Abstract
Background and objectives: The aim of this systematic review with meta-analysis was to assess the performance of short implants in comparison with standard implants and sinus floor elevation in atrophic posterior maxilla. Materials and methods: The protocol of the study was registered in the PROSPERO database (CRD42022375320). An electronic search on three databases (PubMed, Scopus, Web of Science) was performed to find randomized clinical trials (RCTs) with ≥5 years’ follow-up, published until December 2022. Risk of bias (ROB) was calculated using Cochrane ROB. A meta-analysis was performed for primary (implant survival rate, ISR) and secondary outcomes (marginal bone loss, MBL; biological and prosthetic complications). Results: Of 1619 articles, 5 RCTs met the inclusion criteria. The ISR showed a risk ratio (RR) of 0.97 [0.94, 1.00] (CI 95%), p = 0.07. The MBL indicated a WMD of −0.29 [−0.49, −0.09] (CI 95%), p = 0.005. Biological complications showed a RR of 0.46 [0.23, 0.91] (CI 95%), p = 0.03. Prosthetic complications showed a RR of 1.51 [0.64, 3.55] (CI 95%), p = 0.34. Conclusions: The available evidence suggests that short implants might be used as an alternative to standard implants and sinus floor elevation. After 5 years, in terms of ISR, standard implants and sinus floor elevation showed a higher survival rate comparted to short implants, although statistical significance was not achieved. Future RCTs with long-term follow-up are needed to draw a clear conclusion on the advantages of one method over another.
1. Introduction
Atrophic posterior maxilla still represents a challenging situation in implant dentistry. This fact is determined by the resorption of alveolar ridge and maxillary sinus pneumatization [1]. In order to rehabilitate the maxillary edentulous ridge, a variety of prosthetic solutions and surgical techniques have been proposed [1,2]. The removable prosthesis is a common and successful option in rehabilitation of this area with long-term successful outcomes [3]. As with any treatment, the removable prosthesis has several disadvantages, such as being ill-fitting, the adjustment period, loss of retention, ulceration, plaque accumulation, or fracture [3].
To combat the disadvantages of removable prostheses, a variety of surgical techniques have been proposed to reconstruct resorbed posterior maxilla, and then, to use implant-supported fixed prostheses [2]. These techniques are maxillary sinus floor elevation combined with bone grafts, guided bone regeneration (GBR), onlay/inter-positional grafts, short implants, tilted implants, and zygomatic implants [2,4]. Of all these techniques, the maxillary sinus floor elevation is one of the most frequently used techniques in current clinical practice [5]. Sinus lift can be done via a trans-alveolar ridge or via the lateral window technique using different types of bone grafts (autogenous bone, bone substitutes or a mixture of both) in order to maintain space and determine bone regeneration [2,5]. Being a sensitive technique, with this procedure, clinicians may encounter several complications, such as perforation of the Schneiderian membrane, sinusitis, graft failure, post-operative pain, bleeding, or even migration of dental implants in the maxillary sinus [6,7].
Therefore, the use of short implants has been introduced in order to reduce the complications of sinus floor elevation. The definition of a short implant is considered to be one with a length under 8 mm [8]. The use of such implants has the advantages of eliminating the elevation of the maxillary sinus floor, and reducing post-operative complications, treatment time, and cost [8]. However, several studies have mentioned failure of short implants in comparison to standard implants, such as early implant failure, and characteristics of the implant surface [9,10,11,12].
From the current literature, there is no consensus on which therapy is better for atrophic posterior maxilla. The aim of our systematic review with meta-analysis was to assess the clinical performance of short implants in comparison with standard implants and sinus floor elevation in atrophic posterior maxilla.
2. Materials and Methods
2.1. Protocol and Registration of the Study
This systematic analysis was designed according to the PRISMA guidelines [13]. A priori, the protocol details of this review were submitted and registered in the PROSPERO database (code number CRD42022375320).
2.2. Participants, Intervention, Comparison, Outcome (PICO) Question
The question of focus was elaborated according to the PICO question: “In patients with atrophic posterior maxilla (P), what is the ≥5 years’ efficiency of short implants (I) in comparison with sinus floor elevation and standard implants (C) in terms of implant survival rate; marginal bone loss (MBL), complications (O)?”
PICO elements were as follows:
- Participants: healthy systemic patients, ≥18 years, with atrophic posterior maxilla in need of implant placement;
- Intervention: ultra-short/short implants with a length ≤7 mm;
- Comparison: sinus floor elevation and standard implants with a length ≥8 mm;
- Outcome: implant survival rate (primary outcome); MBL, biological complications (i.e., peri-implant mucositis, peri-implantitis), prosthetic complications (i.e., implant supported prosthetic fracture, screw, abutment fracture/loosening, implant fracture) (secondary outcome);
- Study type: randomized clinical trials (RCTs) or prospective controlled clinical trials (CCTs) with a follow-up ≥5 years.
2.3. Inclusion and Exclusion Criteria for RCTs
The inclusion criteria were:
- Randomized clinical trials (RCTs) or controlled clinical trials (CCTs);
- Comparison short implant (≤7 mm) and standard implants (≥8 mm) and sinus floor elevation in the same RCT;
- RCT with a follow-up ≥5 years;
- Implants restored with fixed partial dentures.
The exclusion criteria were:
- In vitro, animal studies; no clinical trials, cross-sectional, cohort studies; systematic or narrative reviews, case reports, case series, monographs, letters to the editor;
- RCT with insufficient, missing or unpublished data;
- RCT with a follow-up of <5 years;
- Articles published in another language than English.
2.4. Search Methods
An electronic search was performed on 4 December 2022 by two independent reviewers (A.M. and F.O.) in the PubMed, Web of Science and Scopus database and included articles published until December 2022. A grey literature search in the OpenGrey and ClinicalTrial.gov database was done. A manual search in journals specialized in implantology was carried out (Journal of Periodontal Research, Journal of Periodontology, Journal of Clinical Periodontology, European Journal of Oral Sciences, European Journal of Oral Implantology, Dental Journal, British Journal of Oral and Maxillofacial Surgery, Clinical Implant Dentistry and Related Research, Clinical Oral Implants Research, Clinical Oral Investigations, Implant Dentistry, International Journal of Oral and Maxillofacial Implants, International Journal of Oral and Maxillofacial Surgery, International Journal of Periodontics and Restorative Dentistry, Journal of Dental Research, Journal of Dentistry, Journal of Implantology, Journal of Maxillofacial and Oral Surgery, Journal of Oral and Maxillofacial Surgery and Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, Journal of Indian Society of Periodontology).
To identify relevant articles, the following search strategy was applied: (‘short implant’ OR ‘ultrashort implant’ OR ‘standard implant’ OR ‘dental implant’) AND (‘sinus lift’ OR ‘sinus floor augmentation’ OR ‘sinus floor elevation’ OR ‘sinus membrane elevation’ OR ‘lateral approach sinus floor elevation’ OR ‘osteotome sinus floor elevation’ OR ‘atrophic posterior maxilla’ OR ‘edentulous posterior maxilla’). Firstly, titles and abstracts from the electronic searches were screened and irrelevant articles were excluded. Secondly, after removing the duplicates, full-text articles previously obtained were examined and those who met to the inclusion criteria were downloaded. If any disagreements related to the selection of the studies was noted, a third reviewer (S.B.) intervened with an additional resolution.
2.5. Data Extraction
The following data from the included studies were taken: first author, year of study, country, reference, type of RCT, patients characteristics and implants, implant treatment modality, type of sinus lift surgery, type of prosthetic restoration, primary outcome, secondary outcomes, and conclusions.
2.6. Risk of Bias
The risk of bias was quantified using the Cochrane Risk of Bias tool version 2.0 [14]. For each RCT included, seven domains were assessed (random sequence generation; allocation concealment; blinding of participants and/or personnel involved in the study; blinding of outcome assessment; incomplete outcome data reporting; selective reporting of outcomes; other sources of bias). Each domain was analyzed by two independent reviewers (A.M., F.O.) and a third reviewer (S.B.) intervened if any disagreement was present. These domains received a quality grade (low, unclear, or high).
2.7. Statistical Analysis
A meta-analysis was performed using RevMan version 5.4 from the Cochrane Collaboration 2020 [15]. A random effect model with a confidence interval (CI) of 95% was used. For the implant survival rate (primary outcome), biological and prosthetic complications (secondary outcomes), risk ratio (RR) (CI 95%) was assessed using a chi-square test [Mantel–Haenszel (M–H)]. Due to the clinical heterogeneity detected between studies, a random-effects model was applied, in order to analyze effect sizes. For MBL (secondary outcome), a weighted mean difference (WMD) (CI 95%) with sample size, inverse variance (IV), and standard error was calculated. The value p < 0.05 was considered statistically significant. The heterogeneity among the studies was evaluated with an I-squared statistic test (I2). I2 values lower than 30% indicated low heterogeneity, values between 30–60% indicated moderate heterogeneity, and values over 60% indicated substantial heterogeneity.
3. Results
3.1. Study Selection
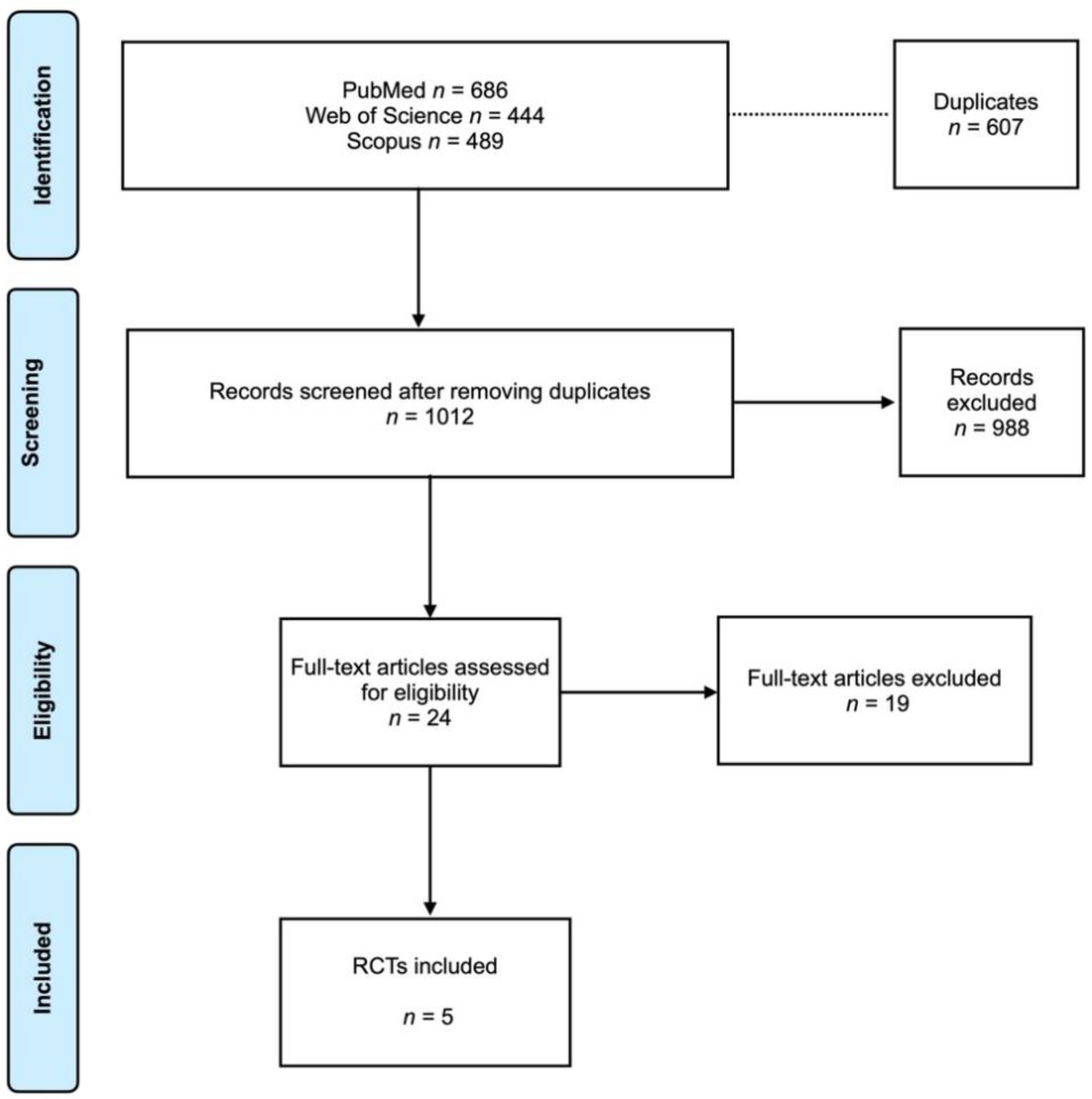
Search results are presented in a Prisma flowchart (Figure 1). The electronic search on the selected database determined a total of 1619 articles. The grey literature was also assessed with no articles to correspond. After removing the duplicates (607 articles), 1012 articles were screened. Of these, 24 articles were full-text assessed for eligibility according to the inclusion criteria. After the evaluation, 19 articles were excluded (reason for exclusion is presented in the Supplementary File, Table S1. In the end, 5 RCTs [16,17,18,19,20] were included in the analysis. The coefficient Cohen’s kappa for inter-reviewer agreement was 0.96.
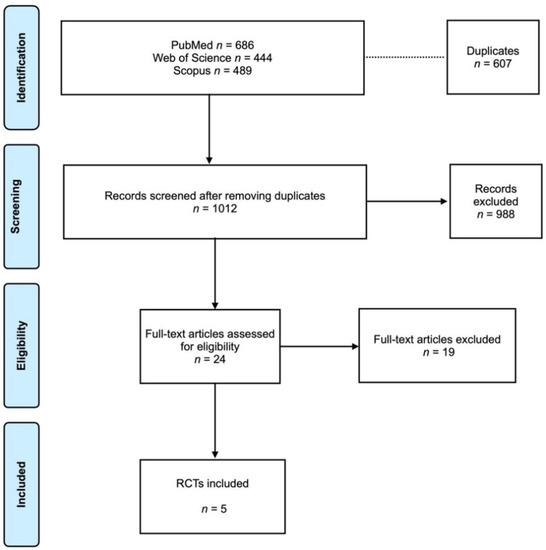
Figure 1.
Prisma flowchart.
3.2. Description of the Included RCTs
The RCTs were published between 2018 and 2019 and conducted in Switzerland, Sweden, Italy and the Netherlands. The study design consisted of an open-prospective RCT multicenter, two-arms parallel group RCT multicenter and split-mouth RCT (Table 1). The total number of patients was 203, of which 84 patients were treated with short implants (S.I.) and 84 with standard implants + sinus lift elevation (Std. I. + S.L.); in regards to the discrepancy of patients’ number, 2 RCTs [18,19] did not report how many patients were in each type of treatment. The total number of inserted implants was 393, of which, 190 were short implants and 203 were standard implants. The length and diameter of short implants was 5–6 mm and 4–6 mm, respectively. The length and diameter of standard implants was 10–15 mm and 4–5 mm, respectively. All RCTs for sinus floor elevation used a lateral window technique using a bone graft with a resorbable collagen membrane [16,17,18,19] or only a bone graft plus an autogenous bone [20]. The majority of the RCTs used cemented crowns as final prosthetics, and only one RCT used also screw-retained crowns.

Table 1.
Characteristics of the included RCTs.
3.3. Risk of Bias Assessment
The results of the Cochrane ROB assessment are presented in Table 2. Most RCTs were considered to have a high ROB.

Table 2.
Cochrane ROB assessment.
3.4. Statistical Analysis of Primary and Secondary Outcomes
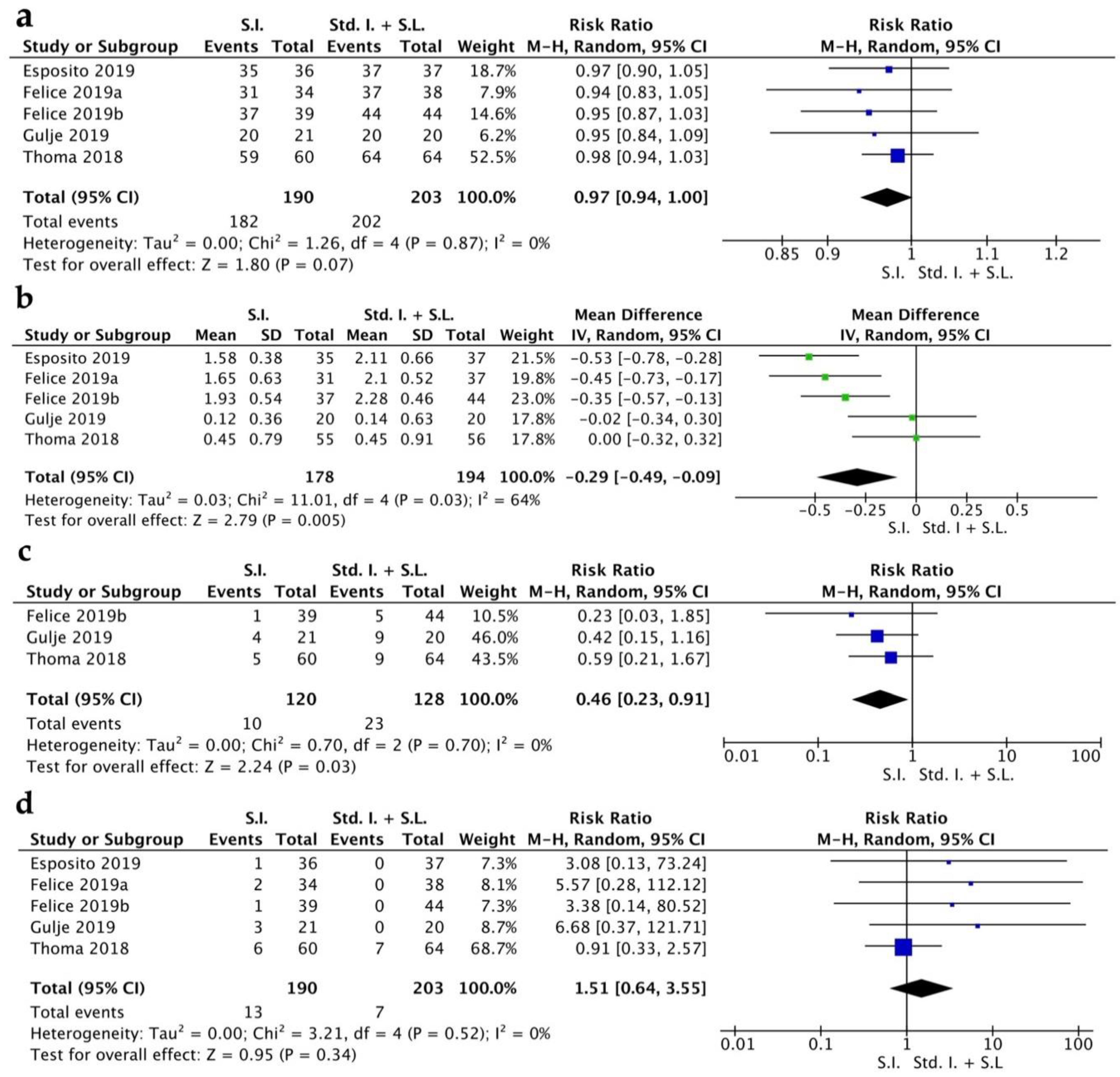
The implant survival rate (ISR) had a RR of 0.97 [0.94, 1.00] (CI 95%). Heterogeneity was low (I2 = 0%) and the random effect model was p = 0.07 (Figure 2a). The MBL indicated a WMD of −0.29 [−0.49, −0.09] (CI 95%) with a high grade of heterogeneity (I2 = 64%) and statistical significance was achieved p = 0.005 (Figure 2b). Biological complications were quantified from three RCTs and showed a RR of 0.46 [0.23, 0.91] (CI 95%), with a low grade of heterogeneity (I2 = 0%) and a random effect model of p = 0.03 (Figure 2c). Prosthetic complications showed a RR of 1.51 [0.64, 3.55] (CI 95%), with a low grade of heterogeneity (I2 = 0%) and a random effect model of p = 0.34 (Figure 2d).
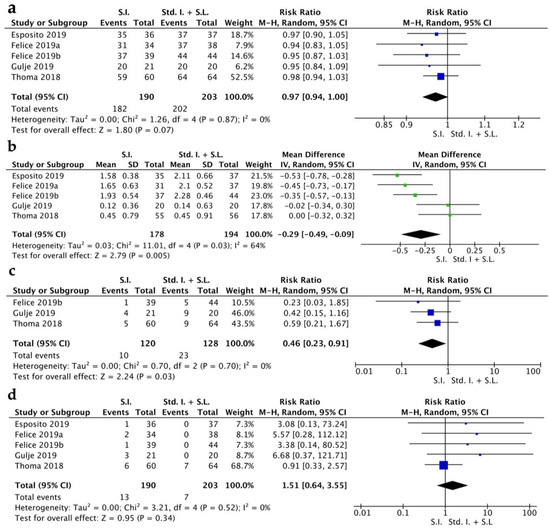
Figure 2.
Forest plot for implant survival rate (a), MBL (b), biological complications (c), prosthetic complications (d). Comparison of short implants (S.I.) versus standard implants and sinus lift (Std. I. + S.L.) [16,17,18,19,20].
4. Discussion
The aim of this systematic review with meta-analysis was to compare the results of short implants and standard implants plus sinus floor elevation in atrophic posterior maxilla in terms of ISR, MBL, biological and prosthetic complications.
The results of ISR indicated no statistical difference between the two therapies; however, survival rate was 95.78% for short implants and 99.5% standard implants during the 5-year follow-up. Guida and coworkers [21] obtained the ISR of 95.40% for short implants and 98.44% for standard implants at 5 years’ follow-up. The authors mentioned that no statistical significance was achieved with a RR of 0.98 [95% CI: (0.94, 1.01); p = 0.21]. In the analysis, the authors included implants inserted in the mandibular region. In the meta-analysis of Toledano and coworkers [22], the authors included RCTs with a follow-up longer than 1 year with a RR value of 1.02 [95% CI: (1.00, 1.05); p = 0.09], suggesting that the ISR was similar for both types of implants. The meta-analysis of Bitinas et al. [9] included 3 RCTs with a follow-up of 5 years and also showed a statistically insignificant difference with a RR value of 0.03 [95% CI: −0.07 to 0.13, (p = 0.52)]. The meta-analysis of Lozano-Carrascal et al. [23] included RCTs with a follow-up longer than 3 years and obtained no statistical significance with a RR value of 1.08 [95% CI: (0.42, 2.83); p = 0.8). Iezzi and coworkers stated in their meta-analysis that high ISR was obtained in short implants in comparison with standard implants [24]. As seen, different results may be found in the electronic literature. Other RCTs have indicated that the ISR for both implants are the same [25,26]. These inconsistencies around systematic reviews may be due to the study population and type of implant system used [22]. However, standard implants with sinus lifts showed better outcomes, and the sinus lift procedure still remains a sensitive technique. Several studies have indicated that this is a safe and predictable technique regardless of the biomaterial used, in terms of clinical and histopathological assessment, and also in patient-reported outcome measurements [27,28].
The MBL indicated a statistical significance between the two therapies. Guida et al. [21] reported a higher MBL at 5 years for standard implants comparted to short implants [0.6 mm (95% CI: 0.42, 0.78; p < 0.00001)]. Toledano et al. reported the MBL of 0.23 mm [95% CI: (0.07, 0.39); p = 0.005], indicating that the MBL was higher for standard implants [22]. Bitinas et al. [9] obtained a MBL of −0.45 mm [95% CI: (−0.87, −0.02); p = 0.04]. Lozano-Carrascal et al. [23] indicated that MBL was in favor of short implants. Iezzi et al. [24] also indicated a significantly lower MBL associated with short implants compared to standard implants. MBL might be influenced by several factors, which includes the type of implant (design, surface configuration), surgical preparation, and surgeon experience [21,22].
The biological complications indicated a statistical significance between the two therapies. Guida et al. [21] reported no statistical significance with a RR value of 1.02 [95% CI: (0.30, 3.47); p = 0.98). Lozano-Carrascal et al. [23] showed a RR of 0.46 [95% CI: (0.22, 0.95); p = 0.037]. As seen, there are not many systematic reviews which report on biological complications. In addition, from our 5 RCTs included, only 3 RCTs mentioned biological complications (peri-implant mucositis or peri-implantitis). Another difficulty seen in the RCTs included was in regard to the diagnosis of peri-implant disease.
The prosthetic complications indicated no statistical differences. Guida et al. [21] also found no statistical significance at 0.80 (95% CI: 0.47, 1.34; p = 0.39). Lozano-Carrascal et al. [23] indicated a RR of 1.52 [95% CI: (0.91, 2.54); p = 1], favoring the standard implants. The variation of the results might have been due to several factors, such as the type of edentulism, prosthetic loading, or type of implant-supported prosthetic (fixed/cemented). Guida and coworkers addressed the issue of other important factors, such as bruxism, smoking, bone quality or implant stability [21].
This systematic review with meta-analysis had several limitations due to heterogeneity and a lack of available information. The main limitation was the low number of the available RCTs with >5 years’ follow-up. Secondly, incomplete information has been reported about biological complications (e.g., peri-implant mucositis, peri-implantitis). In regard to confounding factors, no subgroup analysis could be assessed. Furthermore, in the included RCTs, there was insufficient information in regard to the type of implant-supported reconstruction.
5. Conclusions
The available evidence of this present study suggests that short implants might be used as an alternative to standard implants and sinus floor elevation. After 5 years, in terms of implant survival rate, standard implants and sinus floor elevation showed a higher survival rate compared to short implants; however, statistical significance could not be achieved. Secondary outcomes indicated statistical significance in marginal bone loss and biological complications, and there was no statistical significance in prosthetic complications. Future RCTs with long-term follow-up are needed to draw a clear conclusion on the advantages of one method over another.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13020169/s1, Table S1: Reason for article exclusion [27,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Author Contributions
Conceptualization, A.M., F.O. and S.B.; methodology, A.M., F.O. and S.B.; investigation, A.M., F.O. and S.B.; writing—original draft, A.M., D.D.S., A.P. and A.-M.C.; writing—review and editing, A.M., F.O. and S.B.; supervision, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-term effectiveness of maxillary sinus floor augmentation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S2), 307–318. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, O.; Rudolph, H.; Luthardt, R.G. Clinical performance of removable dental prostheses in the moderately reduced dentition: A systematic literature review. Clin. Oral Investig. 2016, 20, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hüsler, J.; Hämmerle, C.H.F.; Jung, R.E. EAO Supplement Working Group 4–EAO CC 2015 Short implants versus sinus lifting with longer implants to restore the posterior maxilla: A systematic review. Clin. Oral Implant. Res. 2015, 26 (Suppl. S1), 154–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xia, T.; Wang, H.; Cheng, Z.; Shi, B. Outcomes of maxillary sinus floor augmentation without grafts in atrophic maxilla: A systematic review and meta-analysis based on randomised controlled trials. J. Oral. Rehabil. 2019, 46, 282–290. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Padial-Molina, M.; Avila, G.; Rios, H.F.; Hernández-Cortés, P.; Wang, H.-L. Complications associated with implant migration into the maxillary sinus cavity. Clin. Oral Implant. Res. 2012, 23, 1152–1160. [Google Scholar] [CrossRef]
- Moreno Vazquez, J.C.; Gonzalez de Rivera, A.S.; Gil, H.S.; Mifsut, R.S. Complication rate in 200 consecutive sinus lift procedures: Guidelines for prevention and treatment. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2014, 72, 892–901. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Shi, J.-Y.; Gu, Y.-X.; Qiao, S.-C.; Mo, J.-J.; Lai, H.-C. Clinical Investigation and Patient Satisfaction of Short Implants Versus Longer Implants with Osteotome Sinus Floor Elevation in Atrophic Posterior Maxillae: A Pilot Randomized Trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 161–166. [Google Scholar] [CrossRef]
- Bitinas, D.; Bardijevskyt, G. Short implants without bone augmentation vs. long implants with bone augmentation: Systematic review and meta-analysis. Aust. Dent. J. 2021, 66 (Suppl. S1), S71–S81. [Google Scholar] [CrossRef]
- Bolle, C.; Felice, P.; Barausse, C.; Pistilli, V.; Trullenque-Eriksson, A.; Esposito, M. 4 mm long vs longer implants in augmented bone in posterior atrophic jaws: 1-year post-loading results from a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 31–47. [Google Scholar]
- Nisand, D.; Renouard, F. Short implant in limited bone volume. Periodontol. 2000 2014, 66, 72–96. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Derks, J. Etiology, occurrence, and consequences of implant loss. Periodontol. 2000 2022, 88, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Jonathan, J.D.; Julian, P.T.H.; Douglas, G.A.; on behalf of the Collegiate Sports Management Group. Analysing Data and Undertaking Meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Julian, P.T., Higgins, J.T., Jacqueline, C., Miranda, C., Tianjing, L., Matthew, J., Page, V.A.W., Eds.; Wiley Blackwell: London, UK, 2019. [Google Scholar]
- Thoma, D.S.; Haas, R.; Sporniak-Tutak, K.; Garcia, A.; Taylor, T.D.; Hämmerle, C.H.F. Randomized controlled multicentre study comparing short dental implants (6 mm) versus longer dental implants (11–15 mm) in combination with sinus floor elevation procedures: 5-Year data. J. Clin. Periodontol. 2018, 45, 1465–1474. [Google Scholar] [CrossRef]
- Esposito, M.; Barausse, C.; Pistilli, R.; Piattelli, M.; Di Simone, S.; Ippolito, D.R.; Felice, P. Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. Five-year results from a randomised controlled trial. Int. J. Oral Implantol. 2019, 12, 39–54. [Google Scholar]
- Felice, P.; Barausse, C.; Pistilli, R.; Ippolito, D.R.; Esposito, M. Five-year results from a randomised controlled trial comparing prostheses supported by 5-mm long implants or by longer implants in augmented bone in posterior atrophic edentulous jaws. Int. J. Oral Implantol. 2019, 12, 25–37. [Google Scholar]
- Felice, P.; Pistilli, R.; Barausse, C.; Piattelli, M.; Buti, J.; Esposito, M. Posterior atrophic jaws rehabilitated with prostheses supported by 6-mm-long 4-mm-wide implants or by longer implants in augmented bone. Five-year post-loading results from a within-person randomised controlled trial. Int. J. Oral Implantol. 2019, 12, 57–72. [Google Scholar]
- Guljé, F.L.; Raghoebar, G.M.; Vissink, A.; Meijer, H.J.A. Single crowns in the resorbed posterior maxilla supported by either 11-mm implants combined with sinus floor elevation or 6-mm implants:A 5-year randomised controlled trial. Int. J. Oral Implantol. 2019, 12, 315–326. [Google Scholar]
- Guida, L.; Bressan, E.; Cecoro, G.; Volpe, A.D.; Del Fabbro, M.; Annunziata, M. Short versus Longer Implants in Sites without the Need for Bone Augmentation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Materials 2022, 15, 3138. [Google Scholar] [CrossRef]
- Toledano, M.; Fernández-Romero, E.; Vallecillo, C.; Toledano, R.; Osorio, M.T.; Vallecillo-Rivas, M. Short versus standard implants at sinus augmented sites: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 6681–6698. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Carrascal, N.; Anglada-Bosqued, A.; Salomó-Coll, O.; Hernández-Alfaro, F.; Wang, H.-L.; Gargallo-Albiol, J. Short implants (<8mm) versus longer implants (≥8mm) with lateral sinus floor augmentation in posterior atrophic maxilla: A meta-analysis of RCT`s in humans. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e168–e179. [Google Scholar] [PubMed]
- Iezzi, G.; Perrotti, V.; Felice, P.; Barausse, C.; Piattelli, A.; Del Fabbro, M. Are <7-mm long implants in native bone as effective as longer implants in augmented bone for the rehabilitation of posterior atrophic jaws? A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2020, 22, 552–566. [Google Scholar] [PubMed]
- Magdy, M.; Abdelkader, M.A.; Alloush, S.; Fawzy El-Sayed, K.M.; Nawwar, A.A.; Shoeib, M.; ElNahass, H. Ultra-short versus standard-length dental implants in conjunction with osteotome-mediated sinus floor elevation: A randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2021, 23, 520–529. [Google Scholar] [CrossRef]
- Gastaldi, G.; Felice, P.; Pistilli, R.; Barausse, C.; Trullenque-Eriksson, A.; Esposito, M. Short implants as an alternative to crestal sinus lift: A 3-year multicentre randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 391–400. [Google Scholar]
- Velasco-Ortega, E.; Valente, N.A.; Iezzi, G.; Petrini, M.; Derchi, G.; Barone, A. Maxillary sinus augmentation with three different biomaterials: Histological, histomorphometric, clinical, and patient-reported outcomes from a randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 86–95. [Google Scholar] [CrossRef]
- Farina, R.; Franceschetti, G.; Travaglini, D.; Consolo, U.; Minenna, L.; Schincaglia, G.P.; Riccardi, O.; Bandieri, A.; Maietti, E.; Trombelli, L. Morbidity following transcrestal and lateral sinus floor elevation: A randomized trial. J. Clin. Periodontol. 2018, 45, 1128–1139. [Google Scholar] [CrossRef]
- Dasmah, A.; Thor, A.; Ekestubbe, A.; Sennerby, L.; Rasmusson, L. Marginal bone-level alterations at implants installed in block versus particulate onlay bone grafts mixed with platelet-rich plasma in atrophic maxilla. a prospective 5-year follow-up study of 15 patients. Clin. Implant Dent. Relat. Res. 2013, 15, 7–14. [Google Scholar] [CrossRef]
- Cannizzaro, G.; Felice, P.; Minciarelli, A.F.; Leone, M.; Viola, P.; Esposito, M. Early implant loading in the atrophic posterior maxilla: 1-stage lateral versus crestal sinus lift and 8 mm hydroxyapatite-coated implants. A 5-year randomised controlled trial. Eur. J. Oral Implantol. 2013, 6, 13–25. [Google Scholar] [CrossRef]
- Romeo, E.; Storelli, S.; Casano, G.; Scanferla, M.; Botticelli, D. Six-mm versus 10-mm long implants in the rehabilitation of posterior edentulous jaws: A 5-year follow-up of a randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 371–381. [Google Scholar]
- Rossi, F.; Botticelli, D.; Cesaretti, G.; De Santis, E.; Storelli, S.; Lang, N.P. Use of short implants (6 mm) in a single-tooth replacement: A 5-year follow-up prospective randomized controlled multicenter clinical study. Clin. Oral Implants Res. 2016, 27, 458–464. [Google Scholar] [CrossRef]
- Shi, J.-Y.; Gu, Y.-X.; Qiao, S.-C.; Zhuang, L.-F.; Zhang, X.-M.; Lai, H.-C. Clinical evaluation of short 6-mm implants alone, short 8-mm implants combined with osteotome sinus floor elevation and standard 10-mm implants combined with osteotome sinus floor elevation in posterior maxillae: Study protocol for a randomized controlle. Trials 2015, 16, 324. [Google Scholar] [CrossRef] [PubMed]
- Nedir, R.; Nurdin, N.; Abi Najm, S.; El Hage, M.; Bischof, M. Short implants placed with or without grafting into atrophic sinuses: The 5-year results of a prospective randomized controlled study. Clin. Oral Implants Res. 2017, 28, 877–886. [Google Scholar] [CrossRef]
- Toljanic, J.A.; Ekstrand, K.; Baer, R.A.; Thor, A. Immediate Loading of Implants in the Edentulous Maxilla with a Fixed Provisional Restoration without Bone Augmentation: A Report on 5-Year Outcomes Data Obtained from a Prospective Clinical Trial. Int. J. Oral Maxillofac. Implants 2016, 31, 1164–1170. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Sannino, G.; Rapanelli, A.; Crespi, R.; Gastaldi, G.; Capparé, P. Prefabricated Bar System for Immediate Loading in Edentulous Patients: A 5-Year Follow-Up Prospective Longitudinal Study. Biomed Res. Int. 2018, 2018, 7352125. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, G.; Felice, P.; Ippolito, D.R.; Velasco-Ortega, E.; Esposito, M. Immediate loading of fixed cross-arch prostheses supported by flapless-placed 5 mm or 11.5 mm long implants: 5-year results from a randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 295–306. [Google Scholar] [PubMed]
- Storelli, S.; Abbà, A.; Scanferla, M.; Botticelli, D.; Romeo, E. 6 mm vs 10 mm-long implants in the rehabilitation of posterior jaws: A 10-year follow-up of a randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 283–292. [Google Scholar] [PubMed]
- Naenni, N.; Sahrmann, P.; Schmidlin, P.R.; Attin, T.; Wiedemeier, D.B.; Sapata, V.; Hämmerle, C.H.F.; Jung, R.E. Five-Year Survival of Short Single-Tooth Implants (6 mm): A Randomized Controlled Clinical Trial. J. Dent. Res. 2018, 97, 887–892. [Google Scholar] [CrossRef]
- Meloni, S.M.; Lumbau, A.; Spano, G.; Baldoni, E.; Pisano, M.; Tullio, A.; Tallarico, M. Sinus augmentation grafting with anorganic bovine bone versus 50% autologous bone mixed with 50% anorganic bovine bone: 5 years after loading results from a randomised controlled trial. Int. J. oral Implantol. 2019, 12, 483–492. [Google Scholar]
- Testori, T.; Panda, S.; Clauser, T.; Scaini, R.; Zuffetti, F.; Capelli, M.; Taschieri, S.; Mortellaro, C.; Del Fabbro, M. Short implants and platelet-rich fibrin for transcrestal sinus floor elevation: A prospective multicenter clinical study. J. Biol. Regul. Homeost. Agents 2019, 33, 121–135. [Google Scholar]
- Slot, W.; Raghoebar, G.M.; Cune, M.S.; Vissink, A.; Meijer, H.J.A. Four or six implants in the maxillary posterior region to support an overdenture: 5-year results from a randomized controlled trial. Clin. Oral Implants Res. 2019, 30, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.-J.; Mo, J.-J.; Si, M.-S.; Qiao, S.-C.; Shi, J.-Y.; Lai, H.-C. Long-term outcomes of osteotome sinus floor elevation with or without bone grafting: The 10-year results of a randomized controlled trial. J. Clin. Periodontol. 2020, 47, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Guljé, F.L.; Meijer, H.J.A.; Abrahamsson, I.; Barwacz, C.A.; Chen, S.; Palmer, P.J.; Zadeh, H.; Stanford, C.M. Comparison of 6-mm and 11-mm dental implants in the posterior region supporting fixed dental prostheses: 5-year results of an open multicenter randomized controlled trial. Clin. Oral Implants Res. 2021, 32, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, H.H.; Guljé, F.; Palmer, P.J.; Abrahamsson, I.; Chen, S.; Mahallati, R.; Stanford, C.M. Marginal bone level and survival of short and standard-length implants after 3 years: An Open Multi-Center Randomized Controlled Clinical Trial. Clin. Oral Implants Res. 2018, 29, 894–906. [Google Scholar] [CrossRef]
- Barausse, C.; Felice, P.; Pistilli, R.; Buti, J.; Rcsed, M.; Esposito, M. Posterior Jaw Rehabilitation Using Partial Prostheses Supported By Implants 4.0 × 4.0 Mm or Longer: Three-Year Post-Loading Results of a Multicentre Randomised Controlled Trial. Clin. Trials Dent. 2019, 1, 25–36. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).