Paths of Evolution of Progressive Anaplastic Meningiomas: A Clinical and Molecular Pathology Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Enrollment and Collection of Clinical Data
2.2. Molecular Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Follow-Up
3.3. Clinical Prognosticators
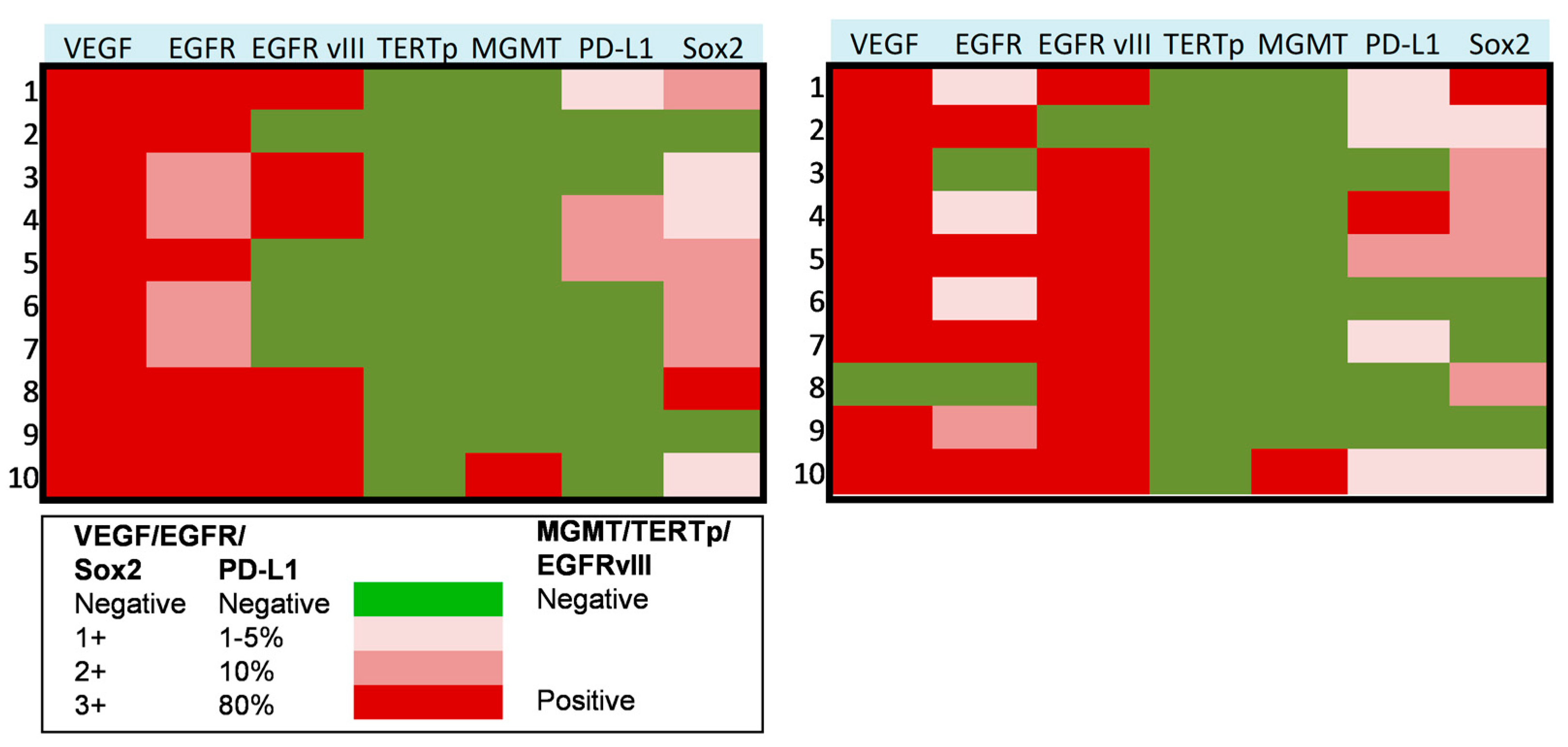
3.4. Molecular Profiling of Progressive Anaplastic Meningiomas
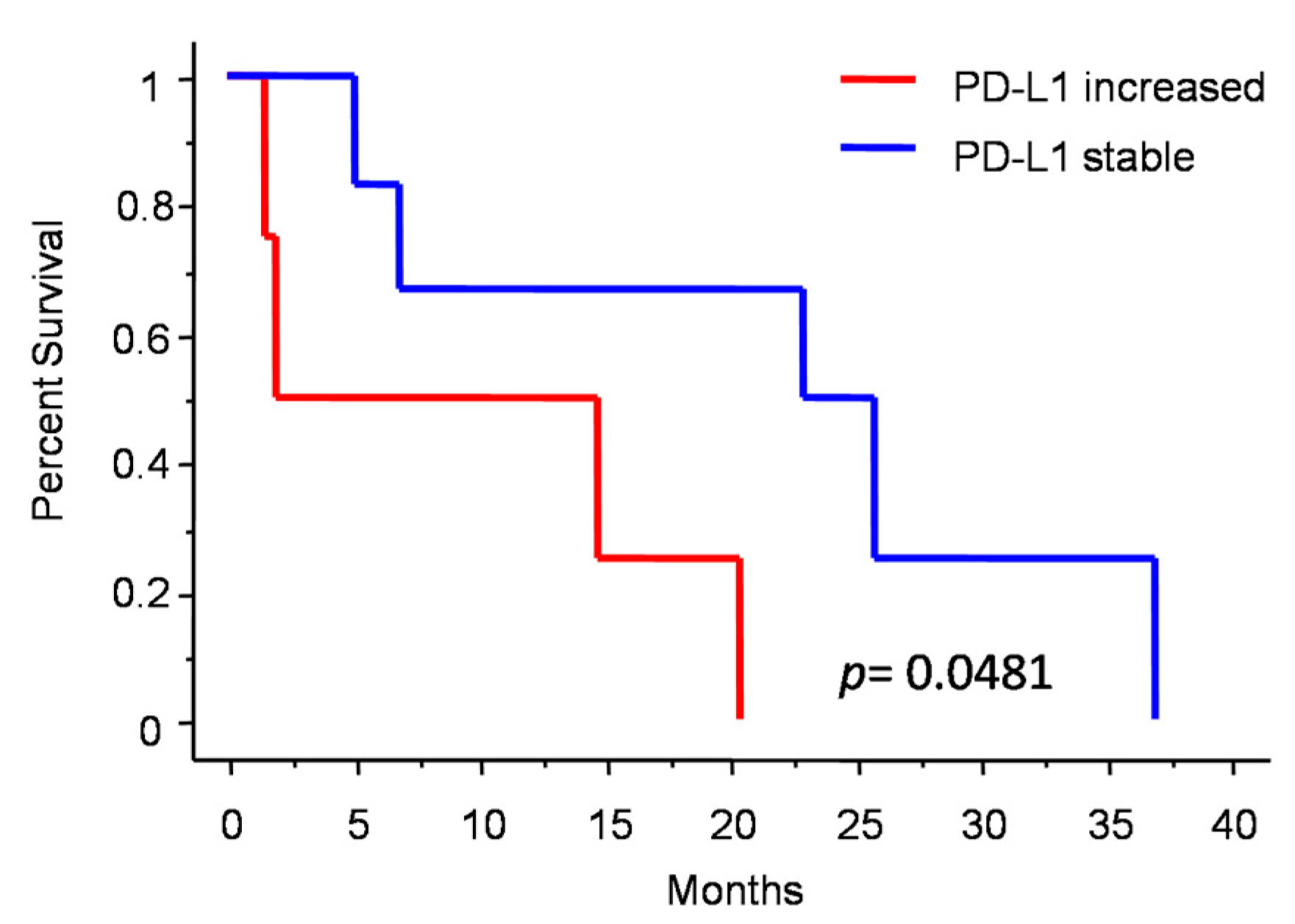
3.5. Molecular Subgrouping and Prognostic Correlation of Progressive Anaplastic Meningiomas
4. Discussion
4.1. Clinical Data
4.2. Molecular Data
4.2.1. Sox2 and EGFRvIII
4.2.2. PD-L1
4.2.3. Other Molecular Markers
4.3. Strengths and Limitations of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Hu, M.; Zhao, M.; Ren, X.; Jiang, Z. Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg. Rev. 2014, 38, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Kang, H.; Song, S.W.; Ha, J.; Won, Y.-J.; Park, C.-K.; Yoo, H.; Jung, K.-W. A Nationwide, Population-Based Epidemiology Study of Primary Central Nervous System Tumors in Korea, 2007-2016: A Comparison with United States Data. Cancer Res. Treat. 2021, 53, 355–366. [Google Scholar] [CrossRef]
- Nakane, Y.; Natsume, A.; Wakabayashi, T.; Oi, S.; Ito, M.; Inao, S.; Saito, K.; Yoshida, J. Malignant transformation-related genes in meningiomas: Allelic loss on 1p36 and methylation status of p73 and RASSF1A. J. Neurosurg. 2007, 107, 398–404. [Google Scholar] [CrossRef]
- Baia, G.S.; Stifani, S.; Kimura, E.T.; McDermott, M.W.; Pieper, R.O.; Lal, A. Notch Activation Is Associated with Tetraploidy and Enhanced Chromosomal Instability in Meningiomas. Neoplasia 2008, 10, 604–612. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Di Bonaventura, R.; Martini, M.; Cenci, T.; Caccavella, V.M.; Barresi, V.; Gessi, M.; Albanese, A.; Lauretti, L.; Pallini, R.; D’Alessandris, Q.G.; et al. Dissecting Stemness in Aggressive Intracranial Meningiomas: Prognostic Role of SOX2 Expression. Int. J. Mol. Sci. 2022, 23, 11690. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Nicholson, R.; Gee, J.; Harper, M. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37, 9–15. [Google Scholar] [CrossRef]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, R.; Cuomo, M.; Buonaiuto, M.; Costabile, D.; Franca, R.A.; Caro, M.D.B.D.; Catapano, G.; Chiariotti, L.; Visconti, R. MGMT and Whole-Genome DNA Methylation Impacts on Diagnosis, Prognosis and Therapy of Glioblastoma Multiforme. Int. J. Mol. Sci. 2022, 23, 7148. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2019, 67, 122–153. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT—Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandris, Q.G.; Biffoni, M.; Martini, M.; Runci, D.; Buccarelli, M.; Cenci, T.; Signore, M.; Stancato, L.; Olivi, A.; De Maria, R.; et al. The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response. Neuro-Oncology 2017, 19, 1097–1108. [Google Scholar] [CrossRef]
- Lauretti, L.; Cenci, T.; Montano, N.; Offi, M.; Giordano, M.; Caccavella, V.M.; Mangraviti, A.; Agostini, L.; Olivi, A.; Gabriele, L.; et al. Molecular Analysis in a Glioblastoma Cohort—Results of a Prospective Analysis. J. Pers. Med. 2022, 12, 685. [Google Scholar] [CrossRef]
- Montano, N.; Cenci, T.; Martini, M.; D’Alessandris, Q.G.; Pelacchi, F.; Ricci-Vitiani, L.; Maira, G.; De Maria, R.; Larocca, L.M.; Pallini, R. Expression of EGFRvIII in Glioblastoma: Prognostic Significance Revisited. Neoplasia 2011, 13, 1113-IN6. [Google Scholar] [CrossRef]
- Konecny, G.E.; Santos, L.; Winterhoff, B.; Hatmal, M.M.; Keeney, G.L.; Mariani, A.; Jones, M.; Neuper, C.; Thomas, B.; Muderspach, L.; et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br. J. Cancer 2008, 100, 89–95. [Google Scholar] [CrossRef]
- Lin, G.; Fan, X.; Zhu, W.; Huang, C.; Zhuang, W.; Xu, H.; Lin, X.; Hu, D.; Huang, Y.; Jiang, K.; et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget 2017, 8, 83986–83994. [Google Scholar] [CrossRef] [PubMed]
- Sughrue, M.E.; Sanai, N.; Shangari, G.; Parsa, A.T.; Berger, M.S.; McDermott, M.W. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J. Neurosurg. 2010, 113, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.K.; Sharma, M.; Silva, D.; Karivedu, V.; Schmitt, P.; Stevens, G.H.; Barnett, G.H.; Prayson, R.A.; Elson, P.; Suh, J.H.; et al. Longitudinal experience with WHO Grade III (anaplastic) meningiomas at a single institution. J. Neuro-Oncol. 2016, 131, 555–563. [Google Scholar] [CrossRef]
- Peyre, M.; Gauchotte, G.; Giry, M.; Froehlich, S.; Pallud, J.; Graillon, T.; Bielle, F.; Cazals-Hatem, D.; Varlet, P.; Figarella-Branger, D.; et al. De novo and secondary anaplastic meningiomas: A study of clinical and histomolecular prognostic factors. Neuro-Oncology 2018, 20, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Sá-Marta, E.; Alves, J.L.; Rebelo, O.; Barbosa, M. World Health Organization Grade III Meningiomas: A Retrospective Study at an Academic Medical Center. World Neurosurg. 2021, 149, e877–e893. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.O.; Song, S.W.; Kim, Y.-H.; Hong, C.-K.; Kim, J.H. Anaplastic Meningioma: Clinical Characteristics, Prognostic Factors and Survival Outcome. Brain Tumor Res. Treat. 2022, 10, 244–254. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, R.; Shukla, N.; Narwal, P.; Katiyar, A.; Mahajan, S.; Sahu, S.; Garg, A.; Sharma, M.C.; Suri, A.; et al. DNA methylation profiling of meningiomas highlights clinically distinct molecular subgroups. J. Neuro-Oncol. 2022, 1–18. [Google Scholar] [CrossRef]
- Pellerino, A.; Bruno, F.; Palmiero, R.; Pronello, E.; Bertero, L.; Soffietti, R.; Rudà, R. Clinical Significance of Molecular Alterations and Systemic Therapy for Meningiomas: Where Do We Stand? Cancers 2022, 14, 2256. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J. Clin. Oncol. 2021, 39, 3839–3852. [Google Scholar] [CrossRef]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.-G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef]
- Maier, A.D.; Stenman, A.; Svahn, F.; Mirian, C.; Bartek, J., Jr.; Juhler, M.; Zedenius, J.; Broholm, H.; Mathiesen, T. TERT promoter mutations in primary and secondary WHO grade III meningioma. Brain Pathol. 2020, 31, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Alshareef, M.; Henderson, F.; Santos, J.L.M.; Vandergrift, W.A.; Lindhorst, S.M.; Varma, A.K.; Infinger, L.; Patel, S.J.; Cachia, D. Ganoderic acid A/DM-induced NDRG2 over-expression suppresses high-grade meningioma growth. Clin. Transl. Oncol. 2019, 22, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Goutagny, S.; Nault, J.C.; Mallet, M.; Henin, D.; Rossi, J.Z.; Kalamarides, M. High Incidence of ActivatingTERTPromoter Mutations in Meningiomas Undergoing Malignant Progression. Brain Pathol. 2013, 24, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D. Transforming Growth Factor Beta Family in the Pathogenesis of Meningiomas. World Neurosurg. 2017, 104, 113–119. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Viaene, A.N.; Zhang, B.; Martinez-Lage, M.; Xiang, C.; Tosi, U.; Thawani, J.P.; Gungor, B.; Zhu, Y.; Roccograndi, L.; Zhang, L.; et al. Transcriptome signatures associated with meningioma progression. Acta Neuropathol. Commun. 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.; Rathi, V.; Chauhan, K.; Jain, K.; Chhabra, S.S.; Acharya, R.; Kalra, S.K.; Gupta, A.; Jain, S.; Ganguly, N.K.; et al. Exploring the role of epidermal growth factor receptor variant III in meningeal tumors. PLoS ONE 2021, 16, e0255133. [Google Scholar] [CrossRef] [PubMed]
- Pallini, R.; Casalbore, P.; Mercanti, D.; Maggiano, N.; Larocca, L.M. Phenotypic Change of Human Cultured Meningioma Cells. J. Neuro-Oncol. 2000, 49, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Wu, W.W.; Santagata, S.; Reardon, D.A.; Dunn, I.F. Checkpoint inhibition in meningiomas. Immunotherapy 2016, 8, 721–731. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Wang, N.; Eichler, A.F.; Chukwueke, U.; Forst, D.A.; Arrillaga-Romany, I.C.; Dietrich, J.; et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat. Commun. 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Perry, A.; Lusis, E.A.; Gutmann, D.H. Meningothelial Hyperplasia: A Detailed Clinicopathologic, Immunohistochemical and Genetic Study of 11 Cases. Brain Pathol. 2006, 15, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A.; Alvarez-Argote, J.; Limonadi, F.M.; Codreanu, I. Bevacizumab in refractory higher-grade and atypical meningioma: The current state of affairs. Expert Opin. Biol. Ther. 2018, 19, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Temozolomide for treatment-resistant recurrent meningioma. Neurology 2004, 62, 1210–1212. [Google Scholar] [CrossRef]
- de Robles, P.; McIntyre, J.; Kalra, S.; Roldán, G.; Cairncross, G.; Forsyth, P.; Magliocco, T.; Hamilton, M.; Easaw, J. Methylation status of MGMT gene promoter in meningiomas. Cancer Genet. Cytogenet. 2008, 187, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Falchetti, M.L.; Pallini, R.; Larocca, L.M.; Verna, R.; D’Ambrosio, E. Telomerase expression in intracranial tumours: Prognostic potential for malignant gliomas and meningiomas. J. Clin. Pathol. 1999, 52, 234–236. [Google Scholar] [CrossRef] [PubMed]



| Parameter | De Novo | Progressive | p |
|---|---|---|---|
| N | 17 (41.5%) | 24 (58.5%) | NA |
| Male sex | 41.2% | 62.5% | 0.2159 * |
| Age at diagnosis (years) | 68.8 (19.2–84.6) | 63.7 (50.3–83.4) | 0.5027 # |
| Tumor location | |||
| Non-skull base | 88.2% | 54.2% | 0.0623 ** |
| Skull base | 5.9% | 33.3% | |
| Intraventricular | 5.9% | 12.5% | |
| Preop mRS ≤ 3 | 88.2% | 79.2% | 0.6786 * |
| Postop mRS ≤ 3 | 81.8% | 58.3% | 0.3707 * |
| Parameter | De Novo | Progressive | p * |
|---|---|---|---|
| Single surgery | 64.7% | 62.5% | >0.99 |
| GTR | 100% | 64.7% | 0.0237 |
| Surgical complication | 21.4% | 47.1% | 0.2580 |
| Adjuvant radiotherapy | 90.9% | 84.6% | >0.99 |
| Chemotherapy | 0 | 20.8% | 0.0650 |
| Extracranial metastasis | 0 | 8.3% | 0.5024 |
| Parameter | Hazard Ratio | Confidence Interval | p |
|---|---|---|---|
| De novo vs. progressive | 0.149 | 0.020–1.123 | 0.0647 |
| mRS ≤3 vs. >3 | 0.484 | 0.091–2.584 | 0.3961 |
| Age ≤70 vs. >70 | 6.479 | 0.815–51.488 | 0.0772 |
| Bilateral vs. monolateral | 3.154 | 0.313–31.797 | 0.3299 |
| GTR vs. STR | 4.591 | 0.3–70.246 | 0.2735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bonaventura, R.; Lauretti, L.; Martini, M.; Cenci, T.; Di Monaco, G.; Palombi, D.; Ceccarelli, G.M.; Chiesa, S.; Gessi, M.; Granitto, A.; et al. Paths of Evolution of Progressive Anaplastic Meningiomas: A Clinical and Molecular Pathology Study. J. Pers. Med. 2023, 13, 206. https://doi.org/10.3390/jpm13020206
Di Bonaventura R, Lauretti L, Martini M, Cenci T, Di Monaco G, Palombi D, Ceccarelli GM, Chiesa S, Gessi M, Granitto A, et al. Paths of Evolution of Progressive Anaplastic Meningiomas: A Clinical and Molecular Pathology Study. Journal of Personalized Medicine. 2023; 13(2):206. https://doi.org/10.3390/jpm13020206
Chicago/Turabian StyleDi Bonaventura, Rina, Liverana Lauretti, Maurizio Martini, Tonia Cenci, Giuliano Di Monaco, Davide Palombi, Giovanni Maria Ceccarelli, Silvia Chiesa, Marco Gessi, Alessia Granitto, and et al. 2023. "Paths of Evolution of Progressive Anaplastic Meningiomas: A Clinical and Molecular Pathology Study" Journal of Personalized Medicine 13, no. 2: 206. https://doi.org/10.3390/jpm13020206
APA StyleDi Bonaventura, R., Lauretti, L., Martini, M., Cenci, T., Di Monaco, G., Palombi, D., Ceccarelli, G. M., Chiesa, S., Gessi, M., Granitto, A., Albanese, A., Larocca, L. M., D’Alessandris, Q. G., Pallini, R., & Olivi, A. (2023). Paths of Evolution of Progressive Anaplastic Meningiomas: A Clinical and Molecular Pathology Study. Journal of Personalized Medicine, 13(2), 206. https://doi.org/10.3390/jpm13020206









