Effect of Radio-Chemotherapy on PD-L1 Immunohistochemical Expression in Head and Neck Squamous Cell Carcinoma
Abstract
:1. Introduction
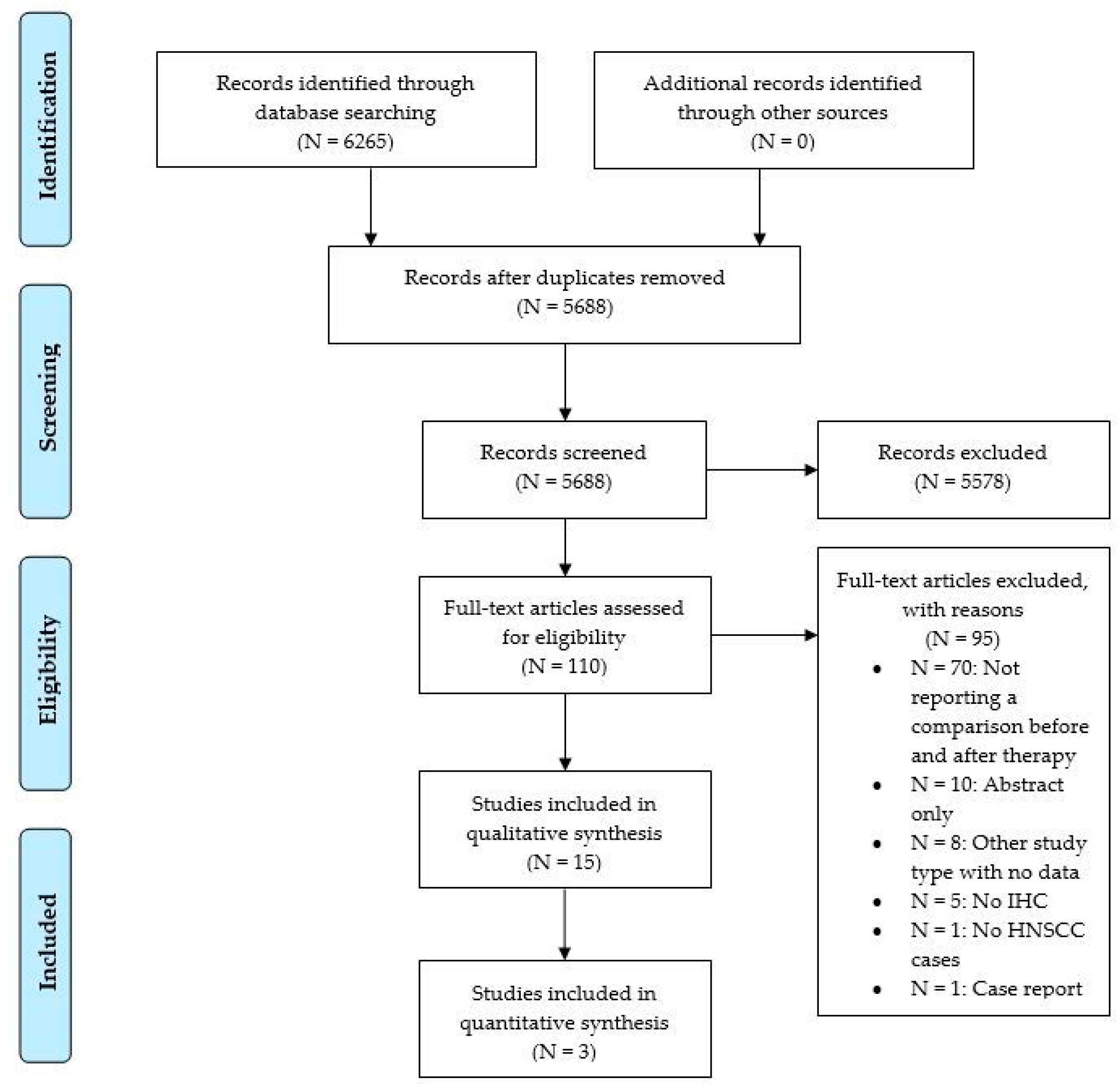
2. Materials and Methods
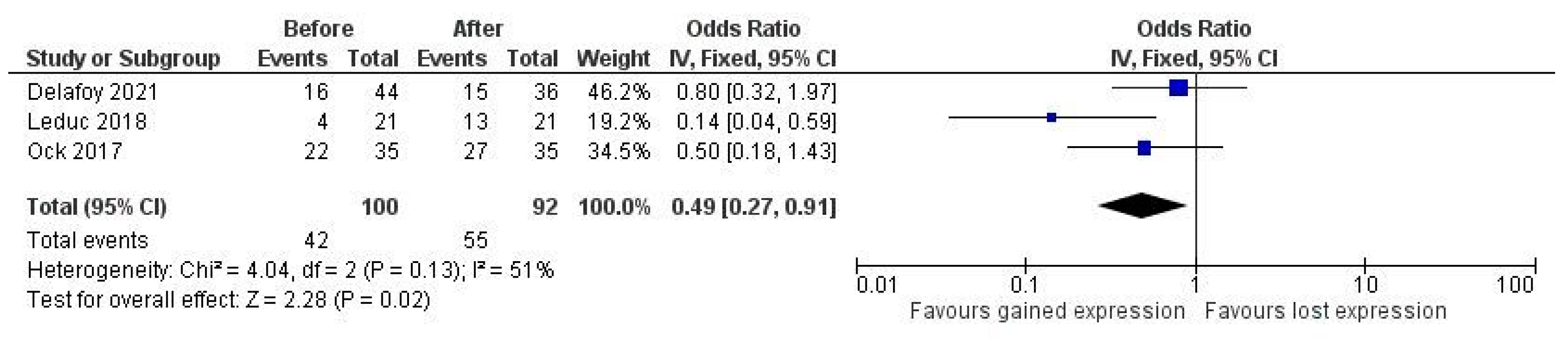
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pulte, D.; Brenner, H. Changes in Survival in Head and Neck Cancers in the Late 20th and Early 21st Century: A Period Analysis. Oncologist 2010, 15, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front. Cell Dev. Biol. 2019, 7, 52. [Google Scholar] [CrossRef]
- Munari, E.; Mariotti, F.R.; Quatrini, L.; Bertoglio, P.; Tumino, N.; Vacca, P.; Eccher, A.; Ciompi, F.; Brunelli, M.; Martignoni, G.; et al. PD-1/PD-L1 in Cancer: Pathophysiological, Diagnostic and Therapeutic Aspects. Int. J. Mol. Sci. 2021, 22, 5123. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.J.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.J.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Licitra, L.; Fayette, J.; Even, C.; Blumenschein, G.J.; Harrington, K.J.; Guigay, J.; Vokes, E.E.; Saba, N.F.; Haddad, R.; et al. Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Efficacy and Safety in CheckMate 141 by Prior Cetuximab Use. Clin. Cancer Res. 2019, 25, 5221–5230. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef]
- Emancipator, K.; Huang, L.; Aurora-Garg, D.; Bal, T.; Cohen, E.E.W.; Harrington, K.; Soulières, D.; Le Tourneau, C.; Licitra, L.; Burtness, B.; et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod. Pathol. 2020, 34, 532–541. [Google Scholar] [CrossRef]
- Concha-Benavente, F.; Srivastava, R.M.; Trivedi, S.; Lei, Y.; Chandran, U.; Seethala, R.R.; Freeman, G.J.; Ferris, R.L. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNγ That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016, 76, 1031–1043. [Google Scholar] [CrossRef] [Green Version]
- de Ruiter, E.J.; Mulder, F.J.; Koomen, B.M.; Speel, E.-J.; van den Hout, M.F.C.M.; de Roest, R.H.; Bloemena, E.; Devriese, L.A.; Willems, S.M. Comparison of three PD-L1 immunohistochemical assays in head and neck squamous cell carcinoma (HNSCC). Mod. Pathol. 2020, 34, 1125–1132. [Google Scholar] [CrossRef]
- Cerbelli, B.; Girolami, I.; Eccher, A.; Costarelli, L.; Taccogna, S.; Scialpi, R.; Benevolo, M.; Lucante, T.; Alò, P.L.; Stella, F.; et al. Evaluating PD-L1 in Head & Neck Squamous Cell Carcinoma: Concordance between 22C3 PharmaDx and SP263 assays on whole sections from a multicenter study. Histopathology 2021, 80, 397–406. [Google Scholar] [CrossRef]
- Guerini Rocco, E.; Eccher, A.; Girolami, I.; Graziano, P.; Fontanini, G.; Vigliar, E.; Troncone, G.; Barberis, M.; Morbini, P.; Martini, M. Concordance between Three PD-L1 Immunohistochemical Assays in Head and Neck Squamous Cell Carcinoma (HNSCC) in a Multicenter Study. Diagnostics 2022, 12, 477. [Google Scholar] [CrossRef]
- Girolami, I.; Pantanowitz, L.; Barberis, M.; Paolino, G.; Brunelli, M.; Vigliar, E.; Munari, E.; Satturwar, S.; Troncone, G.; Eccher, A. Challenges facing pathologists evaluating PD-L1 in head & neck squamous cell carcinoma. J. Oral Pathol. Med. 2021, 50, 864–873. [Google Scholar] [CrossRef]
- Marletta, S.; Fusco, N.; Munari, E.; Luchini, C.; Cimadamore, A.; Brunelli, M.; Querzoli, G.; Martini, M.; Vigliar, E.; Colombari, R.; et al. Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. J. Pers. Med. 2022, 12, 1073. [Google Scholar] [CrossRef]
- Brcic, I.; Gallob, M.; Schwantzer, G.; Zrnc, T.; Weiland, T.; Thurnher, D.; Wolf, A.; Brcic, L. Concordance of tumor infiltrating lymphocytes, PD-L1 and p16 expression in small biopsies, resection and lymph node metastases of oropharyngeal squamous cell carcinoma. Oral Oncol. 2020, 106, 104719. [Google Scholar] [CrossRef]
- Moratin, J.; Metzger, K.; Safaltin, A.; Herpel, E.; Hoffmann, J.; Freier, K.; Hess, J.; Horn, D. Upregulation of PD-L1 and PD-L2 in neck node metastases of head and neck squamous cell carcinoma. Head Neck 2019, 41, 2484–2491. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Creytens, D.; Aspeslagh, S.; Deron, P.; Duprez, F.; Rottey, S.; Van Dorpe, J.A.; Ferdinande, L. Importance of choice of materials and methods in PD-L1 and TIL assessment in oropharyngeal squamous cell carcinoma. Histopathology 2018, 73, 500–509. [Google Scholar] [CrossRef]
- Rasmussen, J.H.; Lelkaitis, G.; Håkansson, K.; Vogelius, I.R.; Johannesen, H.H.; Fischer, B.M.; Bentzen, S.M.; Specht, L.; Kristensen, C.A.; von Buchwald, C.; et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 1003–1006. [Google Scholar] [CrossRef] [Green Version]
- Paolino, G.; Pantanowitz, L.; Barresi, V.; Pagni, F.; Munari, E.; Moretta, L.; Brunelli, M.; Bariani, E.; Vigliar, E.; Pisapia, P.; et al. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol.-Res. Pract. 2021, 226, 153605. [Google Scholar] [CrossRef]
- Karpathiou, G.; Vincent, M.; Dumollard, J.M.; Mobarki, M.; Péoc’h, M. PD-L1 expression in head and neck cancer tissue specimens decreases with time. Pathol. Res. Pract. 2022, 237. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Delafoy, A.; Uguen, A.; Lemasson, G.; Conan-Charlet, V.; Pradier, O.; Lucia, F.; Schick, U. PD-L1 expression in recurrent head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 343–351. [Google Scholar] [CrossRef]
- Doescher, J.; Minkenberg, P.; Laban, S.; Kostezka, U.; von Witzleben, A.; Hoffmann, T.K.; Schuler, P.J.; Weissinger, S.E. Immune checkpoint expression in HNSCC patients before and after definitive chemoradiotherapy. Head Neck 2020, 43, 778–787. [Google Scholar] [CrossRef]
- Gomez-Roca, C.; Even, C.; Le Tourneau, C.; Basté, N.; Delord, J.; Sarini, J.; Vergez, S.; Temam, S.; Hoffmann, C.; Rochaix, P.; et al. Exploratory window-of-opportunity trial to investigate the tumor pharmacokinetics/pharmacodynamics of the IAP antagonist Debio 1143 in patients with head and neck cancer. Clin. Transl. Sci. 2022, 15, 55–62. [Google Scholar] [CrossRef]
- Karabajakian, A.; Bouaoud, J.; Michon, L.; Kamal, M.; Crozes, C.; Zrounba, P.; Auclair-Perossier, J.; Gadot, N.; Attignon, V.; Le Tourneau, C.; et al. Longitudinal assessment of PD-L1 expression and gene expression profiles in patients with head and neck cancer reveals temporal heterogeneity. Oral Oncol. 2021, 119, 105368. [Google Scholar] [CrossRef]
- Leduc, C.; Adam, J.; Louvet, E.; Sourisseau, T.; Dorvault, N.; Bernard, M.; Maingot, E.; Faivre, L.; Cassin-Kuo, M.-S.; Boissier, E.; et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open 2018, 3, e000257. [Google Scholar] [CrossRef] [Green Version]
- Pflumio, C.; Thomas, J.; Salleron, J.; Faivre, J.-C.; Borel, C.; Dolivet, G.; Sastre-Garau, X.; Geoffrois, L. Expression of immune response biomarkers (PD-L1, p16, CD3+ and CD8+ TILs) in recurrent head and neck squamous cell carcinoma within previously irradiated areas. Oncol. Rep. 2021, 45, 1273–1283. [Google Scholar] [CrossRef]
- Chan, O.S.H.; Kowanetz, M.; Ng, W.T.; Koeppen, H.; Chan, L.K.; Yeung, R.M.W.; Wu, H.; Amler, L.; Mancao, C. Characterization of PD-L1 expression and immune cell infiltration in nasopharyngeal cancer. Oral Oncol. 2017, 67, 52–60. [Google Scholar] [CrossRef]
- Long, G.; Li, X.; Yang, L.; Zhao, J.; Lu, X.; Wang, H.; Song, J.; Mei, Q.; Hu, G. The clinical prognostic value of PD-L1 after concurrent chemoradiotherapy in Chinese nasopharyngeal carcinoma patients. Ann. Transl. Med. 2021, 9, 1650. [Google Scholar] [CrossRef]
- Shen, L.-F.; Zhou, S.-H.; Guo, Y. Role of GLUT-1 in the Upregulation of PD-L1 Expression After Radiotherapy and Association of PD-L1 with Favourable Overall Survival in Hypopharyngeal Cancer. OncoTargets Ther. 2020, 13, 11221–11235. [Google Scholar] [CrossRef]
- Naruse, T.; Yanamoto, S.; Okuyama, K.; Ohmori, K.; Tsuchihashi, H.; Furukawa, K.; Yamada, S.-I.; Umeda, M. Immunohistochemical Study of PD-1/PD-L1 Axis Expression in Oral Tongue Squamous Cell Carcinomas: Effect of Neoadjuvant Chemotherapy on Local Recurrence. Pathol. Oncol. Res. 2020, 26, 735–742. [Google Scholar] [CrossRef]
- Ono, T.; Azuma, K.; Kawahara, A.; Kakuma, T.; Sato, F.; Kawaguchi, T.; Akiba, J.; Umeno, H. Changes in immune parameters between pre-treatment and recurrence after (chemo) radiation therapy in patients with head and neck cancer. Sci. Rep. 2020, 10, 11973. [Google Scholar] [CrossRef]
- Seki-Soda, M.; Sano, T.; Ogawa, M.; Yokoo, S.; Oyama, T. CD15+ tumor infiltrating granulocytic cells can predict recurrence and their depletion is accompanied by good responses to S-1 with oral cancer. Head Neck 2021, 43, 2457–2467. [Google Scholar] [CrossRef]
- Ock, C.-Y.; Kim, S.; Keam, B.; Kim, S.; Ahn, Y.-O.; Chung, E.-J.; Kim, J.-H.; Kim, T.M.; Kwon, S.K.; Jeon, Y.K.; et al. Changes in programmed death-ligand 1 expression during cisplatin treatment in patients with head and neck squamous cell carcinoma. Oncotarget 2017, 8, 97920–97927. [Google Scholar] [CrossRef] [Green Version]
- So, Y.K.; Byeon, S.-J.; Ku, B.M.; Ko, Y.H.; Ahn, M.-J.; Son, Y.-I.; Chung, M.K. An increase of CD8+ T cell infiltration following recurrence is a good prognosticator in HNSCC. Sci. Rep. 2020, 10, 20059. [Google Scholar] [CrossRef]
- Wolf, G.T.; Liu, S.; Bellile, E.; Sartor, M.; Rozek, L.; Thomas, D.; Nguyen, A.; Zarins, K.; McHugh, J.B. Tumor infiltrating lymphocytes after neoadjuvant IRX-2 immunotherapy in oral squamous cell carcinoma: Interim findings from the INSPIRE trial. Oral Oncol. 2020, 111, 104928. [Google Scholar] [CrossRef]
- Tuminello, S.; Sikavi, D.; Veluswamy, R.; Gamarra, C.; Lieberman-Cribbin, W.; Flores, R.; Taioli, E. PD-L1 as a prognostic biomarker in surgically resectable non- small cell lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2020, 9, 1343–1360. [Google Scholar] [CrossRef]
- Davey, M.G.; Ryan, É.J.; Davey, M.S.; Lowery, A.J.; Miller, N.; Kerin, M.J. Clinicopathological and prognostic significance of programmed cell death ligand 1 expression in patients diagnosed with breast cancer: Meta-analysis. Br. J. Surg. 2021, 108, 622–631. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, J.; Wang, L.; Li, Z.; Wang, L.; Li, Z. Prognostic and Clinicopathological Significance of PD-L1 in Patients With Bladder Cancer: A Meta-Analysis. Front. Pharmacol. 2019, 10, 962. [Google Scholar] [CrossRef] [Green Version]
- Nocini, R.; Vianini, M.; Girolami, I.; Calabrese, L.; Scarpa, A.; Martini, M.; Morbini, P.; Marletta, S.; Brunelli, M.; Molteni, G.; et al. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin. Exp. Dent. Res. 2022, 8, 690–698. [Google Scholar] [CrossRef]
- Lenouvel, D.; González-Moles, M.Á.; Ruiz-Ávila, I.; Gonzalez-Ruiz, L.; Gonzalez-Ruiz, I.; Ramos-García, P. Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: A systematic review and comprehensive meta-analysis. Oral Oncol. 2020, 106, 104722. [Google Scholar] [CrossRef]
- Girolami, I.; Pantanowitz, L.; Mete, O.; Brunelli, M.; Marletta, S.; Colato, C.; Trimboli, P.; Crescenzi, A.; Bongiovanni, M.; Barbareschi, M.; et al. Programmed Death-Ligand 1 (PD-L1) Is a Potential Biomarker of Disease-Free Survival in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis of PD-L1 Immunoexpression in Follicular Epithelial Derived Thyroid Carcinoma. Endocr. Pathol. 2020, 31, 291–300. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Li, H.; Vujanovic, L.; Zandberg, D.P.; Ferris, R.L.; Bruno, T.C. Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat. Rev. Cancer 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Eichberger, J.; Spoerl, S.; Spanier, G.; Erber, R.; Taxis, J.; Schuderer, J.; Ludwig, N.; Fiedler, M.; Nieberle, F.; Ettl, T.; et al. TIGIT Expression on Intratumoral Lymphocytes Correlates with Improved Prognosis in Oral Squamous Cell Carcinoma. Biomedicines 2022, 10, 3236. [Google Scholar] [CrossRef]
- Hawley, K.M.; Eclov, R.J.; Schnorenberg, M.R.; Tian, Y.; Shah, R.N.; Thomas-Toth, A.T.; Fefferman, M.; Bird, G.H.; Walensky, L.D.; Tirrell, M.V.; et al. Inhibition of FOXP3 by stapled alpha-helical peptides dampens regulatory T cell function. Proc. Natl. Acad. Sci. USA 2022, 119, e2209044119. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Cillo, A.R.; Tabib, T.; Liu, A.; Onkar, S.; Kunning, S.R.; Lampenfeld, C.; Atiya, H.I.; Abecassis, I.; Kürten, C.H.L.; et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 2021, 12, 3349. [Google Scholar] [CrossRef]
- Galeano Niño, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef]
- Tsai, T.-F.; Lin, J.-F.; Lin, Y.-C.; Chou, K.-Y.; Chen, H.-E.; Ho, C.-Y.; Chen, P.-C.; Hwang, T.I.-S. Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway. Biosci. Rep. 2019, 39, BSR20190362. [Google Scholar] [CrossRef] [Green Version]
- Okadome, K.; Baba, Y.; Yasuda-Yoshihara, N.; Nomoto, D.; Yagi, T.; Toihata, T.; Ogawa, K.; Sawayama, H.; Ishimoto, T.; Iwatsuki, M.; et al. PD-L1 and PD-L2 expression status in relation to chemotherapy in primary and metastatic esophageal squamous cell carcinoma. Cancer Sci. 2022, 113, 399–410. [Google Scholar] [CrossRef]
- Li, S.; Ji, J.; Zhang, Z.; Peng, Q.; Hao, L.; Guo, Y.; Zhou, W.; Cui, Q.; Shi, X. Cisplatin promotes the expression level of PD-L1 in the microenvironment of hepatocellular carcinoma through YAP1. Mol. Cell. Biochem. 2020, 475, 79–91. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, M.; Zhou, C.; Zhu, X. Variation of PD-L1 expression in locally advanced cervical cancer following neoadjuvant chemotherapy. Diagn. Pathol. 2020, 15, 67. [Google Scholar] [CrossRef]
- Fournel, L.; Wu, Z.; Stadler, N.; Damotte, D.; Lococo, F.; Boulle, G.; Ségal-Bendirdjian, E.; Bobbio, A.; Icard, P.; Trédaniel, J.; et al. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019, 464, 5–14. [Google Scholar] [CrossRef]
- Fukuoka, E.; Yamashita, K.; Tanaka, T.; Sawada, R.; Sugita, Y.; Arimoto, A.; Fujita, M.; Takiguchi, G.; Matsuda, T.; Oshikiri, T.; et al. Neoadjuvant Chemotherapy Increases PD-L1 Expression and CD8+ Tumor-infiltrating Lymphocytes in Esophageal Squamous Cell Carcinoma. Anticancer Res. 2019, 39, 4539–4548. [Google Scholar] [CrossRef]
| Author, Year (Country) | N Cases | Sites | Therapy | Scoring System | Clone | MAIN RESULTS | Potential Limitations |
|---|---|---|---|---|---|---|---|
| Clone E1L3N (6 studies) | |||||||
| Delafoy, 2021 (France) [30] | 44 | HNSCC NOS | Concurrent cisplatin and RT | TC and IC scored separately, positive if >1% | E1L3N | Positivization in TC in the majority of cases where PD-L1 expression changed after therapy, but decrease after radiation | No correspondence between Bx and Rx nor separation of data |
| Doescher, 2020 (Germany) [31] | 67 | HNSCC excluded sinonasal and RP | Concurrent platinum-based CHT and RT | TC and IC scored separately with H-score | E1L3N | No significant changes in the expression of PD-1 on neither tumor nor TIL during therapy | No use of established CPS cutoffs as in trials, small sample size, heterogeneity in tumor locations, fixation and storage of tissue blocks and treatments with no stratification |
| Gomez-Roca, 2020 (France) [32] | 26 | Oral, OP, L, HP | Inhibitor of apoptosis protein Debio 1143 with and without cisplatin | TC and IC as % positive cells | E1L3N | Increased PD-L1 levels in IC with neoadjuvant target therapy with inhibitor of apoptose protein and in combination with cisplatin, but not with cisplatin alone, not in TC | Pharmacodynamic and pharmacogenomic study with no use of established CPS cutoffs as in trials |
| Leduc, 2018 (France) [34] | 21 | Oral and L | Docetaxel, platinum and fluorouracil (TPF) | TC and IC scored separately with >5% as positive | E1L3N | Significant increase of PD-L1 expression in IC and TC after therapy | Small sample size |
| Long, 2021 (China) [37] | 24 | NP | Concurrent IMRT and cisplatin | TPS, positive if >1%, CPS | E1L3N | Different distribution of changes in PD-L1 expression according to score used whether TPS or CPS | Not full correspondence of data in tables and text of the article |
| Ock, 2017 (Korea) [42] | 35 | OP mainly | Docetaxel, platinum and fluorouracil (TPF) regimens or cisplatin-based regimens and concurrent RT | TPS > 5% as positive | E1L3N | Statistically significant up-regulation of PD-L1 in 69% of originally negative cases and upregulation of PD-L1 after radiotherapy | Small sample size, and unclear detailing of IHC protocol |
| Clone SP142 (2 studies) | |||||||
| Chan, 2017 (China) [36] | 161 | NP | IMRT and CHT with cisplatin | TC and IC with cutoff 1% | SP142 | Reduction of PD-L1 expression in 56% cases in IC and in 33% in TC after treatment | No separated data for patients receiving radiotherapy alone |
| So, 2020 (Korea) [43] | 42 | Oral cavity, L, OP, HP, sinuses | RT only | TC and IC positive if >10%, automated scoring on WSI digitized slides | SP142 | Ratio of recurrent (R) vs. initial (I) PD-L1 expression was <1 in 69% cases with TC turning negative in 23% and positive in 9% | Small sample size, retrospective design, and different treatment characteristics among patients |
| Other clones (7 studies, a clone each) | |||||||
| Karabajakian, 2021 (France) [33] | 35 | Oral, OP, HP, L | RT-CHT NOS | CPS | QR1 | Majority of samples positive at CPS1 remained positive (76%), while majority of negative CPS samples at diagnosis became positive at relapse (75%), but change of different entity at different cutoffs CPS1 and CPS20 | No special limitations |
| Naruse, 2020 (Japan) [39] | 121 | Tongue | Platinum-based CHT | TC only, positive if >5% | Abcam ab156361 | No marked difference in expression of PD-L1 in recurrent patients who had surgery alone or CHT plus surgery | No use of CPS or approved assay and no reporting of CI or p values for comparisons; only reporting for correlation with clinicopathological variables and prognosis |
| Ono, 2020 (Japan) [40] | 30 | L, OP, HP | Cisplatin-based RT-CHT | TPS and IC density score | clone D3 | No significant changes in PD-L1 expression after RT-CHT | Small sample size |
| Pflumio, 2021 (France) [35] | 100 | Oral, OP, L, HP | RT-CHT NOS | TPS > 1% | SP263 | Significantly lower percentage of PD-L1+ TC within the irradiated area cohort than the de novo cohort and significantly fewer tumors with PD-L1+ IC in the irradiated area cohort | Indirect comparison, as 50 irradiated cancers are compared with 50 de novo cancers and no CPS used |
| Seki-Soda, 2021 (Japan) [41] | 71 | Oral cavity | Preoperative fluorouracil-based CHT | TC | 28-8 | PD-L1 positivity in TC significantly decreased by CHT irrespective of clinical response | No use of established CPS cutoffs as in trials |
| Shen, 2020 (China) [38] | 47 age 64 y) | HP | Platinum-based CHT and RT | TC | 66248-1-Ig | PD-L1 expression increased after RT | Not stated a clear cutoff for positivity, only 10% for low vs. high |
| Wolf, 2020 (USA) [44] | 96 | Oral cavity | Multi-cytokine preparation with cyclophosphamide | IC and H-score, CPS | Multiplex fluorescence IHC | No significant changes in PD-L1 expression after CHT | Small sample size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolami, I.; Marletta, S.; Fiorentino, V.; Battocchio, S.; Cerbelli, B.; Fiamengo, B.; Gerosa, C.; Gianatti, A.; Morelli, L.; Riva, G.; et al. Effect of Radio-Chemotherapy on PD-L1 Immunohistochemical Expression in Head and Neck Squamous Cell Carcinoma. J. Pers. Med. 2023, 13, 363. https://doi.org/10.3390/jpm13020363
Girolami I, Marletta S, Fiorentino V, Battocchio S, Cerbelli B, Fiamengo B, Gerosa C, Gianatti A, Morelli L, Riva G, et al. Effect of Radio-Chemotherapy on PD-L1 Immunohistochemical Expression in Head and Neck Squamous Cell Carcinoma. Journal of Personalized Medicine. 2023; 13(2):363. https://doi.org/10.3390/jpm13020363
Chicago/Turabian StyleGirolami, Ilaria, Stefano Marletta, Vincenzo Fiorentino, Simonetta Battocchio, Bruna Cerbelli, Barbara Fiamengo, Clara Gerosa, Andrea Gianatti, Luca Morelli, Giulio Riva, and et al. 2023. "Effect of Radio-Chemotherapy on PD-L1 Immunohistochemical Expression in Head and Neck Squamous Cell Carcinoma" Journal of Personalized Medicine 13, no. 2: 363. https://doi.org/10.3390/jpm13020363
APA StyleGirolami, I., Marletta, S., Fiorentino, V., Battocchio, S., Cerbelli, B., Fiamengo, B., Gerosa, C., Gianatti, A., Morelli, L., Riva, G., Zagami, M. G., Fusco, N., Munari, E., L’Imperio, V., Pagni, F., Morbini, P., Martini, M., & Eccher, A. (2023). Effect of Radio-Chemotherapy on PD-L1 Immunohistochemical Expression in Head and Neck Squamous Cell Carcinoma. Journal of Personalized Medicine, 13(2), 363. https://doi.org/10.3390/jpm13020363











