Abstract
Unambiguous evidence indicates that microbes are closely linked to various human diseases, including cancer. Most prior work investigating the microbiome of breast tissue describes an association between compositional differences of microbial species in benign and malignant tissues, but few studies have examined the relative abundance of microbial communities within human breast tissue at the species level. In this work, a total of 44 breast tissue samples including benign and malignant tissues with adjacent normal breast tissue pairs were collected, and Oxford Nanopore long-read sequencing was employed to assess breast tissue microbial signatures. Nearly 900 bacterial species were detected from the four dominant phyla: Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes. The bacteria with the highest abundance in all breast tissues was Ralstonia pickettii, and its relative abundance increased with decreasing malignancy. We further examined the breast-tissue microbiome composition with different hormone-receptor statuses, and the relative abundance of the genus Pseudomonas increased most significantly in breast tissues. Our study provides a rationale for exploring microbiomes associated with breast carcinogenesis and cancer development. Further large-cohort investigation of the breast microbiome is necessary to characterize a microbial risk signature and develop potential microbial-based prevention therapies.
1. Introduction
Breast cancer has become the most prevalent cancer worldwide and the first leading cause of cancer death in women [1]. The occurrence of breast cancer can be attributed to a variety of related risk factors, such as age, family history, alcohol consumption, and obesity [2], but the exact etiology of breast cancer remains undetermined, and the probability of cancer development, treatment effect, and survival still cannot be accurately predicted [3]. There are additional factors associated with the development of breast cancer waiting to be explored, one of which is the human microbiome. Previous studies have revealed that microbial pathogens are etiological factors in approximately 15–20% of cancers worldwide [4], including gastric [5] and colorectal cancer [6]. Furthermore, multiple tumor types were found to have distinct microbial compositions, although the contribution of these microbes to tumorigenesis and cancer progression is only beginning to be explored and merits further mechanistic investigation [7]. It is currently believed that the ways in which microbes contribute to carcinogenesis fall into several categories: directly damaging host cell DNA, altering cell signaling pathways, modulating immune cells and inflammation, and influencing the metabolism of host-produced factors and pharmaceuticals [8].
In recent years, a greater appreciation of the microbes inhabiting female mammary gland sites has emerged. Due to its high percentage of adipose composition with lymphatic drainage and extensive vasculature, the breast may provide a favorable environment for colonization and growth of bacteria [9]. Accumulating studies have found that the human breast harbors a unique and diverse microbiota [10,11], which not only differs between pathologically-defined normal and malignant breast tissues, but also with clear distinctions among race, tumor subtype, and stage of breast cancer [12,13,14,15]. Recent studies have demonstrated correlations between specific microbes and the clinical outcomes in breast cancer [16], and suggested that tumor-resident microbiota play an important role in promoting breast cancer metastasis [17]. Although progress has been made to support the existence and potential function of the breast tissue microbiome, further in-depth exploration is required to identify the diversity and complexity of breast microbiota composition and determine whether the shifts in microbial assemblages link to breast carcinogenesis [18,19]. In addition, certain microbial communities derived from the mouth and gut have been detected in breast tissue, such as Fusobacterium nucleatum [20] and Bacteroides fragilis [21], which have been shown to promote breast cancer tumor growth and metastasis. Clearly, accurate detection and identification of specific microbial species in the breast are of great importance.
Second-generation sequencing technologies, such as Illumina [22,23] and Ion Torrent [24], have been widely applied in the collection of 16S rRNA sequence data. These platforms provide a wealth of short-read lengths (200–400 bp) with high accuracy (~99%), but also lead to limited species resolution, rarely below genus level [25]. Oxford Nanopore Technologies (Oxford, UK; ONT) has developed a third-generation platform for long-read and direct sequencing of individual strands of DNA [26,27], and the ability to provide a full-length sequence of 16S rRNA genes leads to more accurate identification of microorganisms at the species level [28,29]. Within the last five years, the sequence accuracy of ONT nanopore sequencing has been improved from 60% to >90%, and researchers have begun to use it to characterize the composition of the microbial community in clinical samples, such as human respiratory samples [30], fecal samples [31], and colorectal cancer tumor tissues [32].
In this study, we attempted to investigate the microbiome in human benign and malignant breast tissue (including adjacent normal tissue) using the nanopore sequencing platform, and to determine the bacterial community structures in breast tissue with different hormone-receptor expressions. We sought to address the hypothesis that microbial populations living within the breast display a different relative abundance depending on the benign or malignant state, and that hormone-receptor expression is strongly associated with microbiome profiles. As far as we know, our study is the first to reveal the composition of breast tissue microbes at the species level using near full-length 16S rRNA operon reads. A deeper understanding of the breast microbiome as impacted by disease state and tumor subtype may contribute to facilitating earlier clinical intervention leading to improved breast cancer patient outcomes.
2. Materials and Methods
2.1. Patients Enrollment and Breast Tissue Collection
The study was approved by the institutional ethics committee of Renmin Hospital of Wuhan University (WDRY2020-K194). The research was conducted in accordance with the Declaration of Helsinki, and followed all relevant guidelines and regulations. Fresh-frozen breast tissue was aseptically collected from 26 women undergoing non-mastectomy breast surgery for cancer or benign disease at Renmin Hospital of Wuhan University in Wuhan, Hubei Province, China. Patients included were identified with no history of antibiotic use within 6 months and no other disease or condition that might have affected the study assessments. Written informed consent was obtained from each participant prior to obtaining breast tissue. Breast cancer (Tumor) tissue and adjacent normal breast tissue pairs (Normal Pair) came from the same donor. “Adjacent normal tissue” is defined as non-malignant breast tissue located up to 5 cm away from the edge of the tumor, and was evaluated and confirmed by a pathologist to be histologically free of any tumor cells or lesions. After resection, samples were immediately placed in sterile, study code-labeled tubes and were frozen in liquid nitrogen, then stored in a −80 °C freezer until use.
2.2. Sample Processing and DNA Extraction
All tissue samples were sent to the clinical laboratory for DNA extraction. Tissue samples were cut into small pieces using sterile scissors and homogenized in batches according to their unique group and number. DNA was extracted from 200 μL of pre-treated samples using the Sansure DNA Extraction Kit (Changsha, China) following the manufacturer’s instructions. DNA was also extracted from two samples of 200 μL Tris- EDTA buffer, with each batch of clinical samples as a no-template control.
2.3. Amplification and Nanopore Targeted Sequencing
The universal primers 27F/1492R (for bacterial 16S rRNA gene) [33] were optimized and used in nanopore sequencing; the details of primer design and PCR procedure are described elsewhere [34]. Then, the amplification products of 16S rRNA from the clinical sample and negative controls were pooled for sequencing library construction, using the 1D Ligation Kit (SQK-LSK109, Oxford Nanopore Technologies). Sequencing was performed using an R9.4 flow cell on the GridION x5 (Oxford Nanopore Technologies). Sequencing data was generated continuously after sequencing began. In the default script of the sequencer, a fastq file is generated for every 4000 reads, and real-time data analysis is started every time a fastq file is generated. The whole sequencing process lasted 8 h, and the sequencing data were analyzed using a real-time bioinformatics analysis pipeline.
2.4. Bioinformatics Analysis Pipeline and Bacterial Detection
Basecalling and quality control of raw data sequencing were performed in a high accuracy mode, using Guppy (v. 3.1.5). Reads with undesired lengths (<200 or >2000 nt) were discarded. The clean reads were then mapped to the 16S rRNA reference gene databases collected from the NCBI database. The E-value of BLAST was set to 1e−5, and the taxonomy of each read was assigned according to the taxonomic information of the mapped subject reference with the highest identity and coverage. A consensus sequence of the reads assigned to the same species was generated by Medaka (v. 0.10.1), and the consensus was remapped to the reference database. The species-level taxonomy of the mapped subject reference was detected as the final result. The bacterial detection of the clinical sample was interpreted according to a strict set of rules described elsewhere [34].
2.5. Statistical Analysis
R software (version 3.5.1) was used to analyze the community composition and richness of species. The histogram and heat map based on the relative abundance of species at each classification level were drawn using the ggplot2 and pheatmap packages in R [35,36]. Alpha diversity was described when analyzing the complexity of species diversity using the Chao1 [37] and Shannon indices [38]. If there were only two groups, Student’s t-test was used, but if there were more than two groups, either the Kruskal–Wallis-test or one-way ANOVA was used. Principal Co-ordinates Analysis (PCoA) based on Bray–Curtis distance was performed to obtain principal coordinates and visualize complex and multidimensional data using the vegan package in R [39]. A linear discriminant analysis (LDA) effect size (LEfSe) analysis was used to find the biomarker of each group [40]. A non-parametric factorial Kruskal–Wallis-test was used to detect species with significant differences in abundance among different groups, and a Wilcoxon-rank-sum-test was used to judge the difference between the two groups. Finally, LDA analysis was used to evaluate the impact of significant species (LDA score) by setting LDA score ≥2 and obtaining the biomarkers in different groups.
3. Results
3.1. Patient and Breast Tissue Characteristics
We analyzed a total of 44 breast tissue samples, including benign tissues (n = 8) from women with benign tumors of the breast, and breast cancer tissues (n = 18), as well as adjacent normal breast tissue pairs (n = 18) from women with stage I-IV breast cancer, which were all evaluated and confirmed by pathologists from Renmin Hospital of Wuhan University. The patient demographic and tissue characteristics of the breast tissue samples are shown in Table 1. The average age of breast cancer patients was significantly higher than those in the benign group (p < 0.0001, Student’s t-test), and breast cancer tissues were classified by two major breast tumor subtypes based on hormone-receptor expression: ER/PR positive (n = 9) and ER/PR negative (n = 8). Tumor hormone-receptor status was missing in one breast cancer tissue specimen. Clinical-pathologic characteristics of the patients are listed in Supplementary Table S1.

Table 1.
Demographics of study patients.
3.2. Breast Tissue Microbiome Composition in Breast Tumor/Adjacent Normal/Benign Tissues
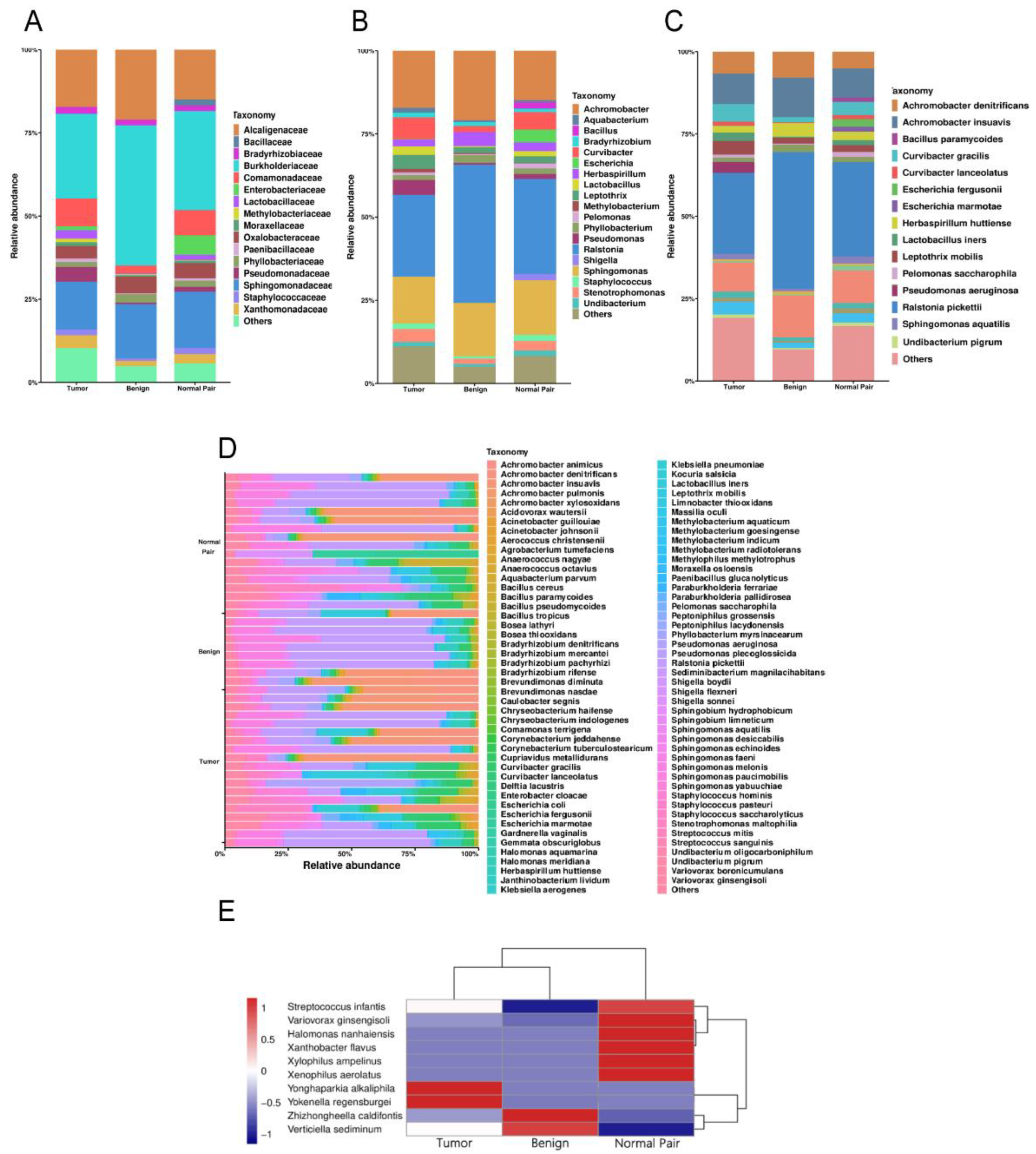
A total of 1,443,997 raw operon reads were obtained from all samples (see Supplementary Table S2 in the Supplemental Material). A total of 1,441,486 clean reads were then generated after processing the raw data (quality control and size selection for 200–2000 nt lengths). Taxonomic classification was carried out on the clean reads that passed processing filters, and the low abundance data of single mapped reads were discarded when considering assigned taxonomic units in order to reduce spurious taxonomic units [41]. Nearly 900 bacterial species were detected overall for all the breast tissue samples (see Supplementary Table S3 in the Supplemental Material). Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes accounted for the most abundant bacterial population at the phylum level (see Supplementary Figure S1 in the Supplemental Material), which was consistent with the results of previous studies [15,42]. At the family level, Burkholderiaceae accounted for the major bulk of bacteria followed by Sphingomonadaceae and Alcaligenaceae across all the breast tissue samples, in that order (Figure 1A). At the genus level, the genus Ralstonia was the most dominant bacterial genus prevalent in all tissues (Figure 1B). The genera Sphingomonas and Achromobacter were also abundant in all the breast tissue collected in this study (Figure 1B). We then focused on the distribution of bacteria at the species level. The bacterial composition of each group was similar, with major differences mainly in rare and/or less abundant lineages (Figure 1C). Interestingly, despite being derived from the same donor, the bacterial composition of breast cancer tissue differed from that of adjacent normal tissue (Figure 1C). The bacterium with the highest abundance in all breast tissues was Ralstonia pickettii, with the relative abundance increasing with decreasing malignancy (24.58% in tumor vs. 28.60% in adjacent normal vs. 41.51% in benign) (Figure 1C). On the contrary, Leptothrix mobilis was more abundant in breast tumors and adjacent tissues (4.17% in tumor vs. 2.01% in adjacent normal vs. 1.77% in benign) (Figure 1C). Other fluctuating microorganisms also merit further attention, and changes in their relative abundance may have a potential impact on breast tissue, which we have included in Supplemental Figure S2 (first 20 species). In addition, we found changes in the relative abundance of some previously reported pathogens under different classifications, such as Pseudomonas aeruginosa, which was highly enriched in cancer tissues and was hardly detected in normal and benign tissues (Figure 1C). In Figure 1D, the bar plots illustrate the relative abundance of microbial communities at the species level of all samples in the study, and show the noticeable individual differences in relative bacterial abundance between different groups. A cluster analysis of species abundance in each group was carried out using a heatmap (Figure 1E). Zhizhongheella caldifontis and Verticiella sediminum were specifically enriched in benign tissues, while enrichment of other bacteria species was found in tumor and adjacent normal tissues, such as Yonghaparkia alkaliphila and Variovorax ginsengisoli (Figure 1E).
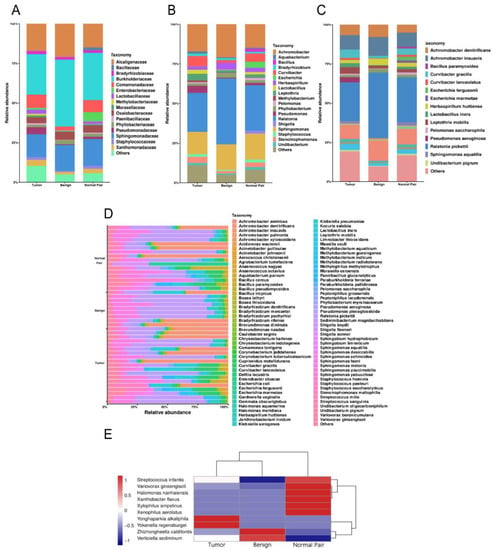
Figure 1.
(A) Bar plots of the taxonomic profiles of the benign (n = 8), normal pair (n = 18), and breast tumor tissue (n = 18) microbiota at the family level, (B) genus level, and (C) species level with a relative abundance >1% are shown. (D) Relative abundance of bacterial species within breast tissue samples across all tissues. Each bar represents a subject and each colored box a bacterial taxon, the height of each colored box represents the relative abundance of that organism within the sample. (E) A heatmap illustrates the cluster analysis of species abundance in each group. A blue color represents rare or absent species while a red color represents abundant species.
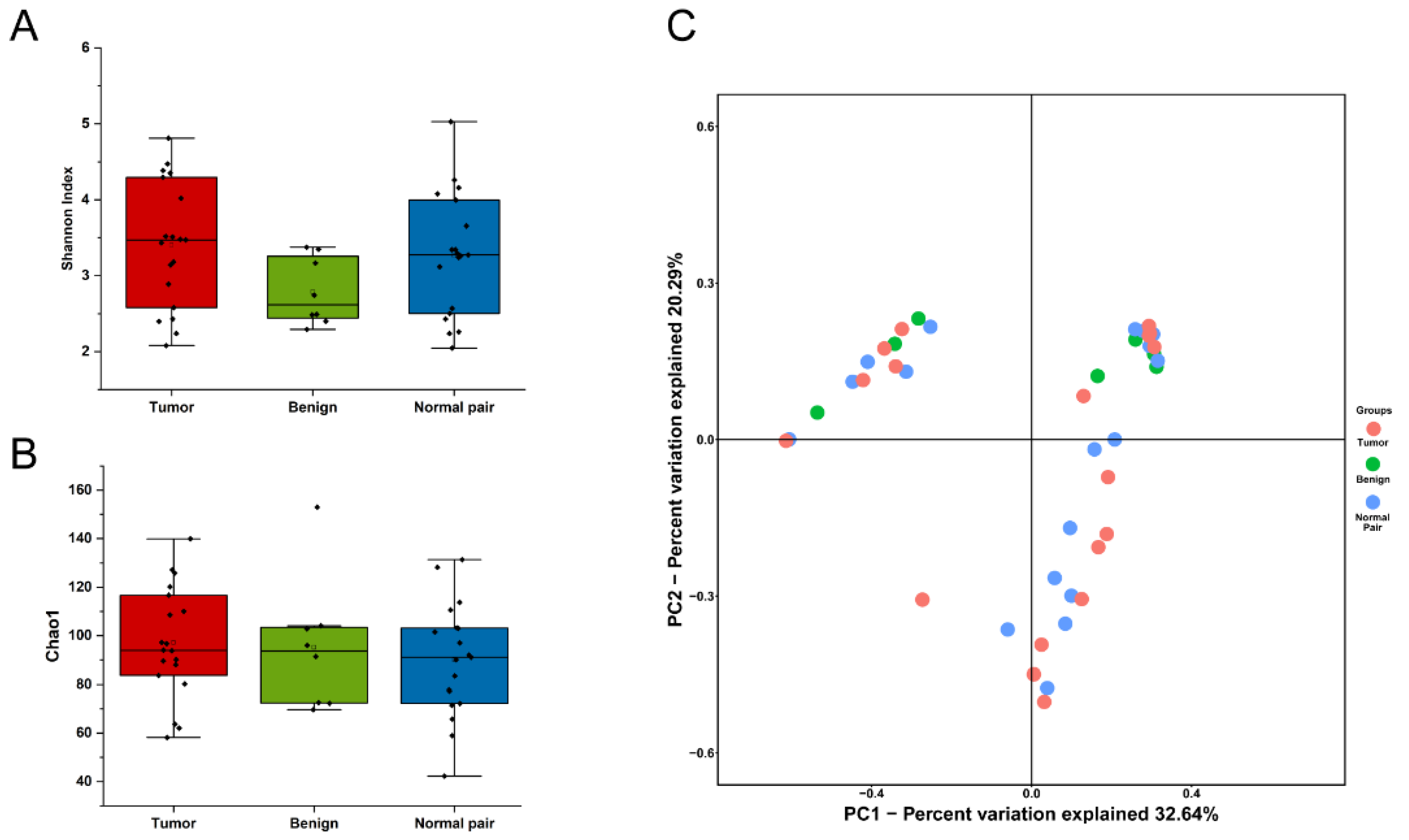
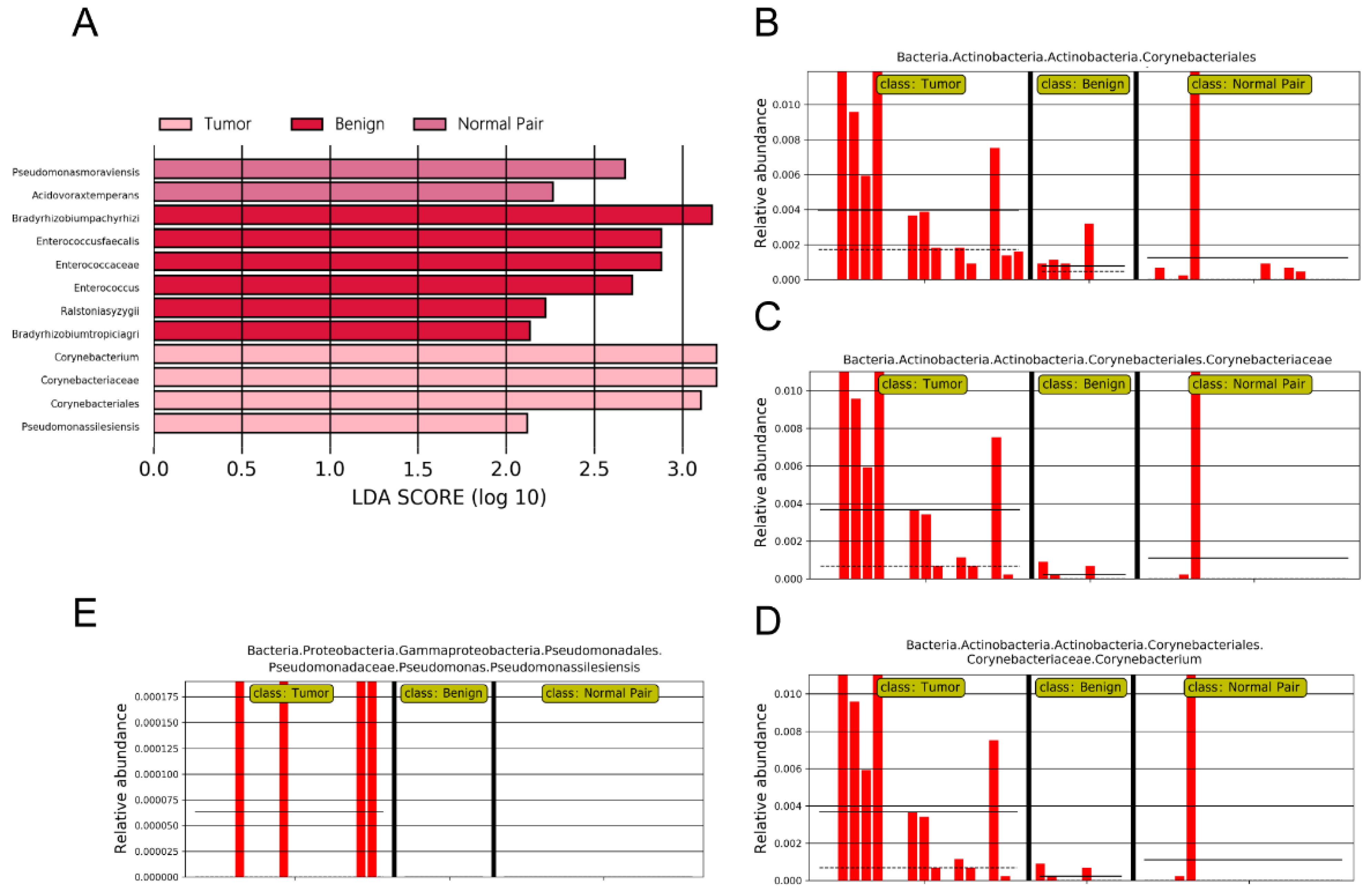
To evaluate microbiome differences between different tissue types, alpha diversity analysis was employed and the results showed that there was no significant difference in Shannon and Chao1 indices between tumor, adjacent normal, and benign tissues (Figure 2A,B). The differences in the composition of microbial communities were visualized using principal coordinate analysis (PCoA) ordination of Bray–Curtis distances. It did not reveal any significant differences in beta diversity, although differences in the microbiome between benign and malignant disease states did exist (Figure 2C). Next, we conducted linear discriminant analysis effect size (LEfSe) analysis to evaluate the microbiome differences between tumor, adjacent normal, and benign tissues, which uncovered different microbiome compositions and identified significant cancer-related biomarkers [14]. The histogram showed that Sphingobium limneticum, Ralstonia solanacearum, and Mesorhizobium huakuii were most abundant in benign samples (Linear Discriminant Analysis (LDA > 2), while Staphylococcus pasteuri, Staphylococcus warneri, and Acidovorax temperans were abundant in normal pairs (LDA > 2) (Figure 3A). Notably, order Corynebacteriales including family Corynebacteriaceae and genus Corynebacterium was enriched in tumor tissues (LDA > 3); the genus Janthinobacterium including Janthinobacterium lividum was also abundant in tumor tissues (LDA > 3) (Figure 3A). The biomarkers which had relative abundance differences between tumor and other different types of tissue arere presented in Figure 3B-E. Further investigation of these bacteria may be helpful to reveal their influence on breast cancer.
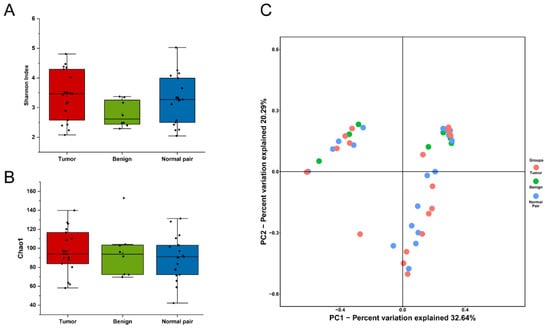
Figure 2.
(A) Boxplot comparing Shannon index and (B) Chao1 between benign, normal pair, and breast tumor tissues (Shannon index, p = 0.171; Chao1, p = 0.645, one-way ANOVA). (C) PCoA plots show the clustering pattern of the three groups based on Bray–Curtis distance (p = 0.699, Adonis).
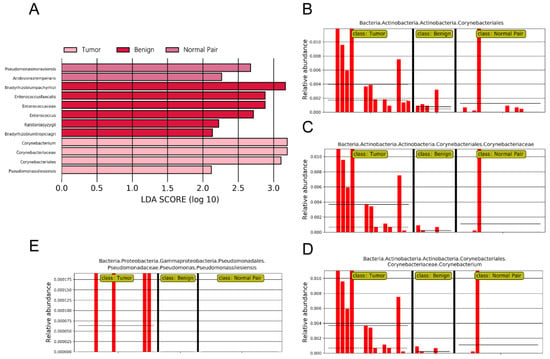
Figure 3.
(A) LDA scores predict microbiota associated with benign, normal pair, and breast tumor tissue microbiomes. (B–E) Bar plots show the potential biomarkers which had relative abundance differences between tumor and other different types of tissues. Each bar represents a sample.
3.3. Breast Tissue Microbiome Composition with Different Hormone-Receptor Statuses
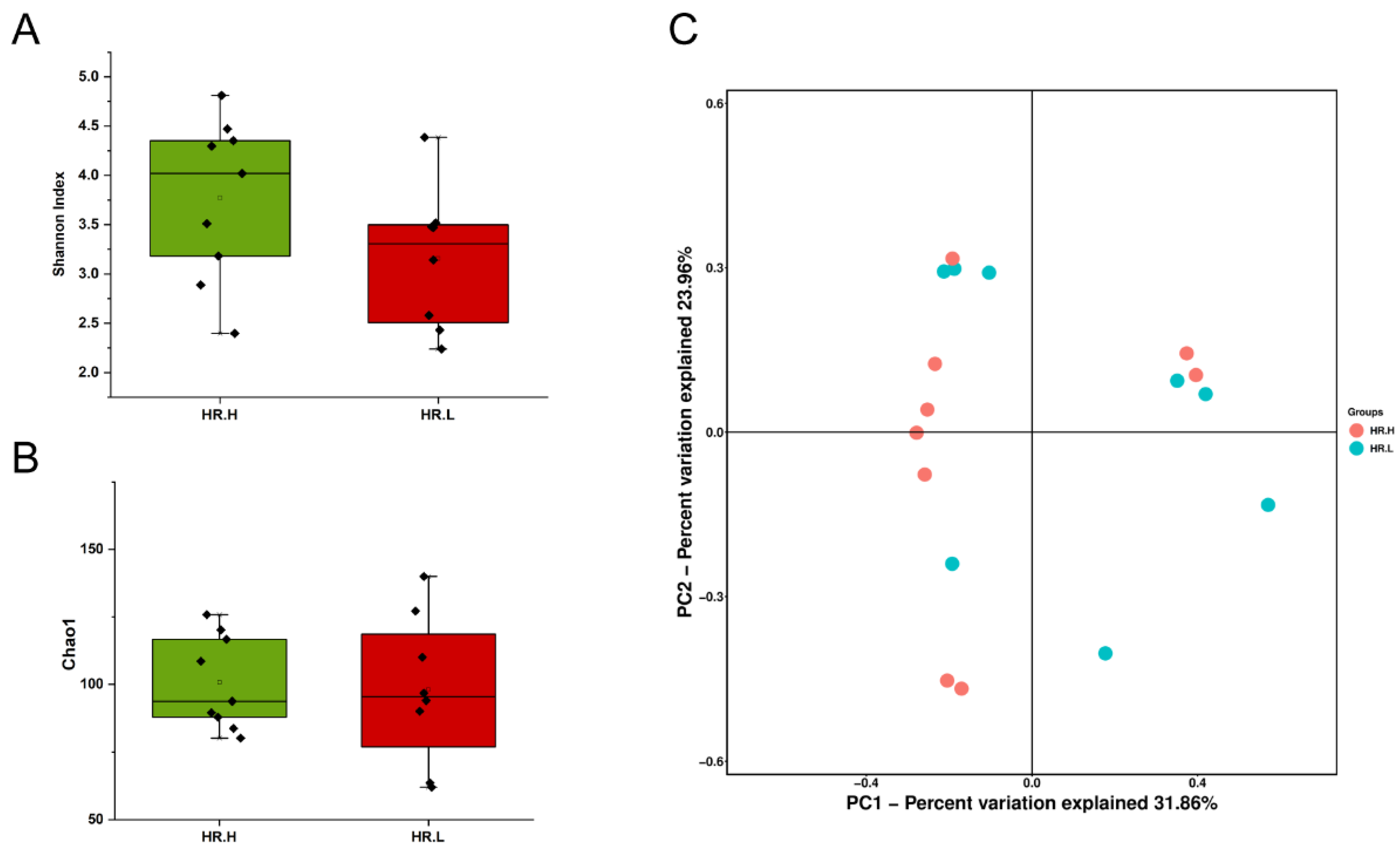
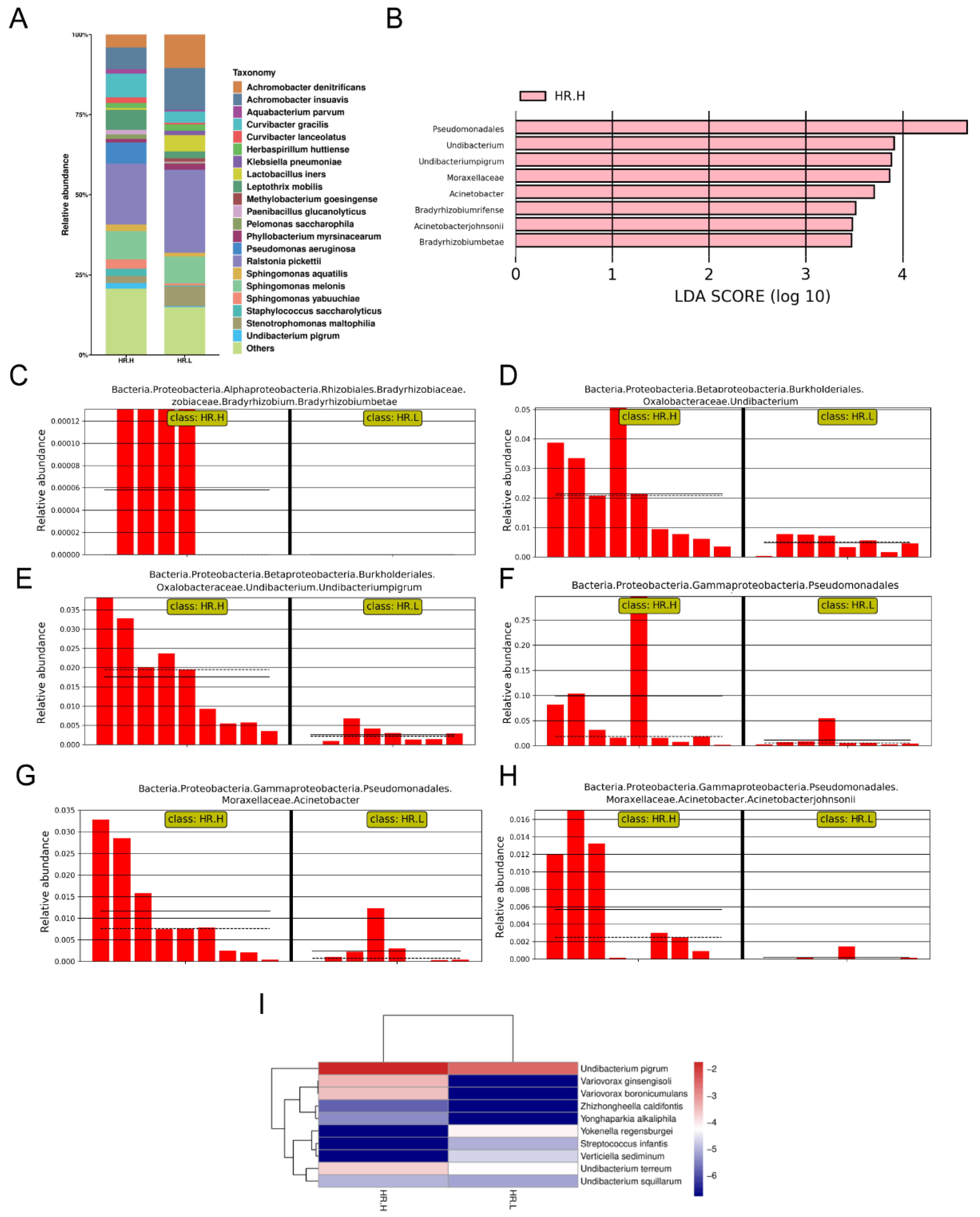
Recent studies have revealed that microbiota in the breast may originate from the skin, gut, or mouth through a variety of mechanisms [9,43]. The gut microbiome has long been involved in development and progression of breast cancer through several pathways, including by altering estrogen levels throughout the body and regulating the host’s immune system and tumor immunity [44]. Therefore, we hypothesized that under different hormone-receptor statuses, some microorganisms might be distinguished which have potential effects on breast cancer through a multitude of pathways, similar to gut microbes. In our study, 17 cancer samples were stratified and further examined based on hormone-receptor expression (one patient was excluded due to incomplete data). The results showed that the overall microbiota of breast tissue between the two states appeared similar. Alpha diversity analysis showed no significant differences in either the Shannon index or Chao1 index (Figure 4A,B), and the PCoA analysis used to reflect the beta diversity revealed that the microbial community in hormone- receptor-positive breast cancer was not different from that in hormone-receptor-negative tumors (Figure 4C). Nevertheless, we found multiple bacterial taxa whose prevalence was different between the subtypes. Ralstonia pickettii still dominated in both types of breast cancer, but its relative abundance was reduced in hormone-receptor-positive breast tissues (19.03% in hormone-receptor-positive vs. 25.83% in hormone receptor-negative) (Figure 5A). On the contrary, Curvibacter gracilis was lower in hormone-receptor-negative breast tissue (7.42% in hormone-receptor-positive vs. 3.54% in hormone-receptor-negative) (Figure 5A). Utilizing a LEfSe analysis to assess differential taxa between the two breast cancer states demonstrated a significantly increased relative abundance in the following species in hormone-receptor-positive breast tissues: Order Pseudomonadales (LDA > 4), Bradyrhizobium betae, Acinetobacter johnsonii, Bradyrhizobium rifense, genus Acinetobacter, family Moraxellaceae, Undibacterium pigrum, and genus Undibacterium (LDA > 3) (Figure 5B). The histogram shows differences in the quantity of these species between the two disease states (Figure 5C–H). Finally, the heatmap demonstrates elevated Undibacterium pigrum in hormone-receptor-positive breast tissues (Figure 5I).
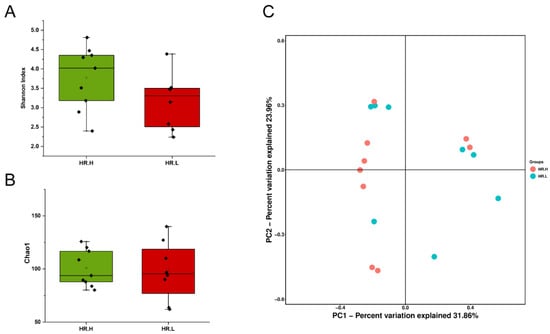
Figure 4.
(A) Boxplot comparing Shannon index and (B) Chao1 between hormone-receptor-positive tumors and hormone-receptor-negative tumors (Shannon index, p = 0.121; Chao1, p = 0.805, Student’s t test). (C) PCoA plots show the clustering pattern of the two groups based on Bray–Curtis distance (p = 0.687, Adonis). HR.H, hormone-receptor-positive tumors; HR.L, hormone-receptor-negative tumors.
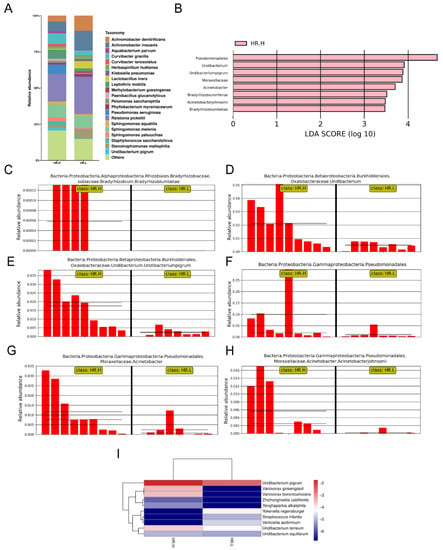
Figure 5.
(A) Bar plots of the taxonomic profiles of hormone-receptor-positive tumors (n = 9) and hormone-receptor-negative tumors (n = 8). Microbiota at species level with a relative abundance >1% are shown. (B) LDA scores computed for the two groups. (C–H) Bar plots show the abundance of the potential biomarkers between the two groups. Each bar represents a sample. (I) A heatmap illustrating the cluster analysis of species abundance in each group. Blue color represents rare or absent species while red color represents abundant species. HR.H, hormone-receptor-positive tumors; HR.L, hormone-receptor-negative tumors.
4. Discussion
Recently, the influence of microorganisms on cancer susceptibility and progression has attracted great attention in the study of cancer, especially in gastrointestinal tumors, but whether changes in microbiota could cause or affect breast cancer remains unclear. In this study, we used near full-length 16S rRNA operon reads to assess the microbial composition of breast tissues and distinguish the bacterial differences in benign, adjacent normal, and tumor tissues accurately to the species level for the first time. Our results indicated distinct microbial communities in tumor and adjacent normal tissues compared to benign tissues, and compared differences in the microbiota between the tumor tissues with different hormone-receptor statuses. Exploration of the core microbial community in breast tissue and microbial dysbiosis in association with benign and malignant diseases will facilitate an understanding of the bacterial impact on breast health and provide advancements in the diagnosis and treatment for early intervention in breast cancer.
Increasing evidence has established the existence of a unique microbiota in human breast tissue over the past decade. Given the nutrient-rich fatty content of the breast and the widespread location of lobules and ducts leading from the nipple to the mammary gland, Urbaniak et al. suggested that bacteria might be widely distributed in the mammary gland, irrespective of lactation [10,43]. A number of studies have since confirmed this speculation by using next-generation sequencing (NGS) and culture methods [12,13,45,46,47]. Nejman et al. reported that breast tumors had a richer and more diverse microbiome than all other tumor types tested after studying 1526 tumors and their adjacent normal tissues across seven cancer types [11]. Hieken et al. explored the microbiome of aseptically collected breast tissue in benign and malignant disease, and they found that the major differences were mainly in rare and/or less abundant lineages since the difference is not significant using the weighted UniFrac distance [48]. Their investigation demonstrated increased relative abundance in the following low-abundant genera in the breast tissue: Fusobacterium, Atopobium, Hydrogenophaga, Gluconacetobacter, and Lactobacillus. Genus Fusobacterium, especially Fusobacterium nucleatum, has already been confirmed to act by secreting virulence factors as well as creating a pro-inflammatory environment that promotes carcinogenesis in colon cancer [49,50], and a recent study has also found its possible impact on breast cancer progression [20]. Meng et al. compared microbiome profiles in the breast tissues collected from Chinese patients with benign and malignant disease and identified characteristic microbial biomarkers [42]. Although alpha diversity analysis revealed that there was no significant difference in Shannon index, and the generated weighted and unweighted UniFrac distance metric also did not show significant differences in beta diversity, the microbial composition of benign and malignant disease states did differ at the phylum and family levels [42], consistent with our findings. They found the enriched microbial biomarkers in tumor tissues included genus Propionicimonas and families Micrococcaceae, Caulobacteraceae, etc. [42] Unfortunately, these investigations using NGS are still in their infancy and lack characteristic microbial biomarkers identified at the species level.
One major finding from our study was the accurate identification of a distinct microbiota associated with benign and malignant disease based on higher species resolution. At the genus level, the bacterium Ralstonia was the most dominant bacterial genus prevalent in the breast. Smith et al. stated that breast tissues from non-Hispanic Black (NHB) women had a higher abundance of genus Ralstonia compared to non-Hispanic White (NHW) tumors [14], but a recent study reported that Ralstonia was the dominant genus with no significant differences in abundance between tumor vs. normal tissues [15]. Possible reasons for the inconsistency of these studies include different eating habits, living environments, and metabolic levels in different races, but there is no doubt that Ralstonia does exist abundantly in the breast tissue, and its possible role in the incidence of breast cancer needs to be further explored by researchers. In our study, we identified that the dominant species is Ralstonia pickettii, of which the relative abundance was higher in benign tissues compared to tumor tissues. It is a non-fermentative, Gram-negative bacterium from the Ralstonia genus, which is emerging as one kind of opportunistic pathogen closely associated with nosocomial infections [51,52]. Multiple nosocomial outbreaks have been caused by medical solutions contaminated with R. pickettii [53,54]. However, the involvement of this bacterium in the carcinogenesis of any organ has not been thoroughly studied. In the study by Higuchi et al. [55], Streptococcus australis and R. pickettii were identified in almost all mesothelioma patients, but it is unclear whether the bacterial profiles with high levels of bacterial composition and abundance are causally associated with oncogenesis. Future studies with larger sample sizes are needed to determine their potential association with tumorigenesis and to explore the underlying mechanisms.
We further examined the breast tissue microbiome composition with different hormone-receptor statuses. Considering that hormone-receptor-positive breast cancer is closely related to hormone secretion, and it has long been proposed that the gut microbiome contributes to breast carcinogenesis by modifying systemic estrogen levels [56,57], we therefore saw the necessity of identifying unique microbial signatures associated with different hormone-receptor statuses, which may play a possibly vital role in local estrogen metabolism and immune regulation similar to that of the gut microbiome. Considering that the research on the relationship between progesterone and the microbiome is still in its infancy, and ER/PR expression was consistent in all samples collected in this study, we hereby refrain from further analysis of the role of progesterone receptors alone. Interestingly, the specific bacterial markers we identified were all concentrated in hormone-receptor-positive breast cancer. Wang H et al. has reported a distinct microbiome in breast cancer with different hormone-receptor statuses, and suggested that Methylobacterium was significantly enriched in hormone-receptor-positive breast cancer [12]. In our study, we observed that order Pseudomonadales was abundant in hormone-receptor-positive breast tissue. Order Pseudomonadales, especially genus Pseudomonas, is widespread in the breast with low proportional abundance [10,15]. In the mouth and vagina, it has been found to be significantly enriched in women without intraepithelial lesions compared to women with squamous intra-epithelial neoplasia [58], but little is known regarding the role of Pseudomonas in breast carcinogenesis. In our study, we found changes in the relative abundance of P. aeruginosa; it was highly enriched in cancer tissues, especially in hormone-receptor-positive tumor tissues, and was hardly detected in normal and benign tissues. It is widely known that pathogen infection is one of the severe complications of malignant tumors, and it has been reported that cancer patients undergoing chemotherapy are at high risk of becoming infected with multi-drug-resistant P. aeruginosa [59], which was also identified in our analysis. However, Chiba et al. [60] reported that neoadjuvant chemotherapy increased the proportional abundance of Pseudomonas to 85%, suggesting chemotherapy induced its preferential growth or survival. They also mentioned that treatment of breast cancer cells with P. aeruginosa-conditioned media differentially effected proliferation and modulated doxorubicin-mediated cell death, suggesting that P. aeruginosa may be related to breast carcinogenesis, but the specific mechanisms underlying these effects are yet to be clarified [60]. In addition, Undibacterium pigrum was also found to be abundant in hormone-receptor-positive breast tissue. This is a Gram-negative bacterium of the genus Undibacterium, which can be isolated from purified water, but unfortunately, its pathogenicity in humans is not yet known [61]. Additional studies will aid in enlightening our observations and further exploring the potential impact of the breast microbiome on cancer development and progression.
ONT nanopore sequencing technology was commercially released in 2014, and since then has become suitable for multiple applications, such as pathogen identification [62], genome assembly [63], and antibiotic-resistance gene detection [64]. Significant advances have been achieved in both the read quantity and accuracy of nanopore sequencing on account of improvements in sequencing chemistry/pore design and better algorithms for basecalling [65]. With the introduction of the Guppy algorithm, ONT indicates a median read accuracy of 89–94% from microbial genomes [66,67]. In addition, the improvements in sequence accuracy from 60% to >90% are illustrated by reason of the changes from R6 to R9 chemistry [68], and the single-read accuracy has increased to >95% for the latest R10.3 flow cells [69,70]. Due to the advantage of generating long reads to allow sequencing of the entire 16S rRNA gene, nanopore sequencing was believed to provide high-resolution results regarding bacterial classification. As one of the earliest reports, Shin et al. used nanopore sequencing to sequence full-length 16S rRNA amplicons from the mouse gut microbiota [41]. They found that both sequencing data were highly similar at all taxonomic resolutions, but at the species level, nanopore sequencing allowed the identification of more species than short-read sequencing. Wei et al. generated 16S rRNA sequences with the same batch of total genomic DNA extracted from fecal samples using Illumina and nanopore technology platforms [31]. Their results suggested that sequencing of the entire 16S rRNA gene improves the accuracy of microbial community classification at both genus and specific levels, compared with the sequencing of the 16S rRNA gene intra-variable region [31]. Taylor et al. compared nanopore sequencing with meta-transcriptomics and Illumina sequencing for microbiome analysis of colorectal tumor tissues [32]. The results showed that long-read sequences could compensate for low read depth for classification purposes [32]. In a word, applying long-read sequencing approaches to study 16S rRNA genes will provide new possibilities for the identification of precise bacterial species in a great variety of microbial compositions.
One drawback of this study was the small sample size. Larger investigations are needed to reduce the within-group differences and further confirm our conclusion. In addition, studies with larger sample sizes and in-depth mechanism analysis are also warranted to systematically elaborate the relationship between breast tissue microbiomes and other indicators strongly associated with breast cancer, such as epidermal growth factor receptor 2 (HER2), and their impact on breast cancer. Another weakness of the present study is the limited demographic data. As a consequence, our analysis may be potentially confounded by the effects of influencing factors such as age and menopausal status, and we presently do not know the exact effect of these confounding factors on the breast microbiota. Furthermore, since the microbiota composition was determined based on the nanopore sequencing data obtained with the taxonomy-supervised analysis, which allocates sequences directly into taxonomic bins instead of using computational operational taxonomic units (OTU) clustering based on sequence similarity, several OTU based analyses cannot be achieved in this study, such as phylogenetic relationship construction and functional predictive analysis, etc. Although improvements for nanopore sequencing have been made in terms of accuracy and data analysis, other sequencing methods including shotgun metagenomics and transcriptomic RNA-seq technology should be employed in further research to reveal deeper characterization, such as the functional genes in the cancer microbiome, and confirm the microbial species discovered in this study. Further investigations with a larger patient cohort on the microflora composition in the mammary gland may be of great help in understanding the multi-faceted roles of the microbiome in breast cancer.
5. Conclusions
In summary, we showed for the first time the differences in the microbiome of human breast tissue with different degrees of malignancy at the species level using Nanopore sequencing. Specially, we compared the microbial composition in breast cancer tissues with different hormone-receptor statuses. It remains to be elucidated whether the identified bacterial profiles are causally associated with carcinogenesis, or merely reflective of the pathological processes in breast cancer. Furthermore, it is currently unclear whether it is a single organism driving the promotion of carcinogenesis, or an interplay of polymicrobial interactions. Further investigation of the potential role of the tissue microbiome in the development of breast cancer is warranted to characterize a microbial risk signature and develop potential microbial-based prevention therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13020174/s1. Table S1: Patient and sample collection characteristics; Table S2: Raw and processed read counts per sample for sequencing; Table S3: Number of different taxa detected in different groups; Figure S1: Relative abundance of bacterial phyla within all breast tissues; Figure S2: The first 20 species of bacteria with relative abundance fluctuate between benign tissue (L), normal tissue (P), and breast tumor tissue (C), and the length of a colored box represents the relative abundance of that organism within each group.
Author Contributions
S.S. and C.C. designed the study. L.L., M.S. and J.H. collected the samples. A.F. and L.L. performed the sequencing and data analysis. L.L. wrote the paper. D.K., T.L. and J.Y. revised critically for content. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Hubei Province (Innovative Group Project, 2020CFA026), Beijing Xisike Clinical Oncology Research Foundation (Y-SY201901-0189) and the Natural Science Foundation of China (8210114078).
Institutional Review Board Statement
The study was approved by the institutional ethics committee of Renmin Hospital of Wuhan University (WDRY2020-K194). The research was conducted in accordance with the Declaration of Helsinki and followed with relevant guidelines and regulations for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from all the patients.
Data Availability Statement
All sequencing data associated with this study can be accessed at the NCBI Sequence Read Archives under BioProject accession No. PRJNA769561, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA769561/ (accessed on 13 October 2021).
Acknowledgments
We thank the patients who generously donated their samples to this project, and Wen Teng, Ni Liao, and Linjuan Yang for processing clinical samples and their technical assistance. Thanks are due to Yuting Chen for valuable discussion. Moreover, we would like to acknowledge Guangdong Magigene Biotechnology Co., Ltd. for their technical help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA-Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Paula, N.; Martins, J.A.M.; Amaral, L.M.; Rhana, P.; Tavares, E.C.; Leite, W.S.; Tavares, G.R.; Rodrigues, A.L.P. Breast cancer: Is grief a risk factor? Rev. Assoc. Med. Bras. 1992 2018, 64, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. The power of small changes: Comprehensive analyses of microbial dysbiosis in breast cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA-Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Stewart, O.A.; Wu, F.; Chen, Y. The role of gastric microbiota in gastric cancer. Gut Microbes 2020, 11, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Vogtmann, E.; Ahlquist, D.A.; Devens, M.E.; Kisiel, J.B.; Taylor, W.R.; White, B.A.; Hale, V.L.; Sung, J.; Chia, N.; et al. Fecal metabolomic signatuRes. in colorectal adenoma patients are associated with gut microbiota and early events of colorectal cancer pathogenesis. mBio 2020, 11, e03186-19. [Google Scholar] [CrossRef] [PubMed]
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Wang, N.; Sun, T.; Xu, J. Tumor-related microbiome in the breast microenvironment and breast cancer. J. Cancer 2021, 12, 4841–4848. [Google Scholar] [CrossRef]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Altemus, J.; Niazi, F.; Green, H.; Calhoun, B.C.; Sturgis, C.; Grobmyer, S.R.; Eng, C. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget 2017, 8, 88122–88138. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct microbial signatures associated with different breast cancer types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Pierre, J.F.; Makowski, L.; Tolley, E.; Lyn-Cook, B.; Lu, L.; Vidal, G.; Starlard-Davenport, A. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci. Rep. 2019, 9, 11940. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, S.; Zhang, Y.; Thapa, S.; Allen, M.S.; Phillips, N.; Chaudhary, P.; Kashyap, M.V.; Vishwanatha, J.K. Comparative analysis of racial differences in breast tumor microbiome. Sci. Rep. 2020, 10, 14116. [Google Scholar] [CrossRef]
- Banerjee, S.; Wei, Z.; Tian, T.; Bose, D.; Shih, N.N.C.; Feldman, M.D.; Khoury, T.; De Michele, A.; Robertson, E.S. Prognostic correlations with the microbiome of breast cancer subtypes. Cell Death Dis. 2021, 12, 831. [Google Scholar] [CrossRef]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y.; et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372.e26. [Google Scholar] [CrossRef]
- Komorowski, A.S.; Pezo, R.C. Untapped “-omics”: The microbial metagenome, estrobolome, and their influence on the development of breast cancer and response to treatment. Breast Cancer Res. Treat. 2019, 179, 287–300. [Google Scholar] [CrossRef]
- Chadha, J.; Nandi, D.; Atri, Y.; Nag, A. Significance of human microbiome in breast cancer: Tale of an invisible and an invincible. Semin. Cancer Biol. 2021, 70, 112–127. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Parida, S.; Wu, S.; Siddharth, S.; Wang, G.; Muniraj, N.; Nagalingam, A.; Hum, C.; Mistriotis, P.; Hao, H.; Talbot, C.C., Jr.; et al. A procarcinogenic colon microbe promotes breast tumorigenesis and metastatic progression and concomitantly activates notch and β-catenin axes. Cancer Discov. 2021, 11, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Costello, E.K.; Berg-Lyons, D.; Gonzalez, A.; Stombaugh, J.; Knight, D.; Gajer, P.; Ravel, J.; Fierer, N.; et al. Moving pictures of the human microbiome. Genome Biol. 2011, 12, R50. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lemons, A.R.; Roseman, J.; Green, B.J.; Cox-Ganser, J.M. Bacterial community assemblages in classroom floor dust of 50 public schools in a large city: Characterization using 16S rRNA sequences and associations with environmental factors. Microbiome 2021, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Hevia, A.; Milani, C.; Lopez, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; Gonzalez, S.; Suarez, A.; Gueimonde, M.; et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 2014, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, L.J.; Dillon, K.P.; Haggblom, M.M.; McGuinness, L.R. Profiling bacterial communities by MinION sequencing of ribosomal operons. Microbiome 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Akeson, M.; Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 2016, 34, 518–524. [Google Scholar] [CrossRef]
- Jain, M.; Olsen, H.E.; Paten, B.; Akeson, M. The Oxford Nanopore MinION: Delivery of nanopore sequencing to the genomics community. Genome Biol. 2016, 17, 239. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Ciuffreda, L.; Rodríguez-Pérez, H.; Flores, C. Nanopore sequencing and its application to the study of microbial communities. Comput. Struct. Biotechnol. J. 2021, 19, 1497–1511. [Google Scholar] [CrossRef]
- Ibironke, O.; McGuinness, L.R.; Lu, S.E.; Wang, Y.Q.; Hussain, S.; Weisel, C.P.; Kerkhof, L.J. Species-level evaluation of the human respiratory microbiome. GigaScience 2020, 9, 10. [Google Scholar] [CrossRef]
- Wei, P.L.; Hung, C.S.; Kao, Y.W.; Lin, Y.C.; Lee, C.Y.; Chang, T.H.; Shia, B.; Lin, J.C. Characterization of fecal microbiota with clinical specimen using long-read and short-read sequencing Platform. Int. J. Mol. Sci. 2020, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.S.; Pearson, J.; Miller, A.; Schmeier, S.; Frizelle, F.A.; Purcell, R.V. MinION Sequencing of colorectal cancer tumour microbiomes-A comparison with amplicon-based and RNA-Sequencing. PLoS ONE 2020, 15, e0233170. [Google Scholar] [CrossRef] [PubMed]
- Calus, S.T.; Ijaz, U.Z.; Pinto, A.J. NanoAmpli-Seq: A workflow for amplicon sequencing for mixed microbial communities on the nanopore sequencing platform. GigaScience 2018, 7, giy140. [Google Scholar] [CrossRef]
- Wang, M.; Fu, A.; Hu, B.; Shen, G.; Liu, R.; Zhao, W.; Jiang, S.; Cai, X.; Li, C.; Wu, Q.; et al. Same-day simultaneous diagnosis of bacterial and fungal infections in clinical practice by nanopore targeted sequencing. medRxiv 2020. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; Available online: https://github.com/tidyverse/ggplot2 (accessed on 1 May 2021).
- Kolde, R.; Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. 2015. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 1 May 2021).
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon-Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Chao, A. Estimating population-size for sparse data in capture recapture experiments. Biometrics 1989, 45, 427–438. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Package Vegan: Community Ecology Package. R Package Version 25-4. 2013. Available online: https://cran.rproject.org/web/packages/vegan/index.html (accessed on 1 May 2021).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.; Go, M.J.; Lee, S.Y.; Kim, S.C.; Lee, C.H.; Cho, B.K. Analysis of the mouse gut microbiome using full-length 16S rRNA amplicon sequencing. Sci. Rep. 2016, 6, 29681. [Google Scholar] [CrossRef]
- Meng, S.; Chen, B.; Yang, J.; Wang, J.; Zhu, D.; Meng, Q.; Zhang, L. Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front. Oncol. 2018, 8, 318. [Google Scholar] [CrossRef]
- Urbaniak, C.; Burton, J.P.; Reid, G. Breast, milk and microbes: A complex relationship that does not end with lactation. Women’s Health 2012, 8, 385–398. [Google Scholar] [CrossRef]
- Alpuim Costa, D.; Nobre, J.G.; Batista, M.V.; Ribeiro, C.; Calle, C.; Cortes, A.; Marhold, M.; Negreiros, I.; Borralho, P.; Brito, M.; et al. Human microbiota and breast cancer—Is there any relevant link? A literature review and new horizons toward personalised medicine. Front. Microbiol. 2021, 12, 584332. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Shamonki, J.M.; Chung, A.; Dinome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial dysbiosis is associated with human breast cancer. PloS ONE 2014, 9, e83744. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C.; et al. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knuston, K.L.; Kalari, K.R.; et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Yu, M.R.; Kim, H.J.; Park, H.R. Fusobacterium nucleatum accelerates the progression of colitis-associated colorectal cancer by promoting EMT. Cancers 2020, 12, 2728. [Google Scholar] [CrossRef]
- Basso, M.; Venditti, C.; Raponi, G.; Navazio, A.S.; Alessandri, F.; Giombini, E.; Nisii, C.; Di Caro, A.; Venditti, M. A case of persistent bacteraemia by Ralstonia mannitolilytica and Ralstonia pickettii in an intensive care unit. Infect. Drug Resist. 2019, 12, 2391–2395. [Google Scholar] [CrossRef]
- Nasir, N.; Sayeed, M.A.; Jamil, B. Ralstonia pickettii bacteremia: An emerging infection in a tertiary care hospital setting. Cureus 2019, 11, e5084. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, W.T.; Chen, C.P.; Sun, S.M.; Kuo, F.M.; Chan, Y.J.; Kuo, S.C.; Wang, F.D. An outbreak of Ralstonia pickettii bloodstream infection associated with an intrinsically contaminated normal saline solution. Infect. Control Hosp. Epidemiol. 2017, 38, 444–448. [Google Scholar] [CrossRef]
- Bedir Demirdag, T.; Ozkaya-Parlakay, A.; Bayrakdar, F.; Gulhan, B.; Kanik Yuksek, S.; Suzuk Yildiz, S.; Mumcuoglu, İ.; Dinc, B.; Yarali, N. An outbreak of Ralstonia pickettii bloodstream infection among pediatric leukemia patients. J. Microbiol. Immunol. Infect. 2021, 55, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Goto, T.; Hirotsu, Y.; Otake, S.; Oyama, T.; Amemiya, K.; Mochizuki, H.; Omata, M. Streptococcus australis and Ralstonia pickettii as major microbiota in mesotheliomas. J. Pers. Med. 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, A.; D’Agostino, Y.; Alexandrova, E.; Lamberti, J.; Pecoraro, G.; Memoli, D.; Rocco, D.; Coviello, E.; Giurato, G.; Nassa, G.; et al. Insights into the role of estrogen receptor β in triple-negative breast cancer. Cancers 2020, 12, 1477. [Google Scholar] [CrossRef]
- Wu, M.; Gao, J.; Wu, Y.; Li, Y.; Chen, Y.; Zhao, F.; Li, C.; Ying, C. Characterization of vaginal microbiota in Chinese women with cervical squamous intra-epithelial neoplasia. Int. J. Gynecol. Cancer 2020, 30, 1500–1504. [Google Scholar] [CrossRef]
- Ahmed, N.; Ali, Z.; Riaz, M.; Zeshan, B.; Wattoo, J.I.; Aslam, M.N. Evaluation of antibiotic resistance and virulence genes among clinical isolates of pseudomonas aeruginosa from cancer patients. Asian Pac J. Cancer Prev. 2020, 21, 1333–1338. [Google Scholar] [CrossRef]
- Chiba, A.; Bawaneh, A.; Velazquez, C.; Clear, K.Y.J.; Wilson, A.S.; Howard-McNatt, M.; Levine, E.A.; Levi-Polyachenko, N.; Yates-Alston, S.A.; Diggle, S.P.; et al. Neoadjuvant chemotherapy shifts breast tumor microbiota populations to regulate drug responsiveness and the development of metastasis. Mol. Cancer Res. 2020, 18, 130–139. [Google Scholar] [CrossRef]
- Eder, W.; Wanner, G.; Ludwig, W.; Busse, H.J.; Ziemke-Kägeler, F.; Lang, E. Description of Undibacterium oligocarboniphilum sp. nov., isolated from purified water, and Undibacterium pigrum strain CCUG 49012 as the type strain of Undibacterium parvum sp. nov., and emended descriptions of the genus Undibacterium and the species Undibacterium pigrum. Int. J. Syst. Evol. Microbiol. 2011, 61 Pt 2, 384–391. [Google Scholar] [CrossRef]
- Wang, M.; Fu, A.; Hu, B.; Tong, Y.; Liu, R.; Liu, Z.; Gu, J.; Xiang, B.; Liu, J.; Jiang, W.; et al. Nanopore targeted sequencing for the accurate and comprehensive detection of SARS-CoV-2 and other respiratory viruses. Small 2020, 16, e2002169. [Google Scholar] [CrossRef]
- O’Donnell, V.K.; Grau, F.R.; Mayr, G.A.; Sturgill Samayoa, T.L.; Dodd, K.A.; Barrette, R.W. Rapid sequence-based characterization of african swine fever virus by use of the Oxford Nanopore MinION sequence sensing device and a companion analysis software Tool. J. Clin. Microbiol. 2019, 58, e01104-19. [Google Scholar] [CrossRef]
- Zhang, C.; Xiu, L.; Li, Y.; Sun, L.; Li, Y.; Zeng, Y.; Wang, F.; Peng, J. Multiplex PCR and nanopore sequencing of genes associated with antimicrobial resistance in neisseria gonorrhoeae directly from clinical samples. Clin. Chem. 2021, 67, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, L.J. Is Oxford Nanopore sequencing ready for analyzing complex microbiomes? FEMS Microbiol. Ecol. 2021, 97, fiab001. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Holt, K.E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 2019, 20, 129. [Google Scholar] [CrossRef]
- Rang, F.J.; Kloosterman, W.P.; de Ridder, J. From squiggle to basepair: Computational approaches for improving nanopore sequencing read accuracy. Genome Biol. 2018, 19, 90. [Google Scholar] [CrossRef]
- Karst, S.M.; Ziels, R.M.; Kirkegaard, R.H.; Sørensen, E.A.; McDonald, D.; Zhu, Q.; Knight, R.; Albertsen, M. High-accuracy long-read amplicon sequences using unique molecular identifiers with Nanopore or PacBio sequencing. Nat. Methods 2021, 18, 165–169. [Google Scholar] [CrossRef]
- Dippenaar, A.; Goossens, S.N.; Grobbelaar, M.; Oostvogels, S.; Cuypers, B.; Laukens, K.; Meehan, C.J.; Warren, R.M.; van Rie, A. Nanopore sequencing for Mycobacterium tuberculosis: A critical review of the literature, new developments, and future opportunities. J. Clin. Microbiol. 2022, 60, e0064621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).