Emergency Care for Burn Patients—A Single-Center Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. Data Collection
2.3. Statistical Analysis
- our study included 93 patients (N)—59 alive patients (a proportion of 0.634 cases) and 34 deceased patients (a proportion of 0.366 cases); therefore, p = 0.366 represents the smallest of the proportions.
- applying the above-mentioned equation, the number of independent variables that can be used in our logistic regression model is k = 3.40; in conclusion, maximum 4 variables can be used.
3. Results
3.1. General Characteristics
3.2. Primary Hospital Admission, Patients’ Residential Areas, Type, and Timing of Admission
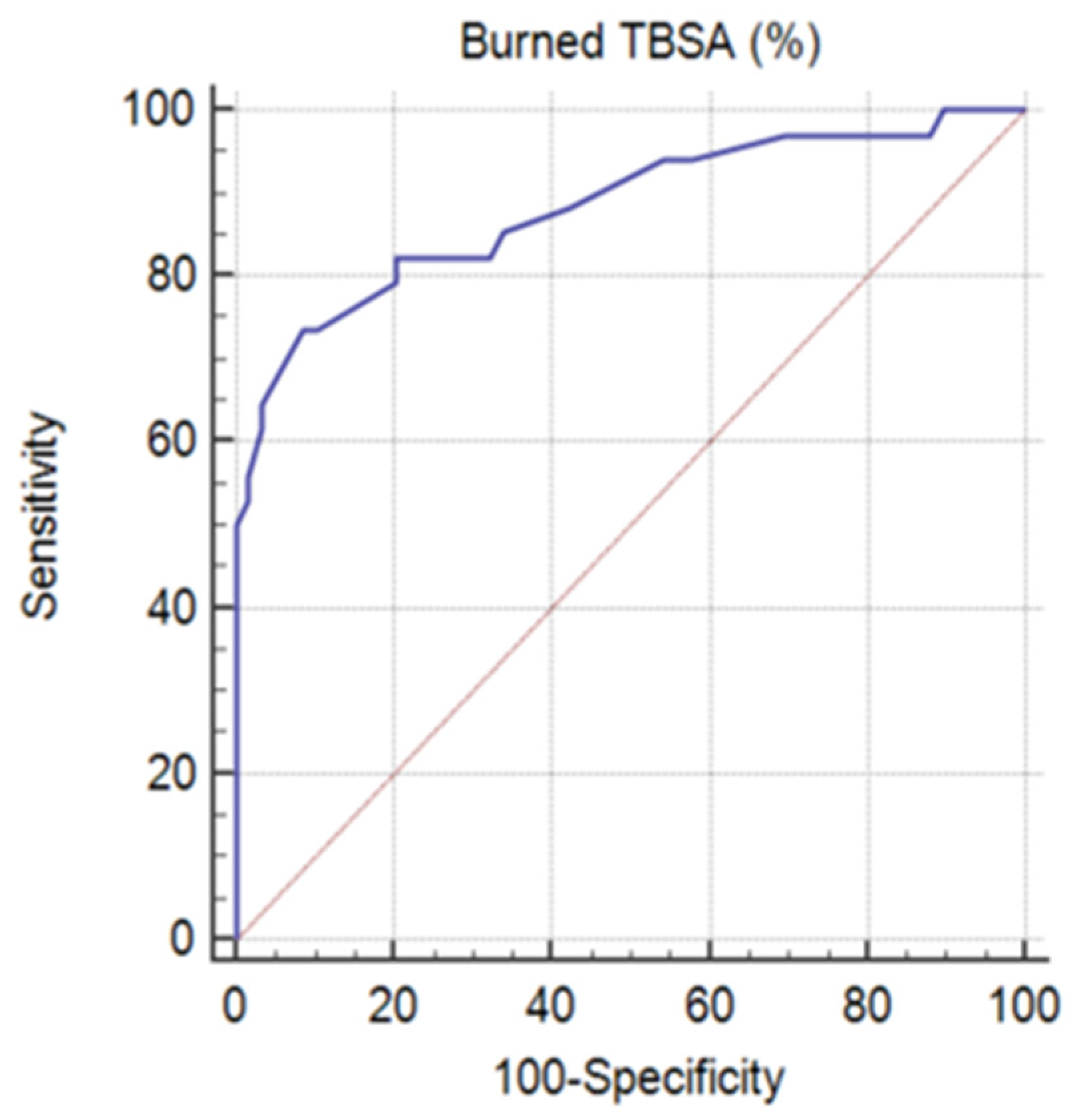
3.3. Burn Etiology, TBSA (%), Type of Burn (Severity), Inflicted Injury, and Body Region
3.4. Inhalation Injury, Type of Ventilation, Tracheostomy, and Days of Mechanical Ventilation
3.5. COVID-19 Status, ABSI Score, Comorbidities, and Bioumoral Parameters
- 1.004 times higher in cases where admission—creatinkinase (U/L) increased by 1 unit, with all other factors remaining unchanged.
- 1.000 times higher in cases where admission—leukocytes (val./uL) increased by 1 unit, with all other factors remaining unchanged.
3.6. Length of Stay and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 2010, 14, R188. [Google Scholar] [CrossRef] [PubMed]
- Latenser, B.A. Critical care of the burn patient: The first 48 hours. Crit. Care Med. 2009, 37, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Vivó, C.; Galeiras, R.; del Caz, M.D. Initial evaluation and management of the critical burn patient. Med. Intensiv. 2016, 40, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lundy, J.B.; Chung, K.K.; Pamplin, J.C.; Ainsworth, C.R.; Jeng, J.C.; Friedman, B.C. Update on Severe Burn Management for the Intensivist. J. Intensive Care Med. 2016, 31, 499–510. [Google Scholar] [CrossRef] [PubMed]
- European Burn Association. Guideline. European Practice Guidelines for Burn Care. Available online: https://www.euroburn.org/documents/ (accessed on 13 November 2022).
- World Health Organization. Burn Key Facts; March, 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 27 June 2022).
- Karimi, H.; Motevalian, S.A.; Momeni, M.; Ghadarjani, M. Financial burden of burn injuries in iran: A report from the burn registry program. Ann. Burn. Fire Disasters 2015, 28, 310–314. [Google Scholar]
- Ehmer-al-lbran; Memon, A.A.; Adil, S.E.; Rao, M.H.; Dawani, O. Post-traumatic stress disorder in patients with acute burn injury. J. Pak. Med. Assoc. 2013, 63, 888–892. [Google Scholar]
- Al-Shamsi, M.; Othman, N. The epidemiology of burns in Basra, Iraq. Ann. Burn. Fire Disasters 2017, 30, 167–171. [Google Scholar]
- D’Abbondanza, J.A.; Shahrokhi, S. Burn Infection and Burn Sepsis. Surg. Infect. 2021, 22, 58–64. [Google Scholar] [CrossRef]
- Folkestad, T.; Brurberg, K.G.; Nordhuus, K.M.; Tveiten, C.K.; Guttormsen, A.B.; Os, I.; Beitland, S. Acute kidney injury in burn patients admitted to the intensive care unit: A systematic review and meta-analysis. Crit. Care 2020, 24, 2. [Google Scholar] [CrossRef]
- Maarouf, R.; Campbell, C. Acute respiratory failure and burn patient outcomes. Curr. Opin. Anaesthesiol. 2021, 34, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Hardin, C.; Corkins, C.J.; Jiwani, A.Z.; Fletcher, J.; Carlsson, A.; Chan, R. Pathophysiologic Mechanisms and Current Treatments for Cutaneous Sequelae of Burn Wounds. Compr. Physiol. 2017, 8, 371–405. [Google Scholar] [CrossRef]
- Baldursdottir, L.; Zoega, S.; Audolfsson, G.; Fridriksdottir, V.; Sigurjonsson, S.Y.; Ingadottir, B. Long term effects of burn injury on health-related quality of life of adult burn survivors in Iceland: A descriptive cross-sectional study and validation of the Icelandic version of the Burn Specific Health Scale-Brief (BSHS-B). Laeknabladid 2021, 107, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Chen, C.C.; Wang, K.Y.; Chung, C.H.; Chang, N.W.; Chien, W.C. Increased risk for sleep disorders in burn patients: A 14-year nationwide, population-based cohort study. Burns 2021, 47, 1408–1415. [Google Scholar] [CrossRef]
- Almarghoub, M.A.; Alotaibi, A.S.; Alyamani, A.; Alfaqeeh, F.A.; Almehaid, F.F.; Al-Qattan, M.M.; Kattan, A.E. The Epidemiology of Burn Injuries in Saudi Arabia: A Systematic Review. J. Burn. Care Res. 2020, 41, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef]
- Guilabert, P.; Usúa, G.; Martín, N.; Abarca, L.; Barret, J.P.; Colomina, M.J. Fluid resuscitation management in patients with burns: Update. Br. J. Anaesth. 2016, 117, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. Management of Burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Romanian Ministry of Health–Press Release. Available online: https://www.ms.ro/2021/03/22/ministerul-sanatatii-3-noi-paturi-pentru-mari-arsi-aprobate-la-spitalul-clinic-de-urgenta-bagdasar-arseni/ (accessed on 13 November 2022).
- Pieptu, V.; Moscalu, R.; Mihai, A.; Moscalu, M.; Pieptu, D.; Azoicăi, D. Epidemiology of hospitalized burns in Romania: A 10-year study on 92,333 patients. Burns 2022, 48, 420–431. [Google Scholar] [CrossRef]
- Santos, J.V.; Oliveira, A.; Costa-Pereira, A.; Amarante, J.; Freitas, A. Burden of burns in Portugal, 2000-2013: A clinical and economic analysis of 26,447 hospitalisations. Burns 2016, 42, 891–900. [Google Scholar] [CrossRef]
- Blom, L.; Klingberg, A.; Laflamme, L.; Wallis, L.; Hasselberg, M. Gender differences in burns: A study from emergency centres in the Western Cape, South Africa. Burns 2016, 42, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.V.; Souza, J.; Amarante, J.; Freitas, A. Burden of Burns in Brazil from 2000 to 2014: A Nationwide Hospital-Based Study. World J. Surg. 2017, 41, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Zúñiga, M.F.; Castro-Delgado, O.E.; Caicedo-Caicedo, J.C.; Merchán-Galvis, Á.M.; Delgado-Noguera, M. Epidemiological profile of minor and moderate burn victims at the University Hospital San José, Popayán, Colombia, 2000–2010. Burns 2013, 39, 1012–1017. [Google Scholar] [CrossRef]
- Ganesamoni, S.; Kate, V.; Sadasivan, J. Epidemiology of hospitalized burn patients in a tertiary care hospital in South India. Burns 2010, 36, 422–429. [Google Scholar] [CrossRef]
- Karki, B.; Rai, S.M.; Nakarmi, K.K.; Basnet, S.J.; Magar, M.G.; Nagarkoti, K.K.; Thapa, S. Clinical Epidemiology of Acute Burn Injuries at Nepal Cleft and Burn Centre, Kathmandu, Nepal. Ann. Plast. Surg. 2018, 80, S95–S97. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Z.; Tan, J.; Zhou, J.; Li, Y.; Wu, J.; Luo, G. Epidemiology and outcome analysis of 6325 burn patients: A five-year retrospective study in a major burn center in Southwest China. Sci. Rep. 2017, 7, 46066. [Google Scholar] [CrossRef]
- Filaj, V.H.; Belba, M.K. Epidemiological trends of severe burns, 2009–2019: A study in the service of burns in Albania. Burns 2021, 47, 930–943. [Google Scholar] [CrossRef]
- Queiroz, L.F.; Anami, E.H.; Zampar, E.F.; Tanita, M.T.; Cardoso, L.T.; Grion, C.M. Epidemiology and outcome analysis of burn patients admitted to an Intensive Care Unit in a University Hospital. Burns 2016, 42, 655–662. [Google Scholar] [CrossRef]
- Hahn, B.; Alex Roh, S.; Price, C.; Fu, W.; DiBello, J.; Barbara, P.; Greenstein, J.; Chacko, J. Demographics and clinical patterns of burns requiring emergency hospitalization at a regional north-eastern us burn center. Hosp. Pract. 2020, 48, 137–145. [Google Scholar] [CrossRef]
- Forjuoh, S.N. Burns in low- and middle-income countries: A review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns 2006, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Lami, F.H.; Al Naser, R.K. Epidemiological characteristics of burn injuries in Iraq: A burn hospital-based study. Burns 2019, 45, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ma, B.; Zeng, D.; Fang, X.; Li, H.; Xiao, S.; Wang, G.; Tang, H.; Xia, Z. Burns in a major burns center in East China from 2005 to 2014: Incidence and outcome. Burns 2017, 43, 1586–1595. [Google Scholar] [CrossRef]
- Palacios García, P.; Pacheco Compaña, F.J.; Rodríguez Pérez, E.; Bugallo Sanz, J.I.; Fernández-Quinto, A.; Avellaneda-Oviedo, E.M. Trends in burn injuries in Galicia (Spain): An epidemiological study. Int. Wound J. 2020, 17, 1717–1724. [Google Scholar] [CrossRef]
- Iqbal, T.; Saaiq, M.; Ali, Z. Epidemiology and outcome of burns: Early experience at the country’s first national burns centre. Burns 2013, 39, 358–362. [Google Scholar] [CrossRef]
- Muller, M.J.; Pegg, S.P.; Rule, M.R. Determinants of death following burn injury. Br. J. Surg. 2001, 88, 583–587. [Google Scholar] [CrossRef]
- Pegg, S.P. Burn epidemiology in the Brisbane and Queensland area. Burns 2005, 31, S27–S31. [Google Scholar] [CrossRef]
- Gilbert, A.D.; Rajha, E.; El Khuri, C.; Bou Chebl, R.; Mailhac, A.; Makki, M.; El Sayed, M. Epidemiology of burn patients presenting to a tertiary hospital emergency department in Lebanon. Burns 2018, 44, 218–225. [Google Scholar] [CrossRef]
- Nickel, K.J.; Omeis, T.; Papp, A. Demographics and clinical outcomes of adult burn patients admitted to a single provincial burn centre: A 40-year review. Burns 2020, 46, 1958–1967. [Google Scholar] [CrossRef]
- Knowlin, L.; Stanford, L.; Moore, D.; Cairns, B.; Charles, A. The measured effect magnitude of co-morbidities on burn injury mortality. Burns 2016, 42, 1433–1438. [Google Scholar] [CrossRef]
- Bagheri, M.; Fuchs, P.C.; Lefering, R.; Grigutsch, D.; Busche, M.N.; Niederstätter, I.; Schiefer, J.L. Effect of comorbidities on clinical outcome of patients with burn injury–An analysis of the German Burn Registry. Burns 2021, 47, 1053–1058. [Google Scholar] [CrossRef]
- Brandão, C.; Meireles, R.; Brito, I.; Ramos, S.; Cabral, L. The Role of Comorbidities On Outcome Prediction In Acute Burn Patients. Ann. Burn. Fire Disasters 2021, 34, 323–333. [Google Scholar]
- Bandeira, N.G.; Barroso, M.V.V.S.; Matos, M.A.A.; Filho, A.L.M.; Figueredo, A.A.; Gravina, P.R.; Klein, S.O.T. Serum Albumin Concentration on Admission as a Predictor of Morbidity and Mortality in Patients with Burn Injuries. J. Burn Care Res. 2021, 42, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Sarhane, K.A.; Fagan, S.P.; Goverman, J. Renal dysfunction in burns: A review. Ann. Burn. Fire Disasters 2013, 26, 16–25. [Google Scholar]
- Emami, A.; Javanmardi, F.; Rajaee, M.; Pirbonyeh, N.; Keshavarzi, A.; Fotouhi, M.; Hosseini, S.M. Predictive Biomarkers for Acute Kidney Injury in Burn Patients. J. Burn Care Res. 2019, 40, 601–605. [Google Scholar] [CrossRef]
- Coban, Y.K. Rhabdomyolysis, compartment syndrome and thermal injury. World J. Crit. Care Med. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Laloë, V. Epidemiology and mortality of burns in a general hospital of Eastern Sri Lanka. Burns 2002, 28, 778–781. [Google Scholar] [CrossRef]
- American Burn Association. Burn Incidence Fact Sheet. Burn Incidence and Treatment in the United States: 2016. Available online: https://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/ (accessed on 30 June 2022).
- Dokter, J.; Felix, M.; Krijnen, P.; Vloemans, J.F.; Baar, M.E.; Tuinebreijer, W.E.; Breederveld, R.S.; Dutch Burn Repository Group. Mortality and causes of death of Dutch burn patients during the period 2006–2011. Burns 2015, 41, 235–240. [Google Scholar] [CrossRef]
- Akerlund, E.; Huss, F.R.; Sjöberg, F. Burns in Sweden: An analysis of 24,538 cases during the period 1987–2004. Burns 2007, 33, 31–36. [Google Scholar] [CrossRef]
- Kallinen, O.; Maisniemi, K.; Böhling, T.; Tukiainen, E.; Koljonen, V. Multiple organ failure as a cause of death in patients with severe burns. J. Burn Care Res. 2012, 33, 206–211. [Google Scholar] [CrossRef]
- Sasor, S.E.; Chung, K.C. Upper Extremity Burns in the Developing World: A Neglected Epidemic. Hand Clin. 2019, 35, 457–466. [Google Scholar] [CrossRef]






| Characteristics | Alive Patients’ Group | Deceased Patients’ Group | Total (N; %) | p-Value | ||
|---|---|---|---|---|---|---|
| Age (mean ± SD, years) | Female | 60.50 ± 17.81 | 62.60 ± 14.46 | 32 (34.4%) | 0.746 | |
| Male | 49.46 ± 15.32 | 58.46 ± 18.40 | 61 (65.6%) | 0.043 | ||
| Gender (N; %) | Female | 22 (37.3%) | 10 (29.4%) | 32 (34.4%) | 0.585 | |
| Male | 37 (62.7%) | 24 (70.6%) | 61 (65.6%) | 0.585 | ||
| Patients’ environment | Urban (N; %) | 37 (62.7%) | 17 (50%) | 54 (58.1%) | 0.328 | |
| Rural (N; %) | 22 (37.3%) | 17 (50%) | 39 (41.9%) | 0.328 | ||
| Variables | Mechanism of injury (N; %) | Thermal | 56 (94.9%) | 31 (91.2%) | 87 (93.5%) | 0.793 |
| Electric | 3 (5.1%) | 3 (8.8%) | 6 (6.5%) | 0.739 | ||
| TBSA (%) | >37% | 5 (8.5%) | 25 (73.5%) | 30 (32.3%) | <0.001 | |
| ≤37% | 54 (91.5%) | 9 (26.5%) | 63 (67.7%) | <0.001 | ||
| Type of burn (degree) | IIA | 4 (6.8%) | 1 (2.9%) | 5 (5.4%) | 0.744 | |
| IIB | 25 (42.4%) | 4 (11.8%) | 29 (31.2%) | 0.004 | ||
| III | 30 (50.8%) | 29 (85.3%) | 59 (63.4%) | 0.001 | ||
| Form of injury | Accident | 56 (94.9%) | 32 (94.1%) | 88 (94.6%) | 0.754 | |
| Self-aggression | 3 (5.1%) | 2 (5.9%) | 5 (5.4%) | 0.754 | ||
| Body region | Head | 44 (74.6%) | 27 (79.4%) | 71 (76.3%) | 0.597 | |
| Neck | 22 (37.3%) | 22 (64.7%) | 44 (47.3%) | 0.011 | ||
| Trunk | 28 (47.5%) | 27 (79.4%) | 55 (59.1%) | 0.003 | ||
| Abdomen | 21 (35.6%) | 16 (47.1%) | 37 (39.8%) | 0.277 | ||
| Pelvic | 24 (40.7%) | 18 (52.9%) | 42 (45.2%) | 0.252 | ||
| Arms | 47 (79.7%) | 33 (97.1%) | 80 (86.0%) | 0.020 | ||
| Legs | 12 (20.3%) | 26 (76.5%) | 38 (40.9%) | 0.004 | ||
| Type of ventilation | Spontaneous breathing | 42 (71.2%) | 4 (11.8%) | 46 (49.5%) | <0.001 | |
| Orotracheal intubation | 16 (27.1%) | 24 (70.6%) | 40 (43.0%) | <0.001 | ||
| Tracheostomy early < 48 h late > 48 h | 1 (1.7%) 1 (1.7%) 0 (0%) | 6 (17.6%) 1 (2.9%) 5 (14.7%) | 7 (7.5%) 2 (2.2%) 5 (5.4%) | 0.016 0.719 0.010 | ||
| Positive RT-PCR for SARS-CoV-2 | 3 (5.1%) | 2 (5.9%) | 5 (5.4%) | 0.870 | ||
| Inhalational injury | yes no | 12 (20.3%) 47 (79.7%) | 26 (76.5%) 8 (23.5%) | 38 (40.9%) 55 (59.1%) | <0.001 0.001 | |
| ABSI score (mean ± SD) | 6.59 ± 1.81 | 11.32 ± 2.28 | NA | <0.001 | ||
| LOS (median, IQR; days) | 23 (16) | 11 (23.75) | NA | 0.008 | ||
| ICU-LOS (median, IQR; days) | 18 (21) | 11 (23.75) | NA | 0.276 | ||
| Comorbidity | Alive Patients’ Group (N = 59; %) | Deceased Patients’ Group (N = 34; %) | p-Value |
|---|---|---|---|
| Without | 34 (57.6%) | 18 (52.9%) | 0.823 |
| Cardiac | 7 (11.9%) | 3 (8.8%) | 0.906 |
| Metabolic | 7 (11.9%) | 2 (5.9%) | 0.563 |
| Respiratory | 2 (3.4%) | 0 (0%) | 0.729 |
| Myasthenia gravis | 0 (0%) | 1 (2.9%) | 0.791 |
| Polyarthritis | 0 (0%) | 1 (2.9%) | 0.791 |
| Neurological | 0 (0%) | 1 (2.9%) | 0.791 |
| Vascular | 1 (1.7%) | 0 (0%) | 0.781 |
| Trauma | 2 (5.9%) | 2 (3.4%) | 0.969 |
| Infection | 1 (1.7%) | 0 (0%) | 0.781 |
| Psychiatric | 1 (1.7%) | 0 (0%) | 0.781 |
| Mixed | 8 (13.6%) | 6 (17.6%) | 0.827 |
| Parameter | Alive Patients’ Group | Deceased Patients’ Group | p-Value | ||
|---|---|---|---|---|---|
| median | IQR | median | IQR | ||
| Admission—albumin (g/dL) | 2.53 | 1.57 | 3.98 | 0.96 | <0.001 |
| Day 1—albumin (g/dL) | 1.84 | 0.87 | 3.20 | 1.20 | <0.001 |
| Admission—creatinkinase (U/L) | 310.50 | 1138.19 | 144.70 | 148 | <0.001 |
| Day 1—creatinkinase (U/L) | 343.59 | 1996.62 | 129 | 102 | <0.001 |
| Admission—creatinine (mg/dL) | 0.96 | 0.55 | 0.81 | 0.29 | 0.092 |
| Day 1—creatinine (mg/dL) | 0.77 | 0.36 | 0.71 | 0.22 | 0.133 |
| Admission—leukocytes (val./uL) | 19,480 | 12,700 | 11,470 | 7910 | <0.001 |
| Day 1—leukocytes (val./uL) | 14,800 | 11,875 | 11,170 | 6970 | 0.032 |
| Admission—hemoglobin (g/dL) | 15.95 | 4.59 | 15.20 | 2.2 | 0.174 |
| Day 1—hemoglobin (g/dL) | 13.77 | 4.88 | 13.60 | 2.9 | 0.895 |
| Admission—thrombocytes (val./uL) | 284,500 | 244,750 | 224,000 | 86,600 | 0.860 |
| Day 1—thrombocytes (val./uL) | 171,450 | 154,650 | 204,000 | 82,000 | 0.137 |
| Admission—potassium (mmol/L) | 4.06 | 1.46 | 4.07 | 0.63 | 0.917 |
| Day 1—potassium (mmol/L) | 4.37 | 1.20 | 4.12 | 0.60 | 0.005 |
| Admission—total proteins (g/dL) | 4.35 | 1.44 | 5.97 | 1.40 | <0.001 |
| Day 1—total proteins (g/dL) | 3.43 | 1.43 | 5.10 | 1.26 | <0.001 |
| Admission—urea (mg/dL) | 40.35 | 38.77 | 32.50 | 14.50 | 0.028 |
| Day 1—urea (mg/dL) | 32.65 | 40.80 | 27.80 | 17.60 | 0.035 |
| Variables | B | Sig. | Exp(B) |
|---|---|---|---|
| Admission—creatinkinase (U/L) | 0.004 | 0.019 | 1.004 |
| Admission—leukocytes (val./uL) | 0.000127 | 0.017 | 1.000 |
| Admission—total proteins (g/dL) | −1.234 | 0.000 | 0.291 |
| Constant | 2.804 | 0.153 | 16.518 |
| Variables | Score | df | Sig. |
|---|---|---|---|
| Admission—albumin (U/L) | 2.277 | 1 | 0.131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculae, A.; Peride, I.; Tiglis, M.; Nechita, A.M.; Petcu, L.C.; Neagu, T.P. Emergency Care for Burn Patients—A Single-Center Report. J. Pers. Med. 2023, 13, 238. https://doi.org/10.3390/jpm13020238
Niculae A, Peride I, Tiglis M, Nechita AM, Petcu LC, Neagu TP. Emergency Care for Burn Patients—A Single-Center Report. Journal of Personalized Medicine. 2023; 13(2):238. https://doi.org/10.3390/jpm13020238
Chicago/Turabian StyleNiculae, Andrei, Ileana Peride, Mirela Tiglis, Ana Maria Nechita, Lucian Cristian Petcu, and Tiberiu Paul Neagu. 2023. "Emergency Care for Burn Patients—A Single-Center Report" Journal of Personalized Medicine 13, no. 2: 238. https://doi.org/10.3390/jpm13020238
APA StyleNiculae, A., Peride, I., Tiglis, M., Nechita, A. M., Petcu, L. C., & Neagu, T. P. (2023). Emergency Care for Burn Patients—A Single-Center Report. Journal of Personalized Medicine, 13(2), 238. https://doi.org/10.3390/jpm13020238






