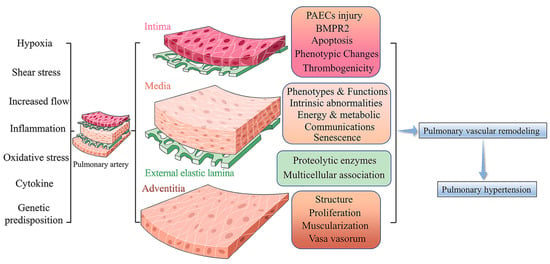
Figure 1.
Pulmonary Vascular Remodeling in PH Pathogenesis. Under a variety of stimuli, such as hypoxia, inflammation, and oxidative stress, the pulmonary artery vasculature undergoes pathological changes in function and structure, leading to pulmonary vascular remodeling and ultimately promoting PH.
2. Intima Remodeling in Pulmonary Vascular Remodeling
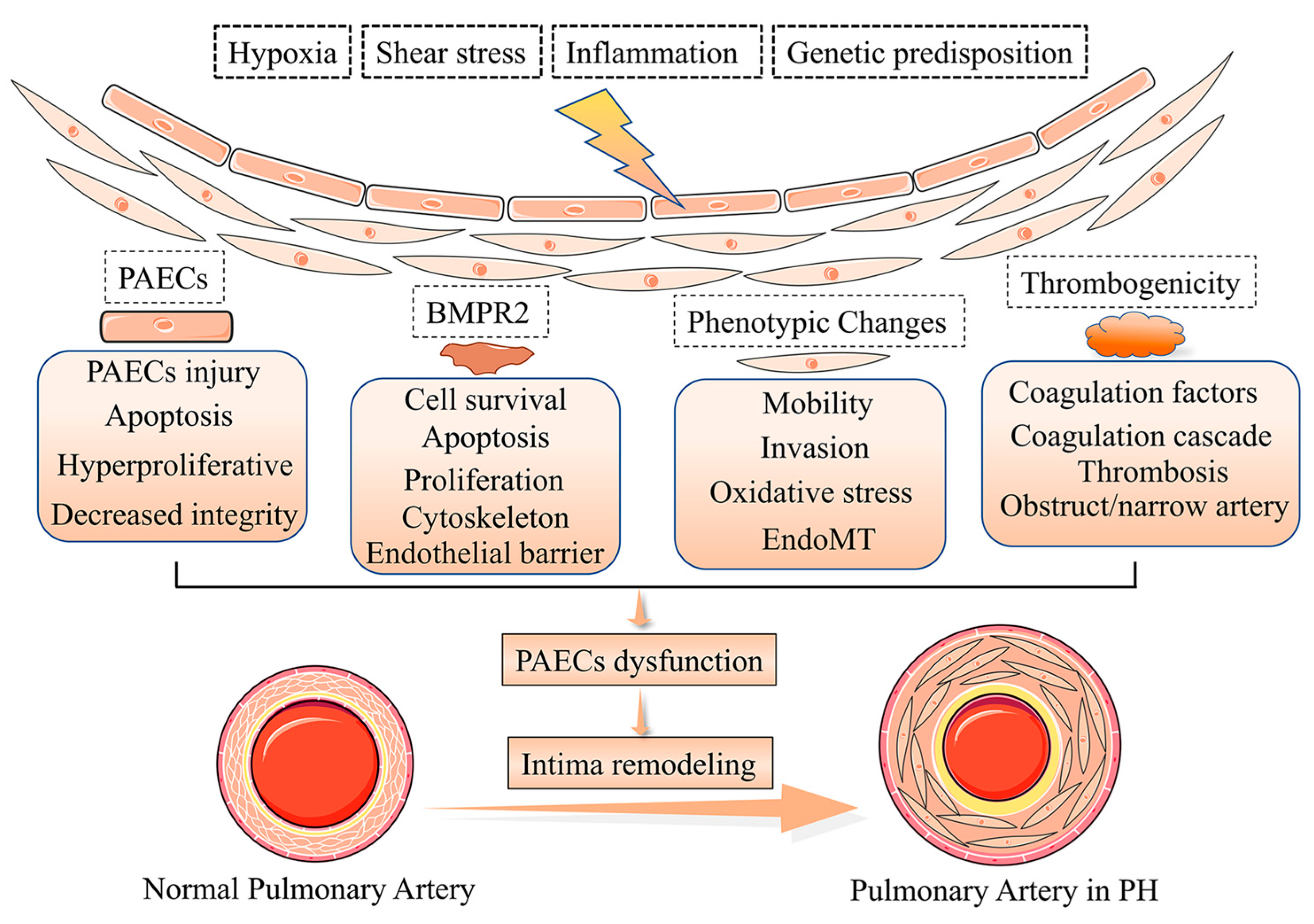
The intima represents the thick interface of endothelial cells between the media and flowing blood. The endothelial cells provide a broad unobstructed flow surface area, contributing to the suitable perfusion pressure that is the normal state of pulmonary circulation [7]. Patients with severe PH have an approximately 3 fold increase in pulmonary intima, but the mechanism of intima damage is unclear [8] and intimal thickening will result in an approximately 40-fold increase in resistance of the pulmonary vascular. There are various types of intimal thickening and PAECs dysfunction, as the most critical component of the intima accelerates the process of intima remodeling [9]. Under normal physiological conditions, PAECs are in a steady state and secrete a variety of active factors that disturb PAECs and PASMCs proliferation, coagulation, the attraction of inflammatory factors and activation of vasoactivity, which leads to dysfunction and pathological changes of PASMCs in PH [10]. Multiple factors trigger PAECs dysfunction in PH [11,12,13,14], such as shear stress, hypoxia, inflammation, PAECs phenotypes, the bone morphogenic type 2 receptor (BMPR2), and cilia length [15,16,17] (Figure 2). Meanwhile, the deterioration of endothelial metabolic function in the pulmonary vascular system is becoming an important driver of PAECs dysfunction and PH development [18].
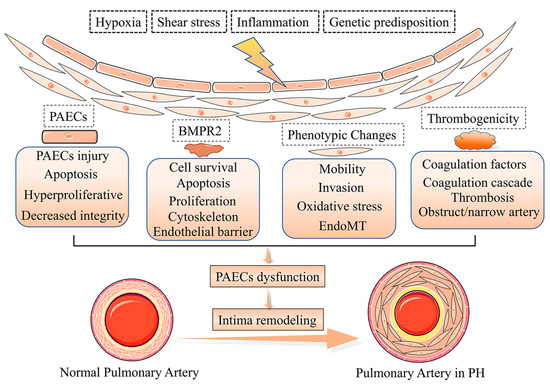
Figure 2.
Pathogenesis of pulmonary vascular intima remodeling in PH. In response to hypoxia, shear stress, inflammation, and genetic predisposition, the function and phenotype of PACEs are altered, resulting in intima remodeling in PH.
2.1. Phenotypes of PAECs Dysfunction in Intima Remodeling
Damage and apoptosis of PAECs can occur in the early stages of PH pathogenesis, while anti-apoptotic PAECs appear later as PH progresses [19]. In late PH, hyperproliferative and anti-apoptotic PAECs predominate and facilitate the formation of plexiform lesions [20]. The pathogenesis of PH is usually associated with abnormal endothelial cell barrier integrity, and patients with idiopathic PH (iPH) often exhibit a hypercoagulable phenotype. Additionally, there is a growing awareness that complex alterations in metabolic and epigenetic pathways facilitate the progression of PH [14]. However, it is essential to note here that PAECs include separate subpopulations of endothelial cells, which are possible exposure to multiple adverse stimuli and physical damages depending on location in the pulmonary vascular system [21].
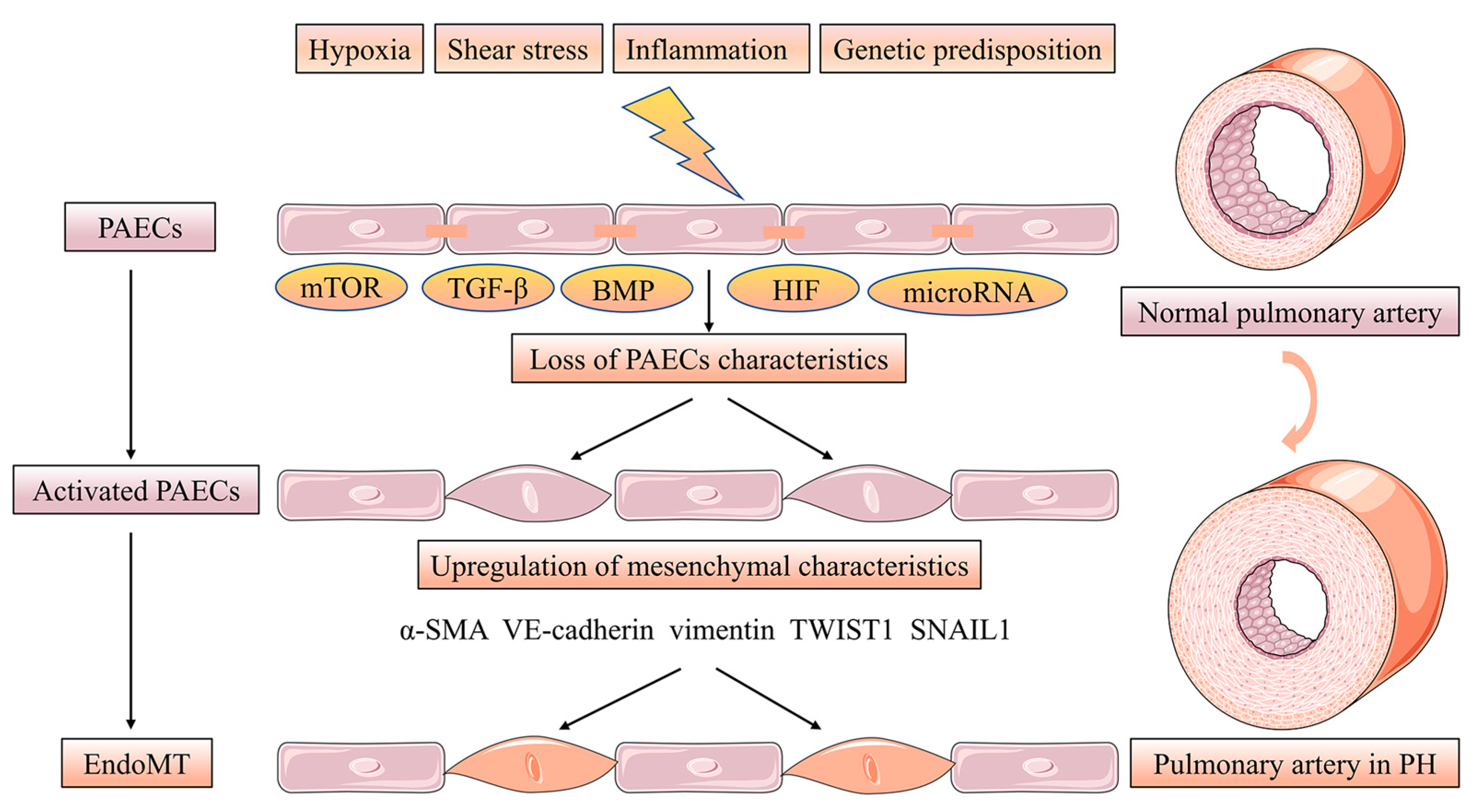
Endothelial-mesenchymal transition (EndoMT) is a phenotypic change in which PAECs manifest a mesenchymal-like phenotype with concomitant endothelial cell characteristics loss while upregulating the level of mesenchymal markers. Furthermore, PAECs adopt highly migratory and invasive cell phenotype characteristics with loss of cell-cell contact [22]. Strongly expressed α-smooth muscle actin (α-SMA), vimentin and VE-cadherin appeared in the PAECs of human PH patients and PH rat models induced by monocrotaline (MCT) hypoxia accompanied EndoMT. PAECs treated with transforming growth factor beta (TGF-β) induce the levels of the EndoMT transcription factors TWIST1, SNAIL1 and the previously mentioned mesenchymal markers involved in this process [23,24]. More interestingly, BMP-7 was abrogated in hypoxia-induced PAECs by the action of EndoMT via inhibiting the mTORC1 signaling pathway [25]. Low BMPR2 expression favors EndoMT leading to over-activated TGF-β signaling [26]. In conclusion, altered TGF-β/BMP signaling is associated with the EndoMT process in PH [27]. Hypoxia acts as an inducer of EndoMT via increasing hypoxia-inducible transcription factor-1α (HIF-1α) and hypoxia-inducible transcription factor-2α (HIF-2α) in PH [28]. Finally, microRNA, such as miR-27a, miR-124 and miR-181b, can be implicated in EndoMT in PH [29,30,31] (Figure 3).
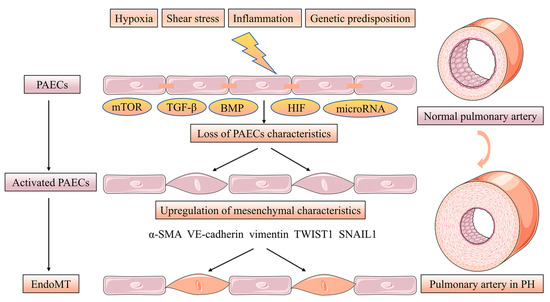
Figure 3.
EndoMT of PACEs in pulmonary vascular intima remodeling. EndoMT is a phenotypic change in which PAECs manifesting a mesenchymal-like phenotype with concomitant endothelial cell characteristics loss while upregulating the level of mesenchymal markers. Furthermore, PAECs adopt highly migratory and invasive cell phenotype characteristics with losing cell-cell contact. The EndoMT process is regulated by the mTOR, TGF-β, BMP and HIF signaling pathway. microRNA, such as miR-27a, miR-124 and miR-181b can be implicated in EndoMT in PH.
2.2. PAECs Survival and Proliferation in Intima Remodeling
BMP receptor signaling, which is encoded by SMAD1, SMAD4 and SMAD9, plays an important role in PH development. BMPR2, as a transmembrane enzyme receptor that regulates TGF-β signaling in PAECs in the lumen of the pulmonary vessels, promotes the survival of PAECs and antagonizes PASMCs proliferation [32,33]. Interestingly, GDF2 encodes the circulating BMP 9, which is a ligand for the BMP2 receptor, and mutations in GDF2 reduced levels of BMP family expression [34].
Additionally, siRNA-mediated silencing of BMPR2 in PAECs contributes to the inhibition of Ras/Raf/ERK and Ras signaling reversing proliferation and hypermotility [35]. With the further development of pathology in PH, PAECs proliferation is a major manifestation resulting in complex arterial structural and functional remodeling, and multiple pathways regulate this transition. Peroxisome proliferator-activated receptor-γ in PAECs inhibits the cell cycle and disrupts endothelial cell barrier function, while antagonizing the migration and angiogenic properties of PAECs [36]. Furthermore, recent studies have suggested a role for endothelial prolyl hydroxylase 2 (PHD2) in PH pathology, and mice with Tie2Core-mediated PHD2 disruption in PAECs exhibited vascular remodeling in PH [37].
The proliferation and survival of PAECs are influenced by several other factors that also exacerbate the pathological condition of PH, such as disruption of Cav1 [38]. mTOR, Nur77 and GDF11 also act as inhibitors of PAECs proliferation and angiogenesis after hypoxia [39,40]. Oxidative, antioxidant and nitrification equally affect endothelial function. Inhibition of reactive oxygen species (ROS)-induced Ca2+ entry also downregulates the migration and proliferation of PAECs [41]. It has been recently shown that endostatin, a cleavage product of Col18A1, inhibits PAECs proliferation and apoptosis via CD47 and ID1/TSP-1/CD36 signaling [42]. The absence of Notch coupling to Sox17 in endothelial cells may exacerbate PH by upregulating the monolayer vulnerability of PAECs [43]. These findings demonstrate the complex relationship between PAECs survival and proliferation in PH.
2.3. PAECs Activation and Thrombogenicity in Intima Remodeling
The presence of thrombotic lesions is a common pathological manifestation of PH. However, the role assumed by thrombus in PH remains controversial [44]. Multiple factors participate in the regulation of PH by affecting PAECs activation and thrombogenicity. A few studies have shown that coagulation factors, represented by activators of the coagulation cascade, lead to the aggregation of fibrin clots and blockage of blood vessels and exacerbate PAECs dysfunction, leading to vascular remodeling [45]. The levels of von Willebrand Factor (vWF) in PH patients also increase in the plasma. PAECs and platelets express and release vWF when activated, facilitating their interaction. The levels of thrombomodulin, as a series of anti-coagulant factors, are decreased in PH patients, which can inhibit this deterioration by ingesting prostacyclin, vasodilators or tadalafil [46]. CD40L is an inflammatory factor that cleaves into sCD40L upon activation, which is known to promote significantly in PH patients, eventually contributing to vascular remodeling in PH [47]. Although substantial evidence suggests that platelets and thrombogenicity exacerbate the pathogenesis of PAECs dysfunction, the molecular mechanisms need further elucidation.
2.4. PAECs Metabolism and Epigenetics in Intima Remodeling
Factors affecting PAECs metabolism and epigenetics participate in the regulation of PH. Metabolic abnormalities, particularly aerobic glycolysis or the Warburg effect, have been proposed as important pathogenic mechanisms in developing PH. PFKFB3 is an essential regulator of glycolysis, and its deficiency inhibits pulmonary vascular remodeling [48]. Endothelin 1/eNOS signaling also serves as an essential pathway that regulates the glycolytic process [49]. Further studies suggest that BolA family member 3 (BOLA3) is involved in the operation of glycolysis and mitochondrial respiratory function [50]. Epigenetic mechanisms are also generally considered to be important in regulating PAECs metabolism. The delivery of glutamine carbon into the tricarboxylic acid (TCA) cycle becomes active in PAECs in the case of mutations in the BMPR2 gene, and the strict requirement for glutamine is driven by the loss of deacetylase sirtuin 3 activities. Additionally, the pharmacological effects of glutaminase can be targeted to reduce the severity of PH pathology [51]. The distribution of other genetic variants showed that variants in ACVRL1, ENG, SMAD9, KCNK3 and TBX4 contributed to PH [52], but only about 1% of the cases in each gene. Therefore, these factors are emerging as promising targets for PH treatment.
3. Media Remodeling in Pulmonary Vascular Remodeling
The media, composed mainly of PASMCs, is the focus of PH because of its property mediating hypoxic pulmonary vasoconstriction. No single property can account for the complexity of the contribution of PASMCs to pulmonary vascular remodeling [53].
3.1. Heterogeneity of PASMCs Phenotypes and Functions
Most PASMCs found during embryogenesis or mesothelium via EndoMT originate from the mesoderm [54,55,56]. Heterogeneity and phenotypic plasticity of different subgroups of PASMCs act as pivotal parts in regulating vascular functions and adapting to changes in microenvironmental cues of pulmonary circulation [57,58]. To acquire or lose proliferative and migratory potential and synthesize ECM, PASMCs can be reversibly converted from resting to contractile or synthetic in response to specific stimuli [59]. Multiple growth factors and inflammatory mediators, such as TGF-β, platelet-derived growth factor (PDGF), angiotensin-II; as well as stimuli, such as mechanical forces, epigenetics and ECM tissue heterogeneity, are essential regulators facilitating PASMCs transformed from the contractile to the synthetic phenotype [60,61,62]. PASMCs acquire migratory and proliferative capacities during this shift to achieve remodeling of the media membrane. There are several PASMCs-specific genes that can be used to determine differentiation status. Most extensively studied α-SMA, smooth muscle myosin heavy chain, and smoothelin, but smooth muscle 22a (SM22α), meta-vinculin, desmin and smooth muscle calponin also characterize the mature contractile PASMCs phenotype [63,64,65]. The majority of such proteins can be used as contractile machinery components and regulators of contraction, whose level expression is downregulated during the switch to the dedifferentiated/synthetic phenotype. Other markers, such as collagen type I, SMemb/non-muscle myosin heavy chain isoform-B, connexin-43, cellular retinol-binding protein-1, osteopontin (OPN) and syndecan-1/-4, are expressed during transforming to the synthetic phenotype [66,67,68]. The phenotypic variability of PASMCs is also regulated by various microRNAs, such as miR-125a-5b, miR-133, and miR-193b [69,70,71].
3.2. Inherent Intrinsic Abnormalities in PASMCs
In the process of PH, pulmonary vascular remodeling involves alterations in the phenotype of the various constricted vascular cells that make up the arterial wall, including PASMCs, PAFs and myofibroblast-like cells [72,73,74]. There is growing evidence that some different types of cells, such as PASMCs, adventitial fibroblasts and myofibroblast-like characteristics cells, exhibit a range of inherent intrinsic abnormalities. The intrinsic abnormalities, including the dysfunction of BMPR2 signaling, dysregulation of K+ channel homeostasis, inhibition of peroxisome proliferator-activated receptor-γ and forkhead box O-1 (FOXO-1) signaling, stimulating activation of the nuclear factor-kappa B (NF-κB) and PHD2/HIF-1α signaling pathway, contribute to intensification of proliferation and phenotype transformation [75,76,77]. The nuclear factor of activated T cells (NFAT) is activated in PASMCs within pulmonary circulation in both animal and human PH suggesting that NFAT plays a critical role in the pathogenesis of PH. NFATc1, NFATc2 and NFATc3 are the main isotypes of NFAT, which is characterized by inflammation, hyperproliferation and metabolic change [78].
Diminished heterozygous function of BMPR2 and several other genes involved in PH, such as activin receptors like kinase 1(ALK1), ALK6 (also known as BMPR1B), endoglin (ENG), and BMP9, have promoted several pathological manifestations of PH, such as depolarization, anti-apoptosis, contraction and proliferation of PASMCs [79,80,81]. In experimental models, the above changes were also shown to be associated with alteration of the ion channel, such as inhibiting RhoA/Rho signaling reduced Ca2+ sensitization and vascular remodeling and lowering pulmonary pressure. Furthermore, a heightened response to PDGF, epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF-2) was also confirmed in the contractile cell type of PH [82,83,84]. It is precisely because the understanding of potential mechanisms is still limited that a better exploration of the inherent intrinsic abnormalities in PASMCs is needed.
3.3. Energy and Metabolic Changes in Media Remodeling
Vasoconstrictor cells from PH patients can adjust their energy and metabolic state to meet changing bioenergetic and biosynthetic demands [85]. It has been demonstrated that the vascular remodeling process of PH involves glycolytic processes regulated by mitochondrial oxidative phosphorylation and glycolysis, dysregulation of amino acid metabolism and lipid oxidation pathways; and NFATc2 plays an important regulatory role in these processes [86]. Furthermore, promoting oxidative metabolism via attenuating pyruvate dehydrogenase kinase (PDK) and lacking malonyl-CoA decarboxylase can reverse pulmonary vascular remodeling in PH mice. PASMCs in iPH also show other pathological metabolic pathways such as activation of hypoxia-inducible factor, enhanced glutamine metabolism and activation of fatty acid oxidation [87,88]. It is evident that energy and metabolic changes have essential effects on vascular remodeling in PH.
3.4. Altered Cell Communications and Senescence in Media Remodeling
Interactions between pulmonary vascular cells, such as PAECs, PASMCs, PAFs, pericytes and immune cells, are the basis for the structure and function of the blood vessels in the lung, including stability of related cellular phenotypes, intrinsic properties and their responsiveness to external microenvironmental stimuli [89]. Alterations of PASMCs communicate with PAECs and play a major regulatory role in the pathological development of PH [90]. PAECs in PH lose the ability to modulate the appropriate moderate balance between vasoconstrictors, such as endothelin-1 (ET-1) and serotonin, and vasodilators, such as nitric oxide (NO) and prostacyclin [91,92,93,94]. The capacity of PAECs is to maintain a regular vascular network by secreting vasoactive factors, which affects the interactions between PAECs and PASMCs, and regulates multicellular contraction, proliferation, and survival. In particular, inflammation often precedes vascular remodeling, which strongly supports the importance of multicellular intercommunication.
Senescent vascular PAECs are characterized by morphologic and metabolic changes, and the acquisition of a senescence-associated secretory phenotype (SASP) [95]. Furthermore, senescent vascular smooth muscle cells have emerged as key triggers of vascular structural disruption and remodeling in PH [96]. OPN, p53 and p16Ink4a signaling are thought to play central roles in the cellular senescence program of PH [97,98]. Moreover, the SASP of PASMCs exacerbates pathological overproliferation of cells and promotes critical media remodeling pathways [99]. How well the properties of senescent PASMCs are understood is critical because cellular senescence leads to irreversible PH-associated pulmonary vascular remodeling.
4. Adventitia Remodeling in Pulmonary Vascular Remodeling
Until recently, the adventitia has somehow been overlooked in this traditional concept, but emerging evidence leads us to recognize the possibility that the adventitia can serve as a staging ground for some of the earliest changes that occur in the vessel wall, especially inflammatory changes [100]. Cumulative results have suggested that the adventitia is not merely a bystander in the pathogenesis of arterial disease but may represent a direct driving force for the development of PH. The adventitia consists mainly of a connective tissue sheath surrounding the pulmonary arteries and PAFs are crucial components [101]. During the period of vascular remodeling induced by PH, PAFs are highly activated and undergo phenotypic transformation characterized by hyperproliferation, migration and inflammatory activities (Figure 4).
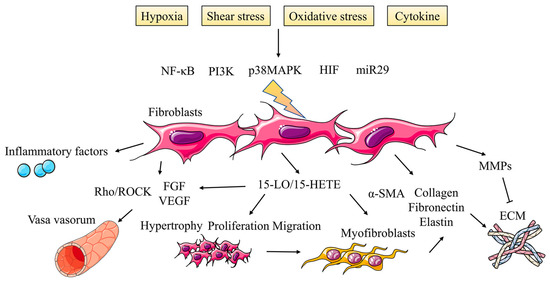
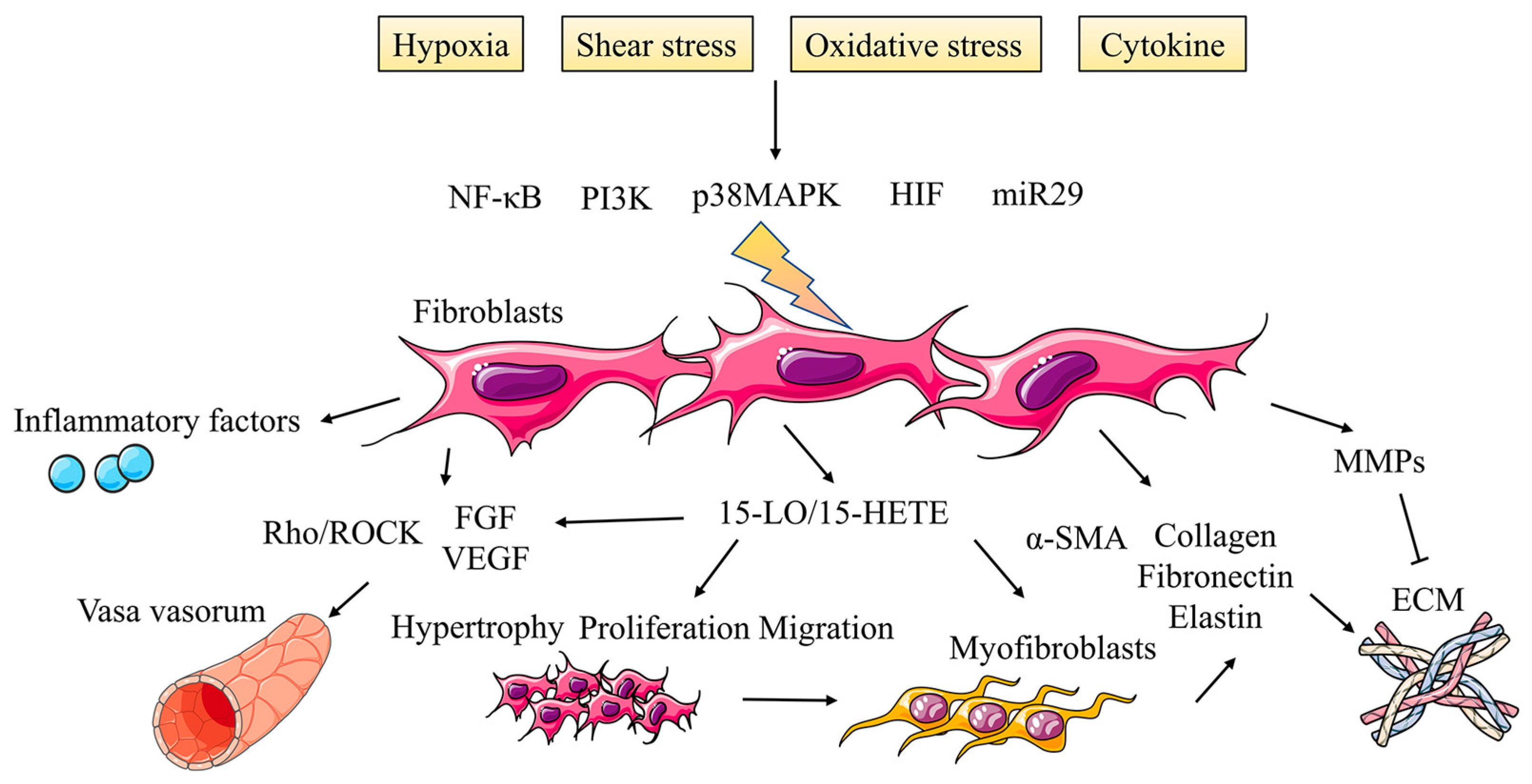
Figure 4.
Activated fibroblasts promote adventitia remodeling in PH. During the period of vascular remodeling induced by PH, PAFs are highly activated and undergo phenotypic transformation characterized by hyperproliferation, migration and inflammatory activities under the regulation of multiple signaling pathways, which also affects vasa vasorum and ECM in adventitia.
4.1. Hypoxia Induces Changes in Adventitia Structure
Hypoxia is the pathological basis of pulmonary vascular remodeling. The thickening of adventitia led to decreased vascular compliance and distensibility and increased blood pressure and right ventricular dysfunction. The adventitia structure change is considered the earliest and most prominent structural change in the PH process [102]. The change of adventitia is earlier than that of intima and media, which can induce the degeneration and thickening of media. Hypoxia causes the thickness of adventitia to increase by more double, among which the hypertrophy of fibroblasts and increase in the number are the main reasons for thickening, and collagen fibers also increase. When stimulated by hypoxia, fibroblasts undergo phenotypic changes, then proliferate, differentiate, and upregulate the expression of contractile protein and ECM protein, while simultaneously releasing PASMCs cytokines that affect media. The fibroproliferative changes of vascular adventitia are related to the decreased lumen and decreased responsiveness of the vessel wall to vasodilation. Local hypoxia can also lead to the upregulation of carbonic anhydrase activity in the adventitia, which leads to artery remodeling and inflammatory reactions [103].
4.2. PAFs Participate in Adventitia Remodeling
PAFs are the most critical cell component of adventitia, which play a vital role in regulating vascular wall function. When blood vessels are stimulated, adventitial PAFs are activated and undergo phenotypic changes, releasing factors that directly affect the phenotype and growth of PASMCs in media, and recruiting of inflammatory factors and hematopoietic progenitor cells [104].
4.2.1. PAFs Proliferation of Adventitia Remodeling
Hypoxia activates some growth factors; stimulates protein kinase C, the mitogen-activated protein kinase family and phosphatidylinositol 3-kinase (PI3K); and regulates PAFs proliferation [105]. The early proliferation of PAFs caused by acute hypoxia exposure relies on p38 mitogen-activated protein kinase (p38 MAPK), and HIF-1α is also a key transcription factor in the biochemical reaction of hypoxia [106,107]. HIF-1α is the downstream effector of p38 MAPK, and p38 MAPK may also contribute to the stability of HIF-1α. HIF-1α is directly related to p38 MAPK phosphorylation, which is crucial for PAFs proliferation under hypoxia. Furthermore, the downregulation of miR-29 is the basis of TGF-β-mediated PAFs, while HIF-1α and Smad3 can jointly inhibit the expression of miR-29 in organ fibrosis [108]. Further research showed that the TGF-1 receptor blocker could inhibit the proliferation of fibroblasts induced by hypoxia, the expression of TGF-β1, MMP-1, α-SMA and NF-κB, and the increase of collagen fibers in adventitia induced by hypoxia [109].
4.2.2. PAFs Muscularization of Adventitia Remodeling
Hypoxia can induce the proliferation of adventitial PAFs and make them change to myofibroblasts. Myofibroblasts migrate from adventitia to intima or media, resulting in the thickening of the blood vessel wall, as the primary source of collagen, fibronectin, tendon protein and elastin. It is considered a critical participant in pulmonary vascular remodeling [110]. Hypoxia can make the expression of HIF-1α, vascular endothelial growth factor-A (VEGF-A) and matrix metalloproteinases (MMPs) in myofibroblasts upregulated, which promotes the proliferation and migration of myofibroblasts into the intima. 15-Hydroxyeicosatetraenoic acid (15-HETE) is an essential regulatory substance, and HETE inhibitor could reverse the extracellular matrix deposition and 15-lipoxygenase (15-LO) expression [111]. The expressions of myofibroblast markers α-SMA, type I collagen and fibronectin increased in fibroblasts cultured in hypoxia in vitro regulated by 15-LO/15-HETE signaling [112]. 15-HETE can upregulate the expressions of α-SMA and FGF-2, which are also vital participants in cell proliferation and differentiation. It is suggested that hypoxia is involved in regulating the transformation of adventitial fibroblasts into myofibroblasts mainly through the 15-HETE/FGF2/TGF-β1/α-SMA signaling pathway [102].
4.3. Vasa Vasorum in Adventitia Remodeling
In arterioles, the intima and media are nourished by the lumen side of the artery wall, while the vasa vasorum nourishes the adventitia. Vasa vasorum originates from the arteries or the arteriovenous nearby [113]. Under hypoxic conditions and early intimal injury, vasa vasorum of the adventitia will generate new blood vessels, which will cause the intima to thicken and penetrate the media and intima. Hypoxia caused the upregulation of HIF-1α/2α expression in adventitia and increased VEGF-A, VEGF-C and FGF-2, promoting angiogenesis. Fibroblasts are the critical regulatory point of adventitia neovascularization. ET-1 is released from fibroblasts activated by hypoxia and vasa vasorum endothelial cells, participating in the regulation of PH with the synergistic effect of VEGF. Furthermore, vasa vasorum in the adventitia of the pulmonary artery with long-term hypoxic expansion secrete ATP, as an essential angiogenic factor. PI3K, Rho and ROCK are involved in ATP-mediated vasa vasorum angiogenesis and assume an important regulatory role in DNA synthesis, migration and tubular structure formation [114]. Adventitia plays an essential role in the development of these diseases. Defining the molecular mechanism of adventitia’s role in hypoxic vascular remodeling and finding the critical action nodes may provide a new target for treating PH.
5. ECM Remodeling in Pulmonary Vascular Remodeling
As an extensive molecular network of the cellular surroundings, the ECM provides for the proper functioning of the structure and function of the vessel wall and plays a crucial role in intercellular and intracellular communication [115]. The ECM is a relatively balanced, dynamic environment composed mainly of collagens and many other proteins, including mainly basement membrane (BM), elastin, laminin, collagen IV, fibronectin, tenascin C, proteoglycan, etc. [116,117,118,119]. In addition to the function of ECM components in maintaining tissue structure [120], studies have shown that reduced pulmonary arterial compliance and increased pulmonary artery ECM remodeling play a regulatory role in the development of PH (Figure 5). Although ECM remodeling is still controversial, it does not prevent us from exploring it further as a potential therapeutic target.
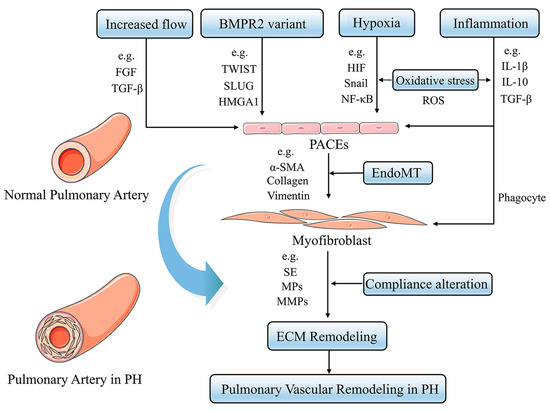
Figure 5.
Pathogenesis of pulmonary vascular ECM remodeling in PH. Increased blood flow, hypoxia, inflammation and BMPR2 signaling lead to EndoMT, in which endothelial cells acquire increased expression of the mesenchymal phenotype. These factors then affect the function and structure of the ECM, including MMPs, collagen, vimentin, etc., resulting in ECM remodeling.
5.1. The Balance between Proteolytic Enzymes in ECM Remodeling
The composition of ECM is regulated by the balance between proteolytic enzymes, including MMPs, metalloproteases (MPs), serine elastases (SE), lysyl oxidases (LOXs), and their endogenous inhibitors tissue inhibitors of metalloproteinase (TIMPs). Dynamic imbalance of protein hydrolases and endogenous inhibitory cytokines promotes worsened collagen deposition and increased elastin breakdown in the pulmonary artery during PH [121]. The exact mechanisms responsible for these imbalances are not yet known with certainty, but several potential mechanisms are becoming accepted. Exacerbation of flow, shear stress and inflammation as triggering events lead to impaired PAECs, weakening barrier function and increasing permeability, resulting in overproduction of serine elastase content by PASMCs [122]. Serine elastase subsequently leads to ECM degradation and activation of growth factors such as FGF and TGF-β, which subsequently upregulates the deposition of fibronectin, collagen, tenascin and elastin by stimulating PASMCs and PAFs. Furthermore, catabolism of ECM and related growth factors also promotes upregulation of biosynthesis of MMPs, accompanied by an imbalance in protein hydrolases and their inhibitors that can be triggered by inflammation, ultimately leading to remodeling of the ECM [123].
5.2. EndoMT in ECM Remodeling
ECM remodeling in PH has been attributed to EndoMT [124,125], in which PAECs develop a mesenchymal phenotype with similar characteristics to PASMCs [126]. Along with reduced expression of endothelial characteristic factors such as endothelial cadherin and platelet endothelial adhesion molecules, intercellular interactions gradually weakened, followed by the separation of PAEC from the intima [127]. Subsequently, PAECs migrate into the media accompanied by upregulated expression levels of α-SMA, collagen, vimentin and MMPs, dedifferentiating into myofibroblast-like mesenchymal cells promoting ECM remodeling by exacerbating the degree of collagen deposition and cross-linking.
Multiple stimuli involved in the course of PH initiate the process of EndoMT. Loss of BMPR2 function has been demonstrated to activate EndoMT [128]. Inhibition of BMPR2 properties in PAECs leads to elevation of high-mobility group AT-hook 1 (HMGA1), a protein that promotes the biosynthesis of a transcriptional regulatory molecule called Slug that increases the amount of α-SMA, accompanied by suppression of endothelial cell genes, as well as the platelet endothelial cell adhesion molecule and vascular endothelial cadherin, ultimately exacerbating the mesenchymal phenotype. Elevated expression levels of the transcription factor Twist can inhibit the biological activity of BMPR2 in EndoMT. Furthermore, stimulation of chronic hypoxia initiates EndoMT by increasing the levels of Snail, β-catenin, NF-κB and STAT3, which regulated by HIF and TGF-β signaling [129]. Inflammation and oxidative stress cytokines such as IL-1β, IL-6, IL-10, TNF-α and ROS contribute to EndoMT via activation of TGF-β mediated inflammatory response and oxidative stress damage.
6. Other Pathological Alterations Present in PH
Although a range of abnormal pathological responses results in PH, including increased alveolar exudates and granulomas, and infiltration of peribronchial inflammatory cells, they are generally considered to be concomitant symptoms. We highlight the main changes in perivascular inflammation, progenitor/stem cells, and other molecular mechanisms of PH vascular remodeling.
6.1. Perivascular Inflammation
It has been well-documented that perivascular inflammation is an essential prominent pathologic feature and driver for pulmonary vascular remodeling in PH [130,131]. Accumulating evidence suggests a functional role of perivascular inflammation in initiating and progressing pulmonary vascular remodeling in PH [132]. High expression of active cellular chemokines and inflammatory factors correlate with clinical outcomes [133]. Multiple immune cell recruitment occurs in the peripulmonary artery, including neutrophils, macrophages, dendritic cells, mast cells, T lymphocytes, and B lymphocytes [134,135,136]. Vascular and parenchymal cells, such as PAECs, PASMCs and PAFs, change their phenotype, resulting in oversensitivity to inflammatory triggers and active secretion of chemokines that promote the outbreak of inflammatory cascade responses [137].
Furthermore, inflammatory factors can also trigger an imbalance in the levels of protein hydrolases and their inhibitors, which exacerbate the remodeling of ECM around the pulmonary vasculature [123]. Inflammatory factor-mediated levels of ROS upregulate the secretion of MMPs from PAECs, PASMCs and PAFs and reduce the secretion of TIMPs, accompanied by the activation and recruitment of macrophages and neutrophils, which further promotes the secretion levels of MMPs and serine elastase. The breakdown products derived from the action of protein hydrolases have pro-inflammatory effects, promoting inflammatory response and forming a positive feedback regulatory mechanism. In summary, multiple studies have recognized that modulating the cross-talk between inflammatory factors and vascular parenchymal cells may serve as a new and effective target for PH immunotherapy.
6.2. Progenitor/Stem Cells in Vascular Remodeling during PH
Explaining the pathological process of PH only by the in situ proliferation of resident vascular cells is considered one-sided; attention should also be paid to the invasion of extravascular cells into the vessel wall. As undifferentiated cells that can be transformed into new vascular cells, these vascular progenitors and stem cells have the ability to self-renew and aggregate together under specific stimuli, which exacerbate the pathological process of pulmonary vascular remodeling in PH under the regulation of related signaling pathways [132]. Given previous studies, as two distinct subpopulations of endothelial progenitor cells (EPCs), early-growing EPCs derived from the hematopoietic lineage and late-growing EPCs originated from the endothelial lineage, have been powerfully demonstrated in PH [138]. Studies have measured a simultaneous increase in expression levels of vascular endothelial cells expressing the markers CD133, CD34 and VEGFR-2 in proportion to mPAP [139,140]. In the pulmonary vascular system of PH patients, an upregulation of the EPCs population with CD31, CD34, vWF, c-kit, eNOS and caveolin-1 as markers was similarly observed [141,142]. Pericytes are used as a source of smooth muscle progenitor cells (SPC), which have also been demonstrated during neomuscularization. Moreover, pulmonary endothelium-pericyte interactions can influence the formation of new blood vessels [143,144]. The side population of progenitor cells labeled with ATP Binding Cassette Subfamily G Member 2 (ABCG2) has the potential to differentiate into α-SMA+ SMC/myofibroblasts and NG2+ pericytes [145]. There is still a need to understand the role that progenitor/stem cells assume in the PH vascular remodeling process.
6.3. Ion Channels
Pulmonary vascular remodeling in PH involves pathobiology such as altered pulmonary arterial tone, endothelial dysfunction, and inflammation, which could all depend on K+, Ca2+, Na+ and Cl− ion channel activities [146]. Membrane permeability to cations and anions control resting membrane potential, intracellular ion homeostasis and cell volume. Pulmonary vascular cells from PH are characterized by an important remodeling of protein expression and function of ion channels [147]. Kv1.5 isoform is a hypoxia-sensitive voltage-gated K+ channel in PASMCs, and its overexpression increases K+ currents and leads to plasma membrane hyperpolarization [148,149,150]. A decrease in mRNA expressions for Kv1.1, Kv1.5, Kv4.3, Ca2+-activated K+ and K2P channel are found in patients of PH. Furthermore, the process of PH is also regulated by voltage-gated Ca2+ channels and Ca2+-activated Cl− channels, which lead to pulmonary vascular remodeling by promoting membrane depolarization, vasomotor tone and cell excitability [151,152,153]. It has also been shown that the Na+ voltage-gated channel subunit 1B (SCN1B) is upregulated in patients with PH, which may contribute to abnormal vasoconstriction and excessive remodeling, and it could be carefully considered as a relevant novel therapeutic target [154].
7. Conclusions
Understanding how all these processes integrate to promote the aberrant proliferative remodeling characteristic of PH will be critical to developing successful antiremodeling therapies. The search for novel therapeutic targets is necessary because currently commonly used treatments associated with adverse side effects for PH are imperfect. Although several promising potential therapies have emerged from studies in non-human models, such as dichloroacetate, inhibitors of BMPR2, ROCK and HIF, their therapeutic effects on patients still need to be validated by large-scale clinical trials. Other questions remain, particularly how best to increase the success rate of translating preclinical discoveries to the bedside, which has been a significant challenge in PH. Further investigation is needed to explore the pathogenesis and regulatory pathways of vascular remodeling and prepare the theoretical basis for developing new drugs to target and reverse the remodeling process in PH.
Author Contributions
Conceptualization, Z.J., X.W. and J.M.; methodology, Z.J., S.W. and H.Y.; writing—original draft preparation, Z.J., S.W. and Y.C.; writing—review and editing, Z.J., X.Z. and L.W.; supervision, Z.Z., S.L. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Inheritance and Innovation Team of Traditional Chinese Medicine for Prevention and Treatment of Cardiovascular Diseases of the National Administration of Traditional Chinese Medicine of the People’s Republic of China (No. ZYYCXTD-C-202203); Clinical Medical Research Center of Traditional Chinese Medicine of Tianjin Municipal Science and Technology Commission (No. 15ZXLCSY00020); Traditional Chinese Medicine & Integrated Traditional Chinese and Western Medicine Project of Tianjin Municipal Health Commission and Tianjin Administration of Traditional Chinese Medicine (No. 2021048).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest regarding this article.
References
- Badesch, D.B.; Raskob, G.E.; Elliott, C.G.; Krichman, A.M.; Farber, H.W.; Frost, A.E.; Barst, R.J.; Benza, R.L.; Liou, T.G.; Turner, M.; et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 2010, 137, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Hess, E.; Maddox, T.M.; Opotowsky, A.R.; Tedford, R.J.; Lahm, T.; Joynt, K.E.; Kass, D.J.; Stephens, T.; Stanislawski, M.A.; et al. Association of Borderline Pulmonary Hypertension With Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016, 133, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef]
- Ruopp, N.F.; Cockrill, B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA 2022, 327, 1379–1391. [Google Scholar] [CrossRef]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef]
- Tuder, R.M. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017, 367, 643–649. [Google Scholar] [CrossRef]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef]
- Evans, C.E.; Cober, N.D.; Dai, Z.; Stewart, D.J. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur. Respir. J. 2021, 58, 2003957. [Google Scholar] [CrossRef]
- Nie, X.; Shen, C.; Tan, J.; Wu, Z.; Wang, W.; Chen, Y.; Dai, Y.; Yang, X.; Ye, S.; Chen, J.; et al. Periostin: A Potential Therapeutic Target For Pulmonary Hypertension? Circ. Res. 2020, 127, 1138–1152. [Google Scholar] [CrossRef]
- Dummer, A.; Rol, N.; Szulcek, R.; Kurakula, K.; Pan, X.; Visser, B.I.; Bogaard, H.J.; DeRuiter, M.C.; Goumans, M.J.; Hierck, B.P. Endothelial dysfunction in pulmonary arterial hypertension: Loss of cilia length regulation upon cytokine stimulation. Pulm. Circ. 2018, 8, 2045894018764629. [Google Scholar] [CrossRef]
- Gorelova, A.; Berman, M.; Al Ghouleh, I. Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension. Antioxid. Redox Signal. 2021, 34, 891–914. [Google Scholar] [CrossRef] [PubMed]
- Rodor, J.; Chen, S.H.; Scanlon, J.P.; Monteiro, J.P. Single-cell RNA sequencing profiling of mouse endothelial cells in response to pulmonary arterial hypertension. Cardiovasc. Res. 2022, 118, 2519–2534. [Google Scholar] [CrossRef]
- Ranchoux, B.; Harvey, L.D.; Ayon, R.J.; Babicheva, A.; Bonnet, S.; Chan, S.Y.; Yuan, J.X.; Perez, V.J. Endothelial dysfunction in pulmonary arterial hypertension: An evolving landscape (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045893217752912. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Rosinus, N.S.; Lankeit, M.; Hobohm, L.; Bremmer, F.; Schütz, E.; Klok, F.A.; Horke, S.; Wiedenroth, C.B.; Münzel, T.; et al. From thrombosis to fibrosis in chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 2017, 117, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Chabert, C.; Khochbin, S.; Rousseaux, S.; Veyrenc, S. Inhibition of BET Proteins Reduces Right Ventricle Hypertrophy and Pulmonary Hypertension Resulting from Combined Hypoxia and Pulmonary Inflammation. Int. J. Mol. Sci. 2018, 19, 2224. [Google Scholar] [CrossRef]
- Hautefort, A.; Mendes-Ferreira, P.; Sabourin, J.; Manaud, G.; Bertero, T.; Rucker-Martin, C.; Riou, M.; Adão, R.; Manoury, B.; Lambert, M.; et al. Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension. Circulation 2019, 139, 932–948. [Google Scholar] [CrossRef]
- Morrell, N.W.; Aldred, M.A.; Chung, W.K.; Elliott, C.G.; Nichols, W.C.; Soubrier, F. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801899. [Google Scholar] [CrossRef]
- Ruffenach, G.; O’Connor, E.; Vaillancourt, M.; Hong, J.; Cao, N.; Sarji, S.; Moazeni, S.; Papesh, J.; Grijalva, V.; Cunningham, C.M.; et al. Oral 15-Hydroxyeicosatetraenoic Acid Induces Pulmonary Hypertension in Mice by Triggering T Cell-Dependent Endothelial Cell Apoptosis. Hypertension 2020, 76, 985–996. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K.; Voelkel, N.F. Endothelial cells and pulmonary arterial hypertension: Apoptosis, proliferation, interaction and transdifferentiation. Respir. Res. 2009, 10, 95. [Google Scholar] [CrossRef]
- Kim, C.; Seedorf, G.J.; Abman, S.H.; Shepherd, D.P. Heterogeneous response of endothelial cells to insulin-like growth factor 1 treatment is explained by spatially clustered sub-populations. Biol. Open 2019, 8, bio045906. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Duffhues, G.; García de Vinuesa, A.; Ten Dijke, P. Endothelial-to-mesenchymal transition in cardiovascular diseases: Developmental signaling pathways gone awry. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2018, 247, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Muyleart, M.; Konduri, G.G.; Mammoto, A. Twist1 in Hypoxia-induced Pulmonary Hypertension through Transforming Growth Factor-β-Smad Signaling. Am. J. Respir. Cell Mol. Biol. 2018, 58, 194–207. [Google Scholar] [CrossRef]
- Ursoli Ferreira, F.; Eduardo Botelho Souza, L.; Hassibe Thomé, C.; Tomazini Pinto, M.; Origassa, C.; Salustiano, S.; Marcel Faça, V.; Olsen Câmara, N.; Kashima, S.; Tadeu Covas, D. Endothelial Cells Tissue-Specific Origins Affects Their Responsiveness to TGF-β2 during Endothelial-to-Mesenchymal Transition. Int. J. Mol. Sci. 2019, 20, 458. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Yan, L.; Du, W.; Zhang, X.; Zhang, M.; Chen, H.; Zhang, Y.; Zhou, J.; Sun, H.; et al. Bone morphogenetic protein-7 inhibits endothelial-mesenchymal transition in pulmonary artery endothelial cell under hypoxia. J. Cell. Physiol. 2018, 233, 4077–4090. [Google Scholar] [CrossRef]
- Hiepen, C.; Jatzlau, J.; Hildebrandt, S.; Kampfrath, B.; Goktas, M. BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics. PLoS Biol. 2019, 17, e3000557. [Google Scholar] [CrossRef] [PubMed]
- Rol, N.; Kurakula, K.B.; Happé, C.; Bogaard, H.J.; Goumans, M.J. TGF-β and BMPR2 Signaling in PAH: Two Black Sheep in One Family. Int. J. Mol. Sci. 2018, 19, 2585. [Google Scholar] [CrossRef]
- Dai, Z.; Zhu, M.M.; Peng, Y.; Machireddy, N.; Evans, C.E.; Machado, R.; Zhang, X.; Zhao, Y.Y. Therapeutic Targeting of Vascular Remodeling and Right Heart Failure in Pulmonary Arterial Hypertension with a HIF-2α Inhibitor. Am. J. Respir. Crit. Care Med. 2018, 198, 1423–1434. [Google Scholar] [CrossRef]
- Liu, T.; Zou, X.Z.; Huang, N.; Ge, X.Y.; Yao, M.Z.; Liu, H.; Zhang, Z.; Hu, C.P. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci. 2019, 227, 64–73. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Li, M.; Plecitá-Hlavatá, L.; D’Alessandro, A.; Tauber, J.; Riddle, S.; Kumar, S.; Flockton, A.; McKeon, B.A.; et al. Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 2017, 136, 2468–2485. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Zhang, X.; Guo, Y.; Wang, X. miR-181b-5p inhibits endothelial-mesenchymal transition in monocrotaline-induced pulmonary arterial hypertension by targeting endocan and TGFBR1. Toxicol. Appl. Pharmacol. 2020, 386, 114827. [Google Scholar] [CrossRef]
- Happé, C.; Kurakula, K.; Sun, X.Q.; da Silva Goncalves Bos, D. The BMP Receptor 2 in Pulmonary Arterial Hypertension: When and Where the Animal Model Matches the Patient. Cells 2020, 9, 1422. [Google Scholar] [CrossRef]
- Bisserier, M.; Mathiyalagan, P.; Zhang, S.; Elmastour, F.; Dorfmüller, P.; Humbert, M.; David, G.; Tarzami, S.; Weber, T.; Perros, F.; et al. Regulation of the Methylation and Expression Levels of the BMPR2 Gene by SIN3a as a Novel Therapeutic Mechanism in Pulmonary Arterial Hypertension. Circulation 2021, 144, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.; Swietlik, E.M.; Salmon, R.M.; Hadinnapola, C.; Nikolic, I.; Wharton, J.; Guo, J.; Liley, J.; Haimel, M.; Bleda, M.; et al. Characterization of GDF2 Mutations and Levels of BMP9 and BMP10 in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Rol, N.; de Raaf, M.A.; Sun, X.Q.; Kuiper, V.P.; da Silva Gonçalves Bos, D.; Happé, C.; Kurakula, K.; Dickhoff, C.; Thuillet, R.; Tu, L.; et al. Nintedanib improves cardiac fibrosis but leaves pulmonary vascular remodelling unaltered in experimental pulmonary hypertension. Cardiovasc. Res. 2019, 115, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Mahon, C.; Sweeney, N.M.; Verschueren, E.; Kantamani, V.; Li, D.; Hennigs, J.K.; Marciano, D.P.; Diebold, I.; Abu-Halawa, O.; et al. PPARγ Interaction with UBR5/ATMIN Promotes DNA Repair to Maintain Endothelial Homeostasis. Cell Rep. 2019, 26, 1333–1343.e1337. [Google Scholar] [CrossRef]
- Tang, H.; Babicheva, A.; McDermott, K.M.; Gu, Y.; Ayon, R.J.; Song, S.; Wang, Z.; Gupta, A.; Zhou, T.; Sun, X.; et al. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. American journal of physiology. Lung Cell. Mol. Physiol. 2018, 314, L256–L275. [Google Scholar] [CrossRef]
- Oliveira, S.D.S.; Chen, J.; Castellon, M.; Mao, M.; Raj, J.U.; Comhair, S.; Erzurum, S.; Silva, C.L.M.; Machado, R.F.; Bonini, M.G.; et al. Injury-Induced Shedding of Extracellular Vesicles Depletes Endothelial Cells of Cav-1 (Caveolin-1) and Enables TGF-β (Transforming Growth Factor-β)-Dependent Pulmonary Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1191–1202. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Zheng, X.D.; Zhang, J.; Zhao, X.; Liu, Y.; Zhang, H.; Zhang, L.; Yu, H.; Zhang, M.; et al. Growth Differentiation Factor 11 Promotes Abnormal Proliferation and Angiogenesis of Pulmonary Artery Endothelial Cells. Hypertension 2018, 71, 729–741. [Google Scholar] [CrossRef]
- Kurakula, K.; Sun, X.Q.; Happé, C.; da Silva Goncalves Bos, D.; Szulcek, R.; Schalij, I.; Wiesmeijer, K.C.; Lodder, K.; Tu, L.; Guignabert, C. Prevention of progression of pulmonary hypertension by the Nur77 agonist 6-mercaptopurine: Role of BMP signalling. Eur. Respir. J. 2019, 54, 1802400. [Google Scholar] [CrossRef]
- Wang, E.L.; Jia, M.M.; Luo, F.M.; Li, T.; Peng, J.J.; Luo, X.J.; Song, F.L.; Yang, J.F.; Peng, J.; Liu, B. Coordination between NADPH oxidase and vascular peroxidase 1 promotes dysfunctions of endothelial progenitor cells in hypoxia-induced pulmonary hypertensive rats. Eur. J. Pharmacol. 2019, 857, 172459. [Google Scholar] [CrossRef]
- Goyanes, A.M.; Moldobaeva, A.; Marimoutou, M.; Varela, L.C.; Wang, L.; Johnston, L.F.; Aladdin, M.M.; Peloquin, G.L.; Kim, B.S.; Damarla, M.; et al. Functional Impact of Human Genetic Variants of COL18A1/Endostatin on Pulmonary Endothelium. Am. J. Respir. Cell Mol. Biol. 2020, 62, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Kim, S.H.; Yang, H.Y.; Kim, J.H.; Schermuly, R.T.; Cho, Y.S.; Kang, H.; Park, J.H.; Lee, E. Sox17 Deficiency Promotes Pulmonary Arterial Hypertension via HGF/c-Met Signaling. Circ. Res. 2022, 131, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Kondababu, K.; Smolders, V.F.E.D.; Olga, T.; Wouter, J.J.; Quax, P.H.A.; MarieJosé, G. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar]
- Sakamaki, F.; Kyotani, S.; Nagaya, N.; Sato, N.; Oya, H.; Satoh, T.; Nakanishi, N. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation 2000, 102, 2720–2725. [Google Scholar] [CrossRef]
- Maeda, N.Y.; Clavé, M.M.; Bydlowski, S.P.; Lopes, A.A. Decreased circulating thrombomodulin is improved by tadalafil therapy in hypoxemic patients with advanced pulmonary arterial hypertension. Thromb. Res. 2016, 146, 15–19. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Yang, J.X.; Mao, W.; Wang, X.X. RNA-binding protein SFPQ cooperates with HDAC1 to suppress CD40 transcription in pulmonary adventitial fibroblasts. Cell Biol. Int. 2019, 44, 166–176. [Google Scholar] [CrossRef]
- Kovacs, L.; Cao, Y.; Han, W.; Meadows, L.; Kovacs-Kasa, A.; Kondrikov, D.; Verin, A.D.; Barman, S.A.; Dong, Z.; Huo, Y.; et al. PFKFB3 in Smooth Muscle Promotes Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 200, 617–627. [Google Scholar] [CrossRef]
- Sun, X.; Kumar, S.; Sharma, S.; Aggarwal, S.; Lu, Q.; Gross, C.; Rafikova, O.; Lee, S.G.; Dasarathy, S.; Hou, Y.; et al. Endothelin-1 induces a glycolytic switch in pulmonary arterial endothelial cells via the mitochondrial translocation of endothelial nitric oxide synthase. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1084–1095. [Google Scholar] [CrossRef]
- Yu, Q.; Tai, Y.Y.; Tang, Y.; Zhao, J.; Negi, V.; Culley, M.K.; Pilli, J.; Sun, W.; Brugger, K.; Mayr, J.; et al. BOLA (BolA Family Member 3) Deficiency Controls Endothelial Metabolism and Glycine Homeostasis in Pulmonary Hypertension. Circulation 2019, 139, 2238–2255. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Thoré, P.; Girerd, B.; Jaïs, X.; Savale, L.; Ghigna, M.R.; Eyries, M.; Levy, M.; Ovaert, C.; Servettaz, A. Phenotype and outcome of pulmonary arterial hypertension patients carrying a TBX4 mutation. Eur. Respir. J. 2020, 55, 1902340. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Frid, M.G.; Graham, B.B.; Tuder, R.M. Dynamic and diverse changes in the functional properties of vascular smooth muscle cells in pulmonary hypertension. Cardiovasc. Res. 2018, 114, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Majesky, M.W. Lineage tracking of origin and fate of smooth muscle cells in atherosclerosis. Cardiovasc. Res. 2018, 114, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Santoro, M.M. The origin and mechanisms of smooth muscle cell development in vertebrates. Development 2021, 148, 197384. [Google Scholar] [CrossRef]
- Shen, M.; Quertermous, T.; Fischbein, M.P.; Wu, J.C. Generation of Vascular Smooth Muscle Cells From Induced Pluripotent Stem Cells: Methods, Applications, and Considerations. Circ. Res. 2021, 128, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Mei, X.; Chen, S.Y. Smooth Muscle Cells in Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e247–e252. [Google Scholar] [CrossRef] [PubMed]
- Lechartier, B.; Berrebeh, N.; Huertas, A.; Humbert, M.; Guignabert, C.; Tu, L. Phenotypic Diversity of Vascular Smooth Muscle Cells in Pulmonary Arterial Hypertension: Implications for Therapy. Chest 2022, 161, 219–231. [Google Scholar] [CrossRef]
- Roostalu, U.; Aldeiri, B.; Albertini, A.; Humphreys, N.; Simonsen-Jackson, M.; Wong, J.K.F.; Cossu, G. Distinct Cellular Mechanisms Underlie Smooth Muscle Turnover in Vascular Development and Repair. Circ. Res. 2018, 122, 267–281. [Google Scholar] [CrossRef]
- Cai, P.; Kovacs, L.; Dong, S.; Wu, G.; Su, Y. BMP4 inhibits PDGF-induced proliferation and collagen synthesis via PKA-mediated inhibition of calpain-2 in pulmonary artery smooth muscle cells. American journal of physiology. Lung Cell. Mol. Physiol. 2017, 312, L638–L648. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Xu, S.L.; Zhang, C.F.; Liu, J.; Zhang, Y.; Yang, J.; Xing, X.Q. PDGF mediates pulmonary arterial smooth muscle cell proliferation and migration by regulating NFATc2. Mol. Med. Rep. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shu, D.; Gong, X.; Lu, M.; Feng, Q.; Zeng, X.B.; Zhang, H.; Gao, J.; Guo, Y.W.; Liu, L.; et al. Platelet-Derived TGF (Transforming Growth Factor)-β1 Enhances the Aerobic Glycolysis of Pulmonary Arterial Smooth Muscle Cells by PKM2 (Pyruvate Kinase Muscle Isoform 2) Upregulation. Hypertension 2022, 79, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Huang, F.J.; Li, Y.; Huang, H.; Wu, Q.C. SEDT2/METTL14-mediated m6A methylation awakening contributes to hypoxia-induced pulmonary arterial hypertension in mice. Aging 2021, 13, 7538–7548. [Google Scholar] [CrossRef]
- Zucker, M.M.; Wujak, L.; Gungl, A.; Didiasova, M.; Kosanovic, D.; Petrovic, A.; Klepetko, W.; Schermuly, R.T.; Kwapiszewska, G.; Schaefer, L.; et al. LRP1 promotes synthetic phenotype of pulmonary artery smooth muscle cells in pulmonary hypertension. Biochimica et biophysica acta. Mol. Basis Dis. 2019, 1865, 1604–1616. [Google Scholar] [CrossRef]
- Bouvard, C.; Genet, N.; Phan, C.; Rode, B.; Thuillet, R.; Tu, L.; Robillard, P.; Campagnac, M.; Soleti, R.; Dumas De La Roque, E.; et al. Connexin-43 is a promising target for pulmonary hypertension due to hypoxaemic lung disease. Eur. Respir. J. 2020, 55, 1900169. [Google Scholar] [CrossRef]
- Liu, P.; Yan, S.; Chen, M.; Chen, A.; Yao, D.; Xu, X.; Cai, X.; Wang, L.; Huang, X. Effects of baicalin on collagen Ι and collagen ΙΙΙ expression in pulmonary arteries of rats with hypoxic pulmonary hypertension. Int. J. Mol. Med. 2015, 35, 901–908. [Google Scholar] [CrossRef]
- Mura, M.; Cecchini, M.J.; Joseph, M.; Granton, J.T. Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension. Respirology 2019, 24, 1104–1110. [Google Scholar] [CrossRef]
- Cai, Z.; Li, J.; Zhuang, Q.; Zhang, X.; Yuan, A.; Shen, L.; Kang, K.; Qu, B.; Tang, Y.; Pu, J.; et al. MiR-125a-5p ameliorates monocrotaline-induced pulmonary arterial hypertension by targeting the TGF-β1 and IL-6/STAT3 signaling pathways. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Khachigian, L.M. Transcription Factors Targeted by miRNAs Regulating Smooth Muscle Cell Growth and Intimal Thickening after Vascular Injury. Int. J. Mol. Sci. 2019, 20, 5445. [Google Scholar] [CrossRef]
- Satoh, T.; Wang, L.; Espinosa-Diez, C.; Wang, B.; Hahn, S.A.; Noda, K.; Rochon, E.R.; Dent, M.R.; Levine, A.R.; Baust, J.J.; et al. Metabolic Syndrome Mediates ROS-miR-193b-NFYA-Dependent Downregulation of Soluble Guanylate Cyclase and Contributes to Exercise-Induced Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction. Circulation 2021, 144, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Bordenave, J.; Tu, L.; Berrebeh, N.; Thuillet, R.; Cumont, A.; Le Vely, B.; Fadel, E.; Nadaud, S.; Savale, L.; Humbert, M.; et al. Lineage Tracing Reveals the Dynamic Contribution of Pericytes to the Blood Vessel Remodeling in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; De Man, F.S.; Girerd, B.; Huertas, A.; Chaumais, M.C.; Lecerf, F.; François, C.; Perros, F.; Dorfmüller, P.; Fadel, E.; et al. A critical role for p130Cas in the progression of pulmonary hypertension in humans and rodents. Am. J. Respir. Crit. Care Med. 2012, 186, 666–676. [Google Scholar] [CrossRef]
- Savai, R.; Al-Tamari, H.M.; Sedding, D.; Kojonazarov, B.; Muecke, C.; Teske, R.; Capecchi, M.R.; Weissmann, N.; Grimminger, F.; Seeger, W.; et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat. Med. 2014, 20, 1289–1300. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, C.; Liu, S.; Lu, W.; Li, Y.; Luo, X.; Ma, R.; Zhang, C.; Chen, H.; Chen, Y.; et al. Dysregulation of BMP9/BMPR2/SMAD signalling pathway contributes to pulmonary fibrosis and pulmonary hypertension induced by bleomycin in rats. Br. J. Pharmacol. 2021, 178, 203–216. [Google Scholar] [CrossRef]
- Mei, L.; Zheng, Y.M.; Song, T.; Yadav, V.R.; Joseph, L.C.; Truong, L.; Kandhi, S. Rieske iron-sulfur protein induces FKBP12.6/RyR2 complex remodeling and subsequent pulmonary hypertension through NF-κB/cyclin D1 pathway. Nat. Commun. 2020, 11, 3527. [Google Scholar] [CrossRef]
- Chen, R.; Yan, J.; Liu, P.; Wang, Z.; Wang, C.; Zhong, W.; Xu, L. The role of nuclear factor of activated T cells in pulmonary arterial hypertension. Cell Cycle (Georget. Tex.) 2017, 16, 508–514. [Google Scholar] [CrossRef]
- Alan, B.; Nalbantgil, S. Genetic, cellular and molecular mechanisms of pulmonary arterial hypertension. Anatol. J. Cardiol. 2010, 10 (Suppl. S1), 9–13. [Google Scholar] [CrossRef]
- Tu, L.; Desroches-Castan, A.; Mallet, C.; Guyon, L.; Cumont, A.; Phan, C.; Robert, F.; Thuillet, R.; Bordenave, J.; Sekine, A.; et al. Selective BMP-9 Inhibition Partially Protects Against Experimental Pulmonary Hypertension. Circ. Res. 2019, 124, 846–855. [Google Scholar] [CrossRef]
- Zhang, H.; Du, L.; Zhong, Y.; Flanders, K.C.; Roberts, J.D., Jr. Transforming growth factor-β stimulates Smad1/5 signaling in pulmonary artery smooth muscle cells and fibroblasts of the newborn mouse through ALK1. American journal of physiology. Lung Cell. Mol. Physiol. 2017, 313, L615–L627. [Google Scholar] [CrossRef]
- Kinoshita, D.; Shishido, T.; Takahashi, T.; Yokoyama, M.; Sugai, T.; Watanabe, K.; Tamura, H.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; et al. Growth Factor Midkine Aggravates Pulmonary Arterial Hypertension via Surface Nucleolin. Sci. Rep. 2020, 10, 10345. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nagaoka, T.; Nagata, Y.; Suzuki, Y.; Tsutsumi, T.; Kuriyama, S.; Watanabe, J. Periostin-related progression of different types of experimental pulmonary hypertension: A role for M2 macrophage and FGF-2 signalling. Respirology 2022, 27, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, W.; Wang, Q.; Wang, J.; Chai, L.; Chen, Y.; Wang, Y.; Liu, J.; Li, M.; Xie, X. PPARγ activation inhibits PDGF-induced pulmonary artery smooth muscle cell proliferation and migration by modulating TERT. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 152, 113233. [Google Scholar] [CrossRef] [PubMed]
- Wujak, M.; Veith, C.; Wu, C.Y.; Wilke, T.; Kanbagli, Z.I.; Novoyatleva, T.; Guenther, A.; Seeger, W. Adenylate Kinase 4-A Key Regulator of Proliferation and Metabolic Shift in Human Pulmonary Arterial Smooth Muscle Cells via Akt and HIF-1α Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 10371. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Gurtu, V.; Webster, L.; Barnes, G.; Watson, G. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci. Transl. Med. 2017, 9, 4583. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Perk, D.; Chan, S.Y. The molecular rationale for therapeutic targeting of glutamine metabolism in pulmonary hypertension. Expert Opin. Ther. Targets 2019, 23, 511–524. [Google Scholar] [CrossRef]
- Chan, S.Y.; Rubin, L.J. Metabolic dysfunction in pulmonary hypertension: From basic science to clinical practice. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2017, 26, 170094. [Google Scholar] [CrossRef]
- Guignabert, C.; Tu, L.; Girerd, B.; Ricard, N.; Huertas, A.; Montani, D.; Humbert, M. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: Importance of endothelial communication. Chest 2015, 147, 529–537. [Google Scholar] [CrossRef]
- Deng, L.; Blanco, F.J.; Stevens, H.; Lu, R.; Caudrillier, A.; McBride, M.; McClure, J.D.; Grant, J.; Thomas, M.; Frid, M.; et al. MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circ. Res. 2015, 117, 870–883. [Google Scholar] [CrossRef]
- Barnes, H.; Yeoh, H.L.; Fothergill, T.; Burns, A.; Humbert, M.; Williams, T. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst. Rev. 2019, 5, Cd012785. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, E.S.; Slee, S.L.; Subhedar, N.V. Variation in the definition of pulmonary hypertension and clinical indications for the use of nitric oxide in neonatal clinical trials. Acta Paediatr. 2020, 109, 930–934. [Google Scholar] [CrossRef]
- MacLean, M.R.; Fanburg, B.; Hill, N.; Lazarus, H.M.; Pack, T.F.; Palacios, M.; Penumatsa, K.C.; Wring, S.A. Serotonin and Pulmonary Hypertension; Sex and Drugs and ROCK and Rho. Compr. Physiol. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Mu, Y.P.; Huang, Q.H.; Zhu, J.L.; Zheng, S.Y. Magnesium attenuates endothelin-1-induced vasoreactivity and enhances vasodilatation in mouse pulmonary arteries: Modulation by chronic hypoxic pulmonary hypertension. Exp. Physiol. 2018, 103, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Milara, J.; Belhadj, N.; Cortijo, J. Senescence Alterations in Pulmonary Hypertension. Cells 2021, 10, 3456. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Saker, M.; Lipskaia, L.; Marcos, E.; Abid, S.; Parpaleix, A.; Houssaini, A.; Validire, P.; Girard, P.; Noureddine, H.; Boyer, L.; et al. Osteopontin, a Key Mediator Expressed by Senescent Pulmonary Vascular Cells in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Houssaini, A.; Breau, M.; Kebe, K.; Abid, S.; Marcos, E.; Lipskaia, L.; Rideau, D.; Parpaleix, A.; Huang, J.; Amsellem, V.; et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 2018, 3, e93203. [Google Scholar] [CrossRef]
- Meijles, D.N.; Pagano, P.J. Nox and Inflammation in the Vascular Adventitia. Hypertension 2016, 67, 14–19. [Google Scholar] [CrossRef]
- Spiekerkoetter, E.; Goncharova, E.A.; Guignabert, C.; Stenmark, K.; Kwapiszewska, G.; Rabinovitch, M.; Voelkel, N. Hot topics in the mechanisms of pulmonary arterial hypertension disease: Cancer-Like pathobiology, the role of the adventitia, systemic involvement, and right ventricular failure. Pulm. Circ. 2019, 9, 2045894019889775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y.; Li, G.; Chen, M.; Huang, W.; Liu, Y.; Li, Y. TGF-β1/FGF-2 signaling mediates the 15-HETE-induced differentiation of adventitial fibroblasts into myofibroblasts. Lipids Health Dis. 2016, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Niinimaki, E.; Muola, P.; Parkkila, S.; Kholová, I.; Haapasalo, H.; Pastorekova, S.; Pastorek, J.; Paavonen, T.; Mennander, A. Carbonic anhydrase IX deposits are associated with increased ascending aortic dilatation. Scand. Cardiovasc. J. SCJ 2016, 50, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, S.C.; Poth, J.M.; Fini, M.A.; Olschewski, A.; El Kasmi, K.C.; Stenmark, K.R. The role of inflammation in hypoxic pulmonary hypertension: From cellular mechanisms to clinical phenotypes. American journal of physiology. Lung Cell. Mol. Physiol. 2015, 308, L229–L252. [Google Scholar] [CrossRef]
- Tang, Y.; Huo, X.; Liu, J.; Tang, Y.; Zhang, M.; Xie, W.; Zheng, Z.; He, J. MicroRNA-325-3p Targets Human Epididymis Protein 4 to Relieve Right Ventricular Fibrosis in Rats with Pulmonary Arterial Hypertension. Cardiovasc. Ther. 2022, 2022, 4382999. [Google Scholar] [CrossRef]
- Church, A.C.; Martin, D.H.; Wadsworth, R.; Bryson, G.; Fisher, A.J.; Welsh, D.J.; Peacock, A.J. The reversal of pulmonary vascular remodeling through inhibition of p38 MAPK-alpha: A potential novel anti-inflammatory strategy in pulmonary hypertension. American journal of physiology. Lung Cell. Mol. Physiol. 2015, 309, L333–L347. [Google Scholar] [CrossRef]
- Johns, R.A.; Takimoto, E.; Meuchel, L.W.; Elsaigh, E.; Zhang, A.; Heller, N.M.; Semenza, G.L.; Yamaji-Kegan, K. Hypoxia-Inducible Factor 1α Is a Critical Downstream Mediator for Hypoxia-Induced Mitogenic Factor (FIZZ1/RELMα)-Induced Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 134–144. [Google Scholar] [CrossRef]
- Luo, Y.; Dong, H.Y.; Zhang, B.; Feng, Z.; Liu, Y.; Gao, Y.Q.; Dong, M.Q.; Li, Z.C. miR-29a-3p attenuates hypoxic pulmonary hypertension by inhibiting pulmonary adventitial fibroblast activation. Hypertension 2015, 65, 414–420. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, W.; Cai, H.; Sun, X.; Yang, D.; Xu, F.; Jin, C. SB-431542, a specific inhibitor of the TGF-β type I receptor inhibits hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts. Die Pharm. 2016, 71, 94–100. [Google Scholar]
- Yang, B.; Janardhanan, R.; Vohra, P.; Greene, E.L.; Bhattacharya, S.; Withers, S.; Roy, B.; Nieves Torres, E.C.; Mandrekar, J.; Leof, E.B.; et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int. 2014, 85, 289–306. [Google Scholar] [CrossRef]
- Li, Q.; Mao, M.; Qiu, Y.; Liu, G.; Sheng, T.; Yu, X.; Wang, S.; Zhu, D. Key Role of ROS in the Process of 15-Lipoxygenase/15-Hydroxyeicosatetraenoiccid-Induced Pulmonary Vascular Remodeling in Hypoxia Pulmonary Hypertension. PLoS ONE 2016, 11, e0149164. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Shi, J.; Wang, N.; Yu, X.; Zhang, C.; Li, J.; Wei, L.; Ma, C.; Zhao, X.; Lian, M.; et al. 15-Lipoxygenase and 15-hydroxyeicosatetraenoic acid regulate intravascular thrombosis in pulmonary hypertension. American journal of physiology. Lung Cell. Mol. Physiol. 2015, 309, L449–L462. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.C.; Sedding, D.G.; Haverich, A. Targeting vasa vasorum dysfunction to prevent atherosclerosis. Vasc. Pharmacol. 2017, 96–98, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Woodward, H.N.; Anwar, A.; Riddle, S.; Taraseviciene-Stewart, L.; Fragoso, M.; Stenmark, K.R.; Gerasimovskaya, E.V. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. American journal of physiology. Lung Cell. Mol. Physiol. 2009, 297, L954–L964. [Google Scholar] [CrossRef]
- Barallobre-Barreiro, J.; Loeys, B.; Mayr, M.; Rienks, M.; Verstraeten, A.; Kovacic, J.C. Extracellular Matrix in Vascular Disease, Part 2/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2189–2203. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Langlois, B.; Belozertseva, E.; Parlakian, A.; Bourhim, M.; Gao-Li, J.; Blanc, J.; Tian, L.; Coletti, D.; Labat, C.; Ramdame-Cherif, Z.; et al. Vimentin knockout results in increased expression of sub-endothelial basement membrane components and carotid stiffness in mice. Sci. Rep. 2017, 7, 11628. [Google Scholar] [CrossRef]
- Murphy, P.A.; Jailkhani, N.; Nicholas, S.A.; Del Rosario, A.M.; Balsbaugh, J.L.; Begum, S.; Kimble, A.; Hynes, R.O. Alternative Splicing of FN (Fibronectin) Regulates the Composition of the Arterial Wall Under Low Flow. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e18–e32. [Google Scholar] [CrossRef]
- Mutgan, A.C.; Jandl, K.; Kwapiszewska, G. Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension? Cells 2020, 9, 2029. [Google Scholar] [CrossRef]
- Ambade, A.S.; Hassoun, P.M.; Damico, R.L. Basement Membrane Extracellular Matrix Proteins in Pulmonary Vascular and Right Ventricular Remodeling in Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2021, 65, 245–258. [Google Scholar] [CrossRef]
- Schäfer, M.; Ivy, D.D.; Nguyen, K.; Boncella, K.; Frank, B.S.; Morgan, G.J. Metalloproteinases and their inhibitors are associated with pulmonary arterial stiffness and ventricular function in pediatric pulmonary hypertension. American journal of physiology. Heart Circ. Physiol. 2021, 321, H242–H252. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Haghighat, L.; Spiekerkoetter, E.; Sawada, H.; Alvira, C.M.; Wang, L.; Acharya, S.; Rodriguez-Colon, G.; Orton, A.; Zhao, M.; et al. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am. J. Pathol. 2011, 179, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Khera, R.; Corrales-Medina, V.F.; Townsend, R.R.; Chirinos, J.A. Inflammation and arterial stiffness in humans. Atherosclerosis 2014, 237, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gong, J.; Dennery, P.A.; Yao, H. Endothelial-to-mesenchymal transition: Pathogenesis and therapeutic targets for chronic pulmonary and vascular diseases. Biochem. Pharmacol. 2019, 168, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Chan, S.Y.; Weir, E.K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. American journal of physiology. Heart Circ. Physiol. 2018, 315, H1322–H1331. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Feng, Z.; Peterson, A.L.; Carr, J.F.; Vang, A. Endothelial to mesenchymal transition during neonatal hyperoxia-induced pulmonary hypertension. J. Pathol. 2020, 252, 411–422. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Frid, M.; Perros, F. Endothelial-to-Mesenchymal Transition: An Evolving Paradigm and a Promising Therapeutic Target in PAH. Circulation 2016, 133, 1734–1737. [Google Scholar] [CrossRef]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Felix, N.S.; de Mendonça, L.; Braga, C.L.; da Silva, J.S.; Samary, C.D.S.; Vieira, J.B.; Cruz, F.; Rocha, N.N.; Zapata-Sudo, G.; Rocco, P.R.M.; et al. Effects of the FGF receptor-1 inhibitor, infigratinib, with or without sildenafil, in experimental pulmonary arterial hypertension. Br. J. Pharmacol. 2019, 176, 4462–4473. [Google Scholar] [CrossRef]
- Karoor, V.; Strassheim, D.; Sullivan, T.; Verin, A.; Umapathy, N.S.; Dempsey, E.C.; Frank, D.N.; Stenmark, K.R. The Short-Chain Fatty Acid Butyrate Attenuates Pulmonary Vascular Remodeling and Inflammation in Hypoxia-Induced Pulmonary Hypertension. Int. J. Mol. Sci. 2021, 22, 9916. [Google Scholar] [CrossRef]
- Kumar, R.; Graham, B. How does inflammation contribute to pulmonary hypertension? Eur. Respir. J. 2018, 51, 1702403. [Google Scholar] [CrossRef] [PubMed]
- Dierick, F.; Solinc, J.; Bignard, J.; Soubrier, F.; Nadaud, S. Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension. Cells 2021, 10, 1338. [Google Scholar] [CrossRef]
- Goldenberg, N.M.; Rabinovitch, M.; Steinberg, B.E. Inflammatory Basis of Pulmonary Arterial Hypertension: Implications for Perioperative and Critical Care Medicine. Anesthesiology 2019, 131, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Kimishima, Y.; Misaka, T.; Yokokawa, T.; Wada, K.; Ueda, K. Clonal hematopoiesis with JAK2V617F promotes pulmonary hypertension with ALK1 upregulation in lung neutrophils. Nat. Commun. 2021, 12, 6177. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Bai, P.; Wan, N.; Liu, J.; Zhu, Q.; He, Y.; Chen, G.; Wang, J.; Chen, H.; Wang, C.; et al. Niacin Attenuates Pulmonary Hypertension Through H-PGDS in Macrophages. Circ. Res. 2020, 127, 1323–1336. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Shao, C.; Zeng, X.; Sun, L.; Kong, H.; Xie, W.; Wang, H. New dynamic viewing of mast cells in pulmonary arterial hypertension (PAH): Contributors or outsiders to cardiovascular remodeling. J. Thorac. Dis. 2018, 10, 3016–3026. [Google Scholar] [CrossRef]
- Berghausen, E.M.; Feik, L.; Zierden, M.; Vantler, M.; Rosenkranz, S. Key inflammatory pathways underlying vascular remodeling in pulmonary hypertension. Herz 2019, 44, 130–137. [Google Scholar] [CrossRef]
- Tura, O.; Skinner, E.M.; Barclay, G.R.; Samuel, K.; Gallagher, R.C.; Brittan, M.; Hadoke, P.W.; Newby, D.E.; Turner, M.L.; Mills, N.L. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. Stem Cells 2013, 31, 338–348. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Liu, J.; Sheng, C.; Zhang, L.; Zeng, Y. Changes of Number and Function of Late Endothelial Progenitor Cells in Peripheral Blood of COPD Patients Combined with Pulmonary Hypertension. Thorac. Cardiovasc. Surg. 2016, 64, 323–329. [Google Scholar] [CrossRef]
- Sun, H.X.; Li, G.J.; Du, Z.H.; Bing, Z.; Ji, Z.X.; Luo, G.; Pan, S.L. The relationship between endothelial progenitor cells and pulmonary arterial hypertension in children with congenital heart disease. BMC Pediatr. 2019, 19, 502. [Google Scholar] [CrossRef]
- Duong, H.T.; Comhair, S.A.; Aldred, M.A.; Mavrakis, L.; Savasky, B.M.; Erzurum, S.C.; Asosingh, K. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm. Circ. 2011, 1, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Perros, F.; Gambaryan, N.; Girerd, B.; Dorfmuller, P.; Price, L.C.; Huertas, A.; Hammad, H.; Lambrecht, B.; Simonneau, G.; et al. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2011, 184, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Liu, Y.; Zhang, Y.; Nathan, A.; Tian, W.; Yu, J.; Sweatt, A.J.; Shamshou, E.A.; Condon, D.; Chakraborty, A.; et al. Mural Cell SDF1 Signaling Is Associated with the Pathogenesis of Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 62, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Shamskhou, E.A.; Orcholski, M.E.; Nathan, A.; Reddy, S.; Honda, H.; Mani, V.; Zeng, Y.; Ozen, M.O.; Wang, L.; et al. Loss of Endothelium-Derived Wnt5a Is Associated With Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension. Circulation 2019, 139, 1710–1724. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.; Fessel, J.P.; Kaoriihida, S.; Schmidt, E.P.; Gaskill, C.; Alvarez, D.; Graham, B.; Harrison, D.G.; Wagner, D.H., Jr.; Nozik-Grayck, E.; et al. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm. Circ. 2013, 3, 31–49. [Google Scholar] [CrossRef]
- Lambert, M.; Capuano, V.; Olschewski, A.; Sabourin, J.; Nagaraj, C.; Girerd, B.; Weatherald, J.; Humbert, M. Ion Channels in Pulmonary Hypertension: A Therapeutic Interest? Int. J. Mol. Sci. 2018, 19, 3162. [Google Scholar] [CrossRef]
- Santos-Gomes, J.; Le Ribeuz, H.; Brás-Silva, C.; Antigny, F.; Adão, R. Role of Ion Channel Remodeling in Endothelial Dysfunction Induced by Pulmonary Arterial Hypertension. Biomolecules 2022, 12, 484. [Google Scholar] [CrossRef]
- Yuan, X.J.; Wang, J.; Juhaszova, M.; Gaine, S.P.; Rubin, L.J. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 1998, 351, 726–727. [Google Scholar] [CrossRef]
- Fu, L.C.; Lv, Y.; Zhong, Y.; He, Q.; Liu, X.; Du, L.Z. Tyrosine phosphorylation of Kv1.5 is upregulated in intrauterine growth retardation rats with exaggerated pulmonary hypertension. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. E Biol. 2017, 50, e6237. [Google Scholar] [CrossRef]
- Lv, Y.; Fu, L.; Zhang, Z.; Gu, W.; Luo, X.; Zhong, Y.; Xu, S.; Wang, Y.; Yan, L.; Li, M.; et al. Increased Expression of MicroRNA-206 Inhibits Potassium Voltage-Gated Channel Subfamily A Member 5 in Pulmonary Arterial Smooth Muscle Cells and Is Related to Exaggerated Pulmonary Artery Hypertension Following Intrauterine Growth Retardation in Rats. J. Am. Heart Assoc. 2019, 8, e010456. [Google Scholar] [CrossRef]
- Balistrieri, A.; Makino, A.; Yuan, J.X. Pathophysiology and Pathogenic Mechanisms of Pulmonary Hypertension: Role of Membrane Receptors, Ion Channels and Ca(2+) Signaling. Physiol. Rev. 2022. [Google Scholar] [CrossRef]
- Leblanc, N.; Forrest, A.S.; Ayon, R.J.; Wiwchar, M.; Angermann, J.E.; Pritchard, H.A.; Singer, C.A.; Valencik, M.L.; Britton, F.; Greenwood, I.A. Molecular and functional significance of Ca(2+)-activated Cl(−) channels in pulmonary arterial smooth muscle. Pulm. Circ. 2015, 5, 244–268. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, I.F.; Davidson, A.B.; Reeves, J.T.; Grover, R.F. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ. Res. 1976, 38, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Platoshyn, O.; Remillard, C.V.; Fantozzi, I.; Sison, T.; Yuan, J.X. Identification of functional voltage-gated Na(+) channels in cultured human pulmonary artery smooth muscle cells. Pflug. Arch. Eur. J. Physiol. 2005, 451, 380–387. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).