Updated Views in Targeted Therapy in the Patient with Non-Small Cell Lung Cancer
Abstract
1. Introduction
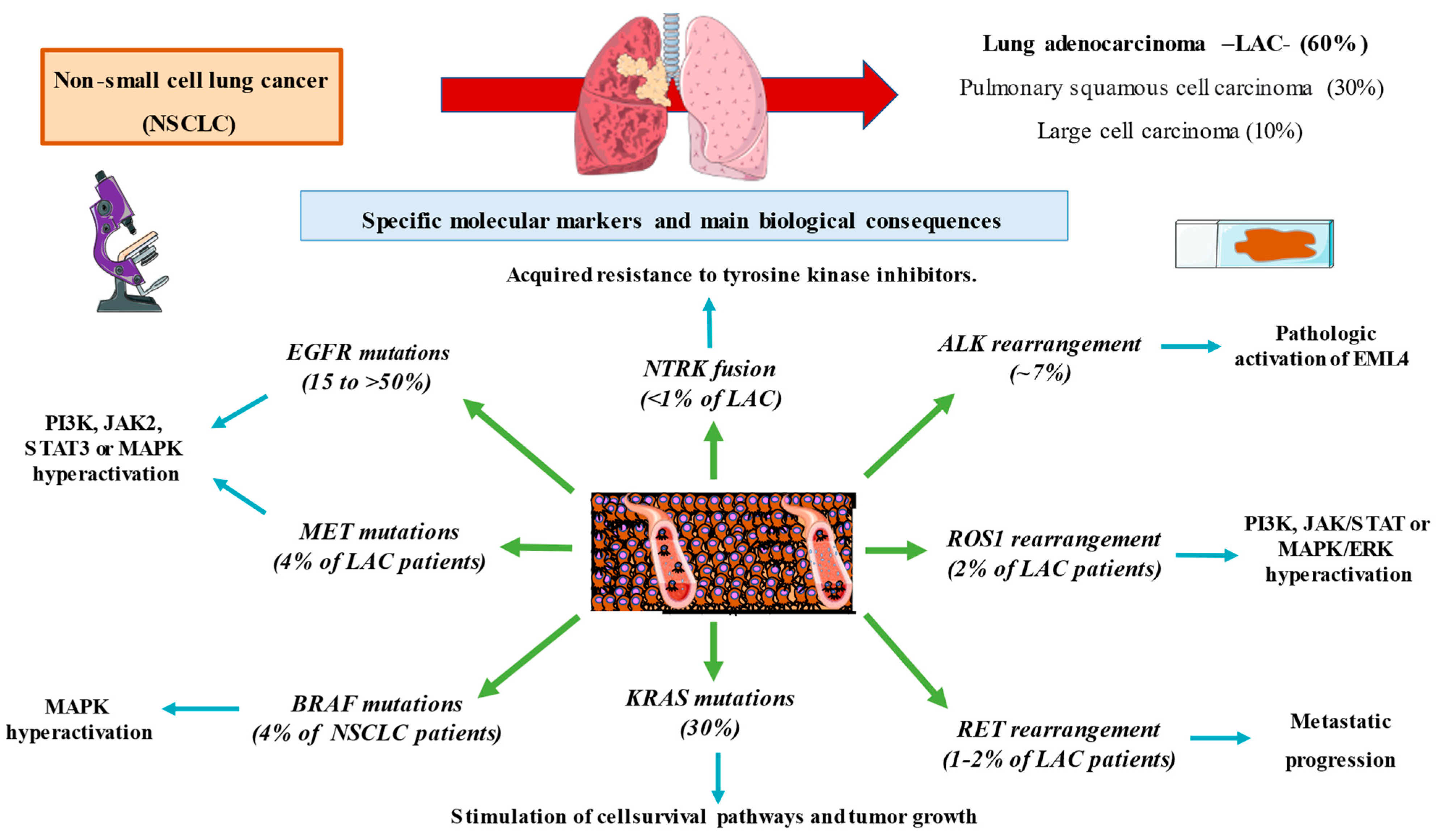
2. Subtypes of Non-Small Cell Lung Cancer
2.1. Squamous Cell Carcinoma
2.2. Lung Adenocarcinoma
2.3. Large Cell Carcinoma
3. Molecular Markers in Lung Cancer
4. Targeted Therapy in NSCLC
4.1. EGFR Mutations
4.2. ALK Rearrangement
4.3. ROS1 Rearrangement
4.4. MET Mutations
4.5. RET Rearrangement
4.6. BRAF Mutations
4.7. NTRK Fusions
4.8. KRAS Mutations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, L.M.; Taylor, G.; Huxley, R.R.; Mitchell, P.; Woodward, M.; Peters, S.A.E. Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis. BMJ Open 2018, 8, e021611. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, M.; Kendrick, P.; Anderson, J.; Arian, N.; Feldman, R.; Gakidou, E.; Gupta, V. Reexamining Rates of Decline in Lung Cancer Risk after Smoking Cessation. A Meta-analysis. Ann. Am. Thorac. Soc. 2020, 17, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Walser, T.; Cui, X.; Yanagawa, J.; Lee, J.M.; Heinrich, E.; Lee, G.; Sharma, S.; Dubinett, S.M. Smoking and Lung Cancer: The Role of Inflammation. Proc. Am. Thorac. Soc. 2008, 5, 811–815. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85. [Google Scholar] [CrossRef]
- Nakazawa, K.; Kurishima, K.; Tamura, T.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in small cell lung cancer. Oncol. Lett. 2012, 4, 617–620. [Google Scholar] [CrossRef]
- Soomro, Z.; Youssef, M.; Yust-Katz, S.; Jalali, A.; Patel, A.J.; Mandel, J. Paraneoplastic syndromes in small cell lung cancer. J. Thorac. Dis. 2020, 12, 6253–6263. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.-W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta (BBA) Rev. Cancer 2015, 1856, 189–210. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Luo, L.; A Wampfler, J.; Wang, Y.; Liu, D.; Chen, Y.-M.; A Adjei, A.; E Midthun, D.; Yang, P. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: A prospective, observational cohort study. Lancet Oncol. 2019, 20, 1098–1108. [Google Scholar] [CrossRef]
- Bilfinger, T.V.; Albano, D.; Perwaiz, M.; Keresztes, R.; Nemesure, B. Survival Outcomes Among Lung Cancer Patients Treated Using a Multidisciplinary Team Approach. Clin. Lung Cancer 2018, 19, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients with Non–Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Carcereny, E.; Rodríguez-Abreu, D.; López-Castro, R.; Guirado, M.; Camps, C.; Bosch-Barrera, J.; García-Campelo, R.; Ortega-Granados, A.L.; González-Larriba, J.L.; et al. Lung cancer in Spain: Information from the Thoracic Tumors Registry (TTR study). Transl. Lung Cancer Res. 2019, 8, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Núñez Hernández, J.C.; Tamariz Campillo, A.F.; Mellado Orellana, R.; Tostado Cosío, R.; Díaz Greene, E.J.; Rodríguez Weber, F.L. Sín-dromes paraneoplásicos asociados a cáncer de pulmón. Acta Médica Grupo Ángeles 2021, 19, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Azizi, A.R.J.; IIIzam, E.L.; Nazirah, A.; Sharifa, S.; Abbas, S.A. Lung Cancer: Risk Factors, Management, And Prognosis. IOSR J. Dent. Med. Sci. 2016, 15, 94–101. [Google Scholar] [CrossRef]
- Goss, G.D.; Spaans, J.N.; Huntsman, D.; Asmis, T.; Wright, N.M.A.; Duciaume, M.; Kaurah, P.; Miller, R.R.; Banerji, S.; Sekhon, H.S.; et al. Histologic and Genotypic Characterization of Lung Cancer in the Inuit Population of the Eastern Canadian Arctic. Curr. Oncol. 2022, 29, 3171–3186. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung Cancer: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Rekhtman, N.; Travis, W.D. Large No More: The Journey of Pulmonary Large Cell Carcinoma from Common to Rare Entity. J. Thorac. Oncol. 2019, 14, 1125–1127. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Provencio-Pulla, M.; Casan Clarà, P. Cribado de cáncer de pulmón con tomografía computarizada de baja dosis. No Es Cuestión De Logística. Arch. Bronconeumol. 2017, 53, 593–594. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Simmons, C.P.; Koinis, F.; Fallon, M.T.; Fearon, K.C.; Bowden, J.; Solheim, T.S.; Gronberg, B.H.; McMillan, D.C.; Gioulbasanis, I.; Laird, B.J. Prognosis in advanced lung cancer – A prospective study examining key clinicopathological factors. Lung Cancer 2015, 88, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2015, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.-I.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 10299, 535–554. [Google Scholar] [CrossRef]
- Wodarz, D.; Newell, A.C.; Komarova, N.L. Passenger mutations can accelerate tumour suppressor gene inactivation in cancer evo-lution. J. R. Soc. Interface 2018, 15, 20170967. [Google Scholar] [CrossRef]
- VanderLaan, P.A.; Rangachari, D.; Costa, D.B. The rapidly evolving landscape of biomarker testing in non–small cell lung cancer. Cancer Cytopathol. 2020, 129, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lescure, A.; De La Peña, F.A.; Aranda, E.; Calvo, A.; Felip, E.; Garrido, P.; Vera, R. Study of the Spanish Society of Medical Oncology (SEOM) on the access to oncology drugs and predictive biomarkers in Spain. Clin. Transl. Oncol. 2020, 22, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.; Martín-López, J.; Martínez-Pozo, A.; Hernández-Iglesias, T.; Carcedo, D.; de Alda, L.R.; García, J.F.; Rojo, F. Real-world biomarker testing rate and positivity rate in NSCLC in Spain: Prospective Central Lung Cancer Biomarker Testing Registry (LungPath) from the Spanish Society of Pathology (SEAP). J. Clin. Pathol. 2021, 75, 193–200. [Google Scholar] [CrossRef]
- Normanno, N.; Barberis, M.; De Marinis, F.; Gridelli, C. Molecular and Genomic Profiling of Lung Cancer in the Era of Precision Medicine: A Position Paper from the Italian Association of Thoracic Oncology (AIOT). Cancers 2020, 12, 1627. [Google Scholar] [CrossRef] [PubMed]
- Cainap, C.; Balacescu, O.; Cainap, S.S.; Pop, L.-A. Next Generation Sequencing Technology in Lung Cancer Diagnosis. Biology 2021, 10, 864. [Google Scholar] [CrossRef]
- Vanderpoel, J.; Stevens, A.L.; Emond, B.; Lafeuille, M.-H.; Hilts, A.; Lefebvre, P.; Morrison, L. Total cost of testing for genomic alterations associated with next-generation sequencing versus polymerase chain reaction testing strategies among patients with metastatic non-small cell lung cancer. J. Med Econ. 2022, 25, 457–468. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Yang, C.; Xia, B.-R.; Jin, W.-L.; Lou, G. Circulating tumor cells in precision oncology: Clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int. 2019, 19, 341. [Google Scholar] [CrossRef]
- Scharpenseel, H.; Hanssen, A.; Loges, S.; Mohme, M.; Bernreuther, C.; Peine, S.; Lamszus, K.; Goy, Y.; Petersen, C.; Westphal, M.; et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci. Rep. 2019, 9, 7406. [Google Scholar] [CrossRef]
- Kloten, V.; Lampignano, R.; Krahn, T.; Schlange, T. Circulating Tumor Cell PD-L1 Expression as Biomarker for Therapeutic Efficacy of Immune Checkpoint Inhibition in NSCLC. Cells 2019, 8, 809. [Google Scholar] [CrossRef]
- Maly, V.; Maly, O.; Kolostova, K.; Bobek, V. Circulating Tumor Cells in Diagnosis and Treatment of Lung Cancer. In Vivo 2019, 33, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Sacher, A.G.; Paweletz, C.; Dahlberg, S.E.; Alden, R.S.; O’Connell, A.; Feeney, N.; Mach, S.L.; Jänne, P.A.; Oxnard, G.R. Prospective Validation of Rapid Plasma Genotyping for the Detection ofEGFRandKRASMutations in Advanced Lung Cancer. JAMA Oncol. 2016, 2, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Thress, K.S.; Alden, R.S.; Lawrance, R.; Paweletz, C.P.; Cantarini, M.; Yang, J.C.-H.; Barrett, J.C.; Jänne, P.A. Association Between Plasma Genotyping and Outcomes of Treatment with Osimertinib (AZD9291) in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- Juan, O.; Vidal, J.; Gisbert, R.; Muñoz, J.; Maciá, S.; Gómez-Codina, J. Prognostic significance of circulating tumor cells in advanced non-small cell lung cancer patients treated with docetaxel and gemcitabine. Clin. Transl. Oncol. 2013, 16, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Adam, J.; Barthélémy, A.; Oulhen, M.; Auger, N.; Valent, A.; Borget, I.; Planchard, D.; Taylor, M.; André, F.; et al. Detection of Circulating Tumor Cells Harboring a Unique ALK Rearrangement in ALK-Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2013, 31, 2273–2281. [Google Scholar] [CrossRef]
- Zhang, Z.; Ramnath, N.; Nagrath, S. Current Status of CTCs as Liquid Biopsy in Lung Cancer and Future Directions. Front. Oncol. 2015, 5, 209. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Zhang, S.; Wang, M.; Yang, S.; Li, N.; Wu, G.; Liu, W.; Liao, G.; Cai, K.; et al. Molecular Epidemiology of EGFR Mutations in Asian Patients with Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology – Mainland China Subset Analysis of the PIONEER study. PLoS ONE 2015, 10, e0143515. [Google Scholar] [CrossRef]
- Jin, R.; Peng, L.; Shou, J.; Wang, J.; Jin, Y.; Liang, F.; Zhao, J.; Wu, M.; Li, Q.; Zhang, B.; et al. EGFR-Mutated Squamous Cell Lung Cancer and Its Association with Outcomes. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR Mutations and Lung Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 49–69. [Google Scholar] [CrossRef]
- Lohinai, Z.; Hoda, M.A.; Fabian, K.; Ostoros, G.; Raso, E.; Barbai, T.; Timar, J.; Kovalszky, I.; Cserepes, M.; Rozsas, A.; et al. Distinct Epidemiology and Clinical Consequence of Classic Versus Rare EGFR Mutations in Lung Adenocarcinoma. J. Thorac. Oncol. 2015, 10, 738–746. [Google Scholar] [CrossRef]
- Yoon, H.-Y.; Ryu, J.-S.; Sim, Y.S.; Kim, D.; Lee, S.Y.; Choi, J.; Park, S.; Ryu, Y.J.; Lee, J.H.; Chang, J.H. Clinical significance of EGFR mutation types in lung adenocarcinoma: A multi-centre Korean study. PLoS ONE 2020, 15, e0228925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sheng, J.; Kang, S.; Fang, W.; Yan, Y.; Hu, Z.; Hong, S.; Wu, X.; Qin, T.; Liang, W.; et al. Patients with Exon 19 Deletion Were Associated with Longer Progression-Free Survival Compared to Those with L858R Mutation after First-Line EGFR-TKIs for Advanced Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2014, 9, e107161. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Nakagawa, K. First- and Second-Generation EGFR-TKIs Are All Replaced to Osimertinib in Chemo-Naive EGFR Mutation-Positive Non-Small Cell Lung Cancer? Int. J. Mol. Sci. 2019, 20, 146. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, K.; Kim, S.; Lee, D. A randomized phase III study of gefitinib (IRESSA) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never smokers with advanced or metastatic adenocarcinoma of the lung. In Proceedings of the 13th Biennial World Conference on Lung Cancer of the International Association of the Study of Lung Cancer (IASLC), San Francisco, CA, USA, 14 September 2009. [Google Scholar]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J.; et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non–Small-Cell Lung Cancer and EGFR-Activating Mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [CrossRef]
- Liang, S.-K.; Hsieh, M.-S.; Lee, M.-R.; Keng, L.-T.; Ko, J.-C.; Shih, J.-Y. Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma. Oncotarget 2017, 8, 90430–90443. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.-P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Yang, X.; Chu, L.; Wang, S.; Tong, T.; Chu, X.; Yu, F.; Zeng, Y.; Guo, T.; et al. Overall survival benefit of osimertinib and clinical value of upfront cranial local therapy in untreated EGFR -mutant nonsmall cell lung cancer with brain metastasis. Int. J. Cancer. 2022, 150, 1318–1328. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Provencio, M.; Terrasa, J.; Garrido, P.; Campelo, R.G.; Aparisi, F.; Diz, P.; Aguiar, D.; García-Giron, C.; Hidalgo, J.; Aguado, C.; et al. Osimertinib in advanced EGFR-T790M mutation-positive non-small cell lung cancer patients treated within the Special Use Medication Program in Spain: OSIREX-Spanish Lung Cancer Group. BMC Cancer 2021, 21, 230. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Tsai, C.-M.; Shepherd, F.A.; Bazhenova, L.; Sequist, L.V.; Hida, T.; Yang, J.C.H.; Ramalingam, S.S.; Mitsudomi, T.; Jänne, P.A.; et al. Osimertinib in patients with T790M mutation-positive, advanced non–small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019, 125, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Hochmair, M.J.; Buder, A.; Schwab, S.; Burghuber, O.C.; Prosch, H.; Hilbe, W.; Cseh, A.; Fritz, R.; Filipits, M. Liquid-Biopsy-Based Identification of EGFR T790M Mutation-Mediated Resistance to Afatinib Treatment in Patients with Advanced EGFR Mutation-Positive NSCLC, and Subsequent Response to Osimertinib. Target. Oncol. 2018, 14, 75–83. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Ortega, M.A.; Navarro, F.; Pekarek, L.; Fraile-Martínez, O.; García-Montero, C.; Saez, M.A.; Arroyo, M.; Monserrat, J.; Alvarez-Mon, M. Exploring histopathological and serum biomarkers in lung adenocarcinoma: Clinical applications and translational opportunities (Review). Int. J. Oncol. 2022, 61, 154. [Google Scholar] [CrossRef]
- Pekarek, L.; De la Torre-Escuredo, B.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Cobo-Prieto, D.; Guijarro, L.G.; Saz, J.V.; De Castro-Martinez, P.; Torres-Carranza, D.; et al. Towards the Search for Potential Biomarkers in Osteosarcoma: State-of-the-Art and Translational Expectations. Int. J. Mol. Sci. 2022, 23, 14939. [Google Scholar] [CrossRef] [PubMed]
- Mamesaya, N.; Nakashima, K.; Naito, T.; Nakajima, T.; Endo, M.; Takahashi, T. ALK-rearranged lung squamous cell carcinoma re-sponding to alectinib: A case report and review of the literature. BMC Cancer 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- I Selinger, C.; Rogers, T.-M.; A Russell, P.; O’Toole, S.; Yip, P.; Wright, G.M.; Wainer, Z.; Horvath, L.G.; Boyer, M.; McCaughan, B.; et al. Testing for ALK rearrangement in lung adenocarcinoma: A multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod. Pathol. 2013, 26, 1545–1553. [Google Scholar] [CrossRef]
- Griesinger, F.; Roeper, J.; Pöttgen, C.; Willborn, K.C.; Eberhardt, W.E.E. Brain metastases in ALK-positive NSCLC - time to adjust current treatment algorithms. Oncotarget 2018, 9, 35181–35194. [Google Scholar] [CrossRef]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical Features and Outcome of Patients With Non–Small-Cell Lung Cancer Who Harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bang, Y.-J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M.; Fox, S.B.; Riely, G.J.; Solomon, B.; Ou, S.-H.I.; Kim, D.-W.; et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study. Lancet Oncol. 2012, 13, 1011–1019. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Diagnosis and Treatment of Anaplastic Lymphoma Kinase–Positive Non–Small Cell Lung Cancer. Hema-Tology/Oncol. Clin. North Am. 2017, 31, 101–111. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.-W.; Mehra, R.; Tan, D.S.; Felip, E.; Chow, L.Q.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; et al. Ceritinib inALK-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef]
- Tang, H.; Jin, L.; Zhang, Z.; Jiang, Z.; Malik, Z. Comparison of Clinical Efficacy of Alectinib Versus Crizotinib in ALK-Positive Non-Small Cell Lung Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 646526. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Camidge, D.; Gadgeel, S.; Rosell, R.; Dziadziuszko, R.; Kim, D.-W.; Pérol, M.; Ou, S.-H.; Ahn, J.; Shaw, A.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Zou, Z.; Xing, P.; Hao, X.; Wang, Y.; Song, X.; Shan, L.; Zhang, C.; Liu, Z.; Ma, K.; Dong, G.; et al. Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases—a multicenter retrospective study. BMC Med. 2022, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in ALK Inhibitor–Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 2021, 16, 2091–2108. [Google Scholar] [CrossRef] [PubMed]
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef]
- Joshi, A.; Pande, N.; Noronha, V.; Patil, V.; Kumar, R.; Chougule, A.; Vaishakhi, T.; Janu, A.; Mahajan, A.; Prabhash, K. ROS1 mutation non-small cell lung cancer—access to optimal treatment and outcomes. Ecancermedicalscience 2019, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.; Riely, G.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.; Solomon, B.; Varella-Garcia, M.; Iafrate, A.; Shapiro, G.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef]
- Michels, S.; Massutí, B.; Schildhaus, H.-U.; Franklin, J.; Sebastian, M.; Felip, E.; Grohé, C.; Rodriguez-Abreu, D.; Abdulla, D.S.; Bischoff, H.; et al. Safety and Efficacy of Crizotinib in Patients With Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. J. Thorac. Oncol. 2019, 14, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Dziadziuszko, R.; Krebs, M.G.; De Braud, F.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion–Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 1253–1263. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; A Soo, R.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef]
- Salgia, R. MET in Lung Cancer: Biomarker Selection Based on Scientific Rationale. Mol. Cancer Ther. 2017, 16, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.E.; Salgia, R. MET molecular mechanisms and therapies in lung cancer. Cell Adhes. Migr. 2010, 4, 146–152. [Google Scholar] [CrossRef]
- Liang, H.; Wang, M. MET Oncogene in Non-Small Cell Lung Cancer: Mechanism of MET Dysregulation and Agents Targeting the HGF/c-Met Axis. OncoTargets Ther. 2020, 13, 2491–2510. [Google Scholar] [CrossRef]
- Fujino, T.; Suda, K.; Mitsudomi, T. Lung Cancer with MET exon 14 Skipping Mutation: Genetic Feature, Current Treatments, and Future Challenges. Lung Cancer Targets Ther. 2021, 12, 35–50. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.; Tan, D.S.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib inMETExon 14–Mutated orMET-Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.-H.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S.; et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Cappuzzo, F.; Ou, S.-H.; Camidge, D.R. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J. Thorac. Oncol. 2017, 12, 15–26. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.; Groen, H.; Tan, D.; Hida, T.; De Jonge, M.; Orlov, S.; et al. Results of the GEOMETRY mono-1 phase II study for evaluation of the MET inhibitor capmatinib (INC280) in patients (pts) with METΔex14 mutated advanced non-small cell lung cancer (NSCLC). Ann. Oncol. 2018, 29, viii741–viii742. [Google Scholar] [CrossRef]
- Drusbosky, L.M.; Rodriguez, E.; Dawar, R.; Ikpeazu, C.V. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J. Hematol. Oncol. 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Ulivi, P.; Verlicchi, A.; Cravero, P.; Delmonte, A.; Crinò, L. Targeting RET-rearranged non-small-cell lung cancer: Future prospects. Lung Cancer: Targets Ther. 2019, 10, 27–36. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.; Loong, H.H.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. New Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; A Cassier, P.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Cascetta, P.; Sforza, V.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Montanino, A.; Costanzo, R.; Sandomenico, C.; De Cecio, R.; et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415. [Google Scholar] [CrossRef]
- Bustamante, J.G.; Otterson, G.A. Agents to treat BRAF-mutant lung cancer. Drugs Context 2019, 8, 212566. [Google Scholar] [CrossRef]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Besse, B.; Groen, H.J.; Hashemi, S.M.; Mazieres, J.; Kim, T.M.; Quoix, E.; Souquet, P.-J.; Barlesi, F.; Baik, C.; et al. Phase 2 Study of Dabrafenib Plus Trametinib in Patients With BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J. Thorac. Oncol. 2021, 17, 103–115. [Google Scholar] [CrossRef]
- Abuali, I.; Lee, C.-S.; Seetharamu, N. A narrative review of the management of BRAF non-V600E mutated metastatic non-small cell lung cancer. Precis. Cancer Med. 2022, 13, 13. [Google Scholar] [CrossRef]
- Xia, H.; Xue, X.; Ding, H.; Ou, Q.; Wu, X.; Nagasaka, M.; Shao, Y.W.; Hu, X.; Ou, S.I. Evidence of NTRK1 Fusion as Resistance Mechanism to EGFR TKI in EGFR+ NSCLC: Results from a Large-Scale Survey of NTRK1 Fusions in Chinese Patients with Lung Cancer. Clin Lung Cancer 2020, 21, 247–254. [Google Scholar] [CrossRef]
- Liu, F.; Wei, Y.; Zhang, H.; Jiang, J.; Zhang, P.; Chu, Q. NTRK Fusion in Non-Small Cell Lung Cancer: Diagnosis, Therapy, and TRK Inhibitor Resistance. Front. Oncol. 2022, 12, 864666. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Tan, D.S.W.; Lassen, U.N.; Leyvraz, S.; Liu, Y.; Patel, J.D.; Rosen, L.; Solomon, B.; Norenberg, R.; Dima, L.; et al. Efficacy and Safety of Larotrectinib in Patients With Tropomyosin Receptor Kinase Fusion–Positive Lung Cancers. JCO Precis. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef]
- Karachaliou, N.; Mayo, C.; Costa, C.; Magrí, I.; Gimenez-Capitan, A.; Molina-Vila, M.A.; Rosell, R. KRAS Mutations in Lung Cancer. Clin. Lung Cancer. 2013, 14, 205–214. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.-H.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired Resistance to KRASG12C Inhibition in Cancer. N. Engl. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef] [PubMed]

| Therapeutic Target (Percentage in NSCLC) | Targeted Therapy | Effectiveness | Reference |
|---|---|---|---|
| EGFR mutation (15–50%) | Erlotinib | ORR 83% PFS 13.1 months | [54] |
| Gefitinib | ORR 84.6% PFS 8.4 months OS 30.6 months | [55] | |
| Dacomitinib | ORR 75.6% PFS 18.2 months. OS 34.1 months | [56] | |
| Afatinib | ORR 67.2% PFS 11.8 months | [57] | |
| Osimertinib | ORR 80% PFS 18.9 months. | [63,64] | |
| ALK rearrangement (7%) | Crizotinib | ORR 60% PFS 9.7 months. | [73] |
| Ceritinib | ORR 58% PFS 7 months. | [76] | |
| lorlatinib | ORR 76% PFS 18 months. | [75] | |
| Alectinib | ORR 90% PFS 34.8 months. | [77,78,79] | |
| Brigatinib | ORR 74% PFS 29.4 months | [80] | |
| ROS1 rearrangement (2%) | Crizotinib | ORR 70% PFS 20 months | [83,84] |
| Lorlatinib | ORR 62% PFS 21 months. | [86] | |
| Etrectinib | ORR 67% PFS 15.7 months. | [85] | |
| MET mutation (2%) | Capmatinib | ORR 68% PFS 12,4 months | [91] |
| Tepotinib | ORR 50% PFS 11 months | [92] | |
| RET rearrangement (2%) | Selpercatinib | ORR 64% PFS 16.5 months | [98] |
| Pralsetinib | ORR 61% PFS 7.3 months. | [99] | |
| BRAF mutation (4%) | Dabrafenib and trametinib | ORR 68.4% PFS 10 months | [103] |
| NTRK fussion (<1%) | Larotrectinib | ORR 73% PFS 35.4 months. | [107] |
| Entrectinib | ORR 57% PFS 11 months | [108] | |
| KRAS mutation (30%) | Sotorasib | ORR 37.1% PFS 6.8 months | [111] |
| Adagrasib | ORR 42.9% PFS 6.5 months | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.A.; Pekarek, L.; Navarro, F.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.Á.; Diez-Pedrero, R.; Boyano-Adánez, M.d.C.; Guijarro, L.G.; Barrena-Blázquez, S.; et al. Updated Views in Targeted Therapy in the Patient with Non-Small Cell Lung Cancer. J. Pers. Med. 2023, 13, 167. https://doi.org/10.3390/jpm13020167
Ortega MA, Pekarek L, Navarro F, Fraile-Martínez O, García-Montero C, Álvarez-Mon MÁ, Diez-Pedrero R, Boyano-Adánez MdC, Guijarro LG, Barrena-Blázquez S, et al. Updated Views in Targeted Therapy in the Patient with Non-Small Cell Lung Cancer. Journal of Personalized Medicine. 2023; 13(2):167. https://doi.org/10.3390/jpm13020167
Chicago/Turabian StyleOrtega, Miguel A., Leonel Pekarek, Fátima Navarro, Oscar Fraile-Martínez, Cielo García-Montero, Miguel Ángel Álvarez-Mon, Raúl Diez-Pedrero, María del Carmen Boyano-Adánez, Luis G. Guijarro, Silvestra Barrena-Blázquez, and et al. 2023. "Updated Views in Targeted Therapy in the Patient with Non-Small Cell Lung Cancer" Journal of Personalized Medicine 13, no. 2: 167. https://doi.org/10.3390/jpm13020167
APA StyleOrtega, M. A., Pekarek, L., Navarro, F., Fraile-Martínez, O., García-Montero, C., Álvarez-Mon, M. Á., Diez-Pedrero, R., Boyano-Adánez, M. d. C., Guijarro, L. G., Barrena-Blázquez, S., Gómez-Lahoz, A. M., Haro, S., Arroyo, M., Monserrat, J., Saez, M. A., & Alvarez-Mon, M. (2023). Updated Views in Targeted Therapy in the Patient with Non-Small Cell Lung Cancer. Journal of Personalized Medicine, 13(2), 167. https://doi.org/10.3390/jpm13020167









