Vascularized versus Free Nerve Grafts: An Experimental Study on Rats
Abstract
:1. Background
2. Materials and Methods
2.1. Surgical Procedure:
2.2. Evaluation
2.3. Muscle Weight
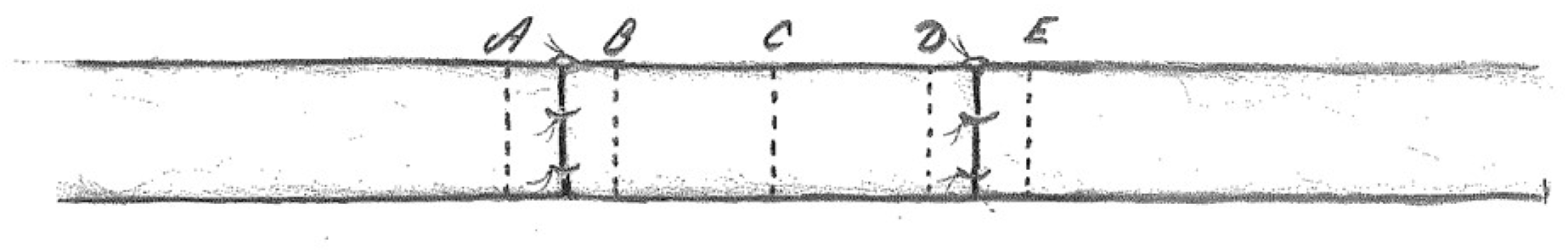
2.4. Histomorphology
- 2 mm proximal and 2 mm distal to the proximal nerve suture;
- in the middle part of the nerve graft;
- 2 mm proximal and 2 mm distal to the distal nerve suture (Figure 1).
- transverse nerve diameter;
- diameter and density of myelinated axons (axons/mm2);
- diameter and density of nerve fibers (fibers/mm2);
- neural/connective tissue area rate;
- number of axons/nerve fibers rate.
- microvascular density of the nerve.
2.5. Statistical Analysis
3. Results
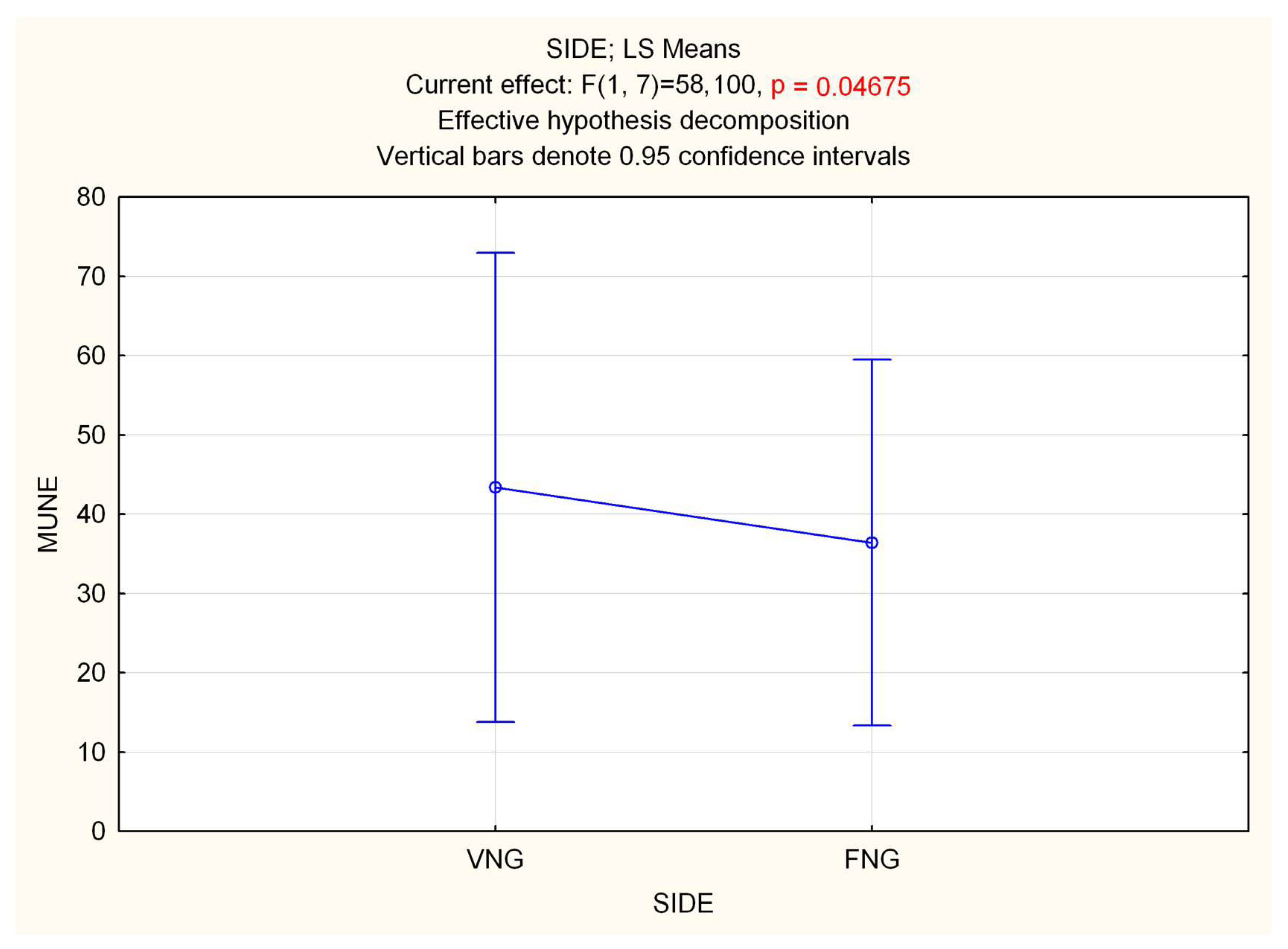
3.1. Electrodiagnostic Studies
3.2. Muscle Weight
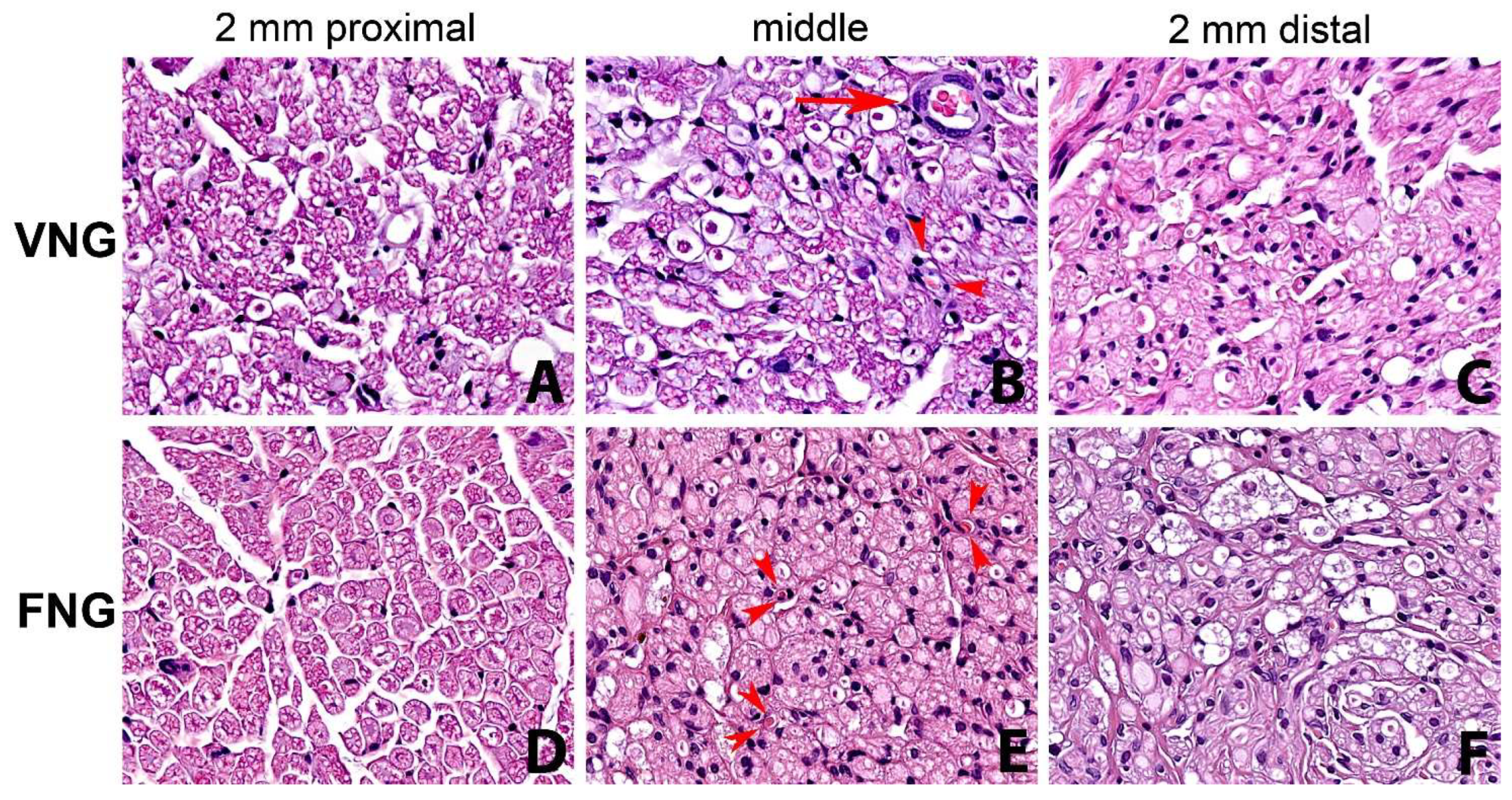
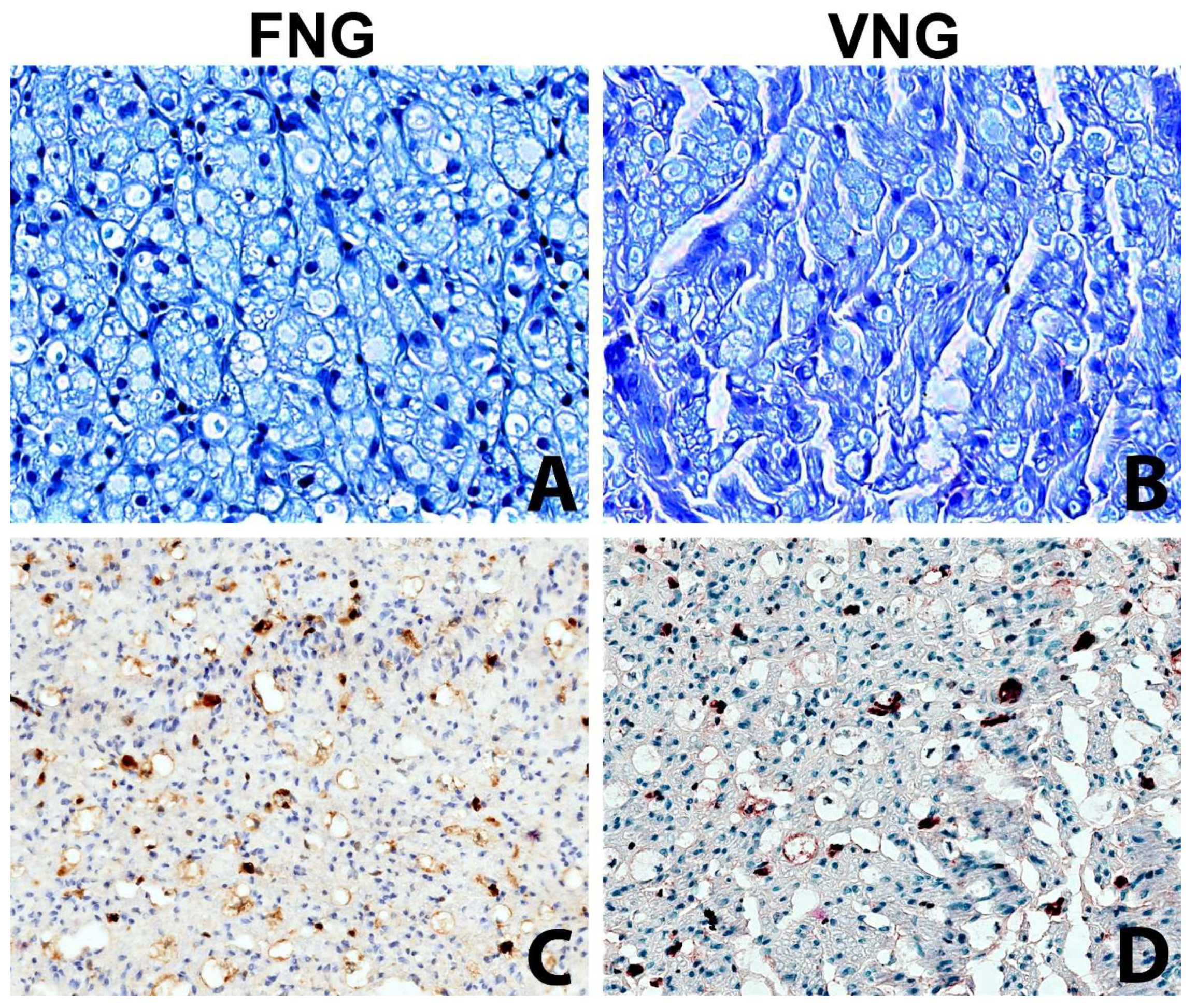
3.3. Histomorphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| VNG | Vascularized nerve grafts |
| FNG | Free nerve grafts |
| TA | Tibialis Anterior |
| GM | Gastrocnemius Medialis |
| SFI | Sciatic Functional Index |
References
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef] [PubMed]
- Broeren, B.O.; Duraku, L.S.; Hundepool, C.A.; Walbeehm, E.T.; Zuidam, J.M.; Hooijmans, C.R.; De Jong, T. Nerve recovery from treatment with a vascularized nerve graft compared to an autologous non-vascularized nerve graft in animal models: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252250. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Wu, L.P.; Zhao, M. Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering. Polymers 2023, 15, 2015. [Google Scholar] [CrossRef] [PubMed]
- Atti, V.N.; Fernandes, M.; Figueiredo, G.S.D.L.; Roth, F.; Valente, S.G.; Nakachima, L.R.; Fernandes, C.H.; Dos Santos, J.B.G. Peripheral nerve regeneration in rats using nerve graft in a vein conduit pre-filled with platelet-rich fibrin (PRF). Hand Surg. Rehabil. 2023, 42, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, Y.; Merle, M.; Michon, J.; Folliguet, B.; Barrat, E. Free vascularized nerve grafts: An experimental study in the rabbit. Microsurgery 1985, 6, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Tsai, T.M.; Firrell, J.; Breidenbach, W.C. Experimental comparison of vascularized and nonvascularized nerve grafting. J. Hand Surg. Am. 1988, 13, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.T.; Jiang, Q.W.; Su, G.L.; Shen, J.Z.; Shen, X. Free vascularized bone graft. Chin. Med. J. 1980, 93, 753–757. [Google Scholar]
- Yanagishita, S.; Otani, N.; Seike, S.; Tomita, K.; Kubo, T. Reconstruction of a Spinal Accessory Nerve Defect Using Vascularized Vastus Lateralis Motor Nerve Graft. Plast. Reconstr. Surg.-Glob. Open 2023, 11, E5174. [Google Scholar] [CrossRef]
- Usami, S.; Kawahara, S.; Inami, K.; Hirase, Y. Use of a vascularized dorsal sensory branch of an ulnar nerve flap for repairing a proper digital nerve with coverage of a volar soft tissue defect: Report of two cases. Microsurgery 2019, 39, 647–650. [Google Scholar] [CrossRef]
- Muangsanit, P.; Shipley, R.J. Vascularization Strategies for Peripheral Nerve Tissue Engineering. Anat. Rec. 2018, 1667, 1657–1667. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tsukuura, R.; Yamamoto, T.; Daniel, B.W. Nerve vascularity in free vascularized nerve flaps. Glob. Health Med. 2020, 2, 263–264. [Google Scholar] [CrossRef]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C. V Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, K.; Zemoodeh, H.R.; Zarenezhad, M.; Owji, M. “In Situ Vascular Nerve Graft” for Restoration of Intrinsic Hand Function: An Anatomical Study. J. Hand Surg. 2018, 23, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Poppler, L.H.; Davidge, K.; Lu, J.C.Y.; Armstrong, J.; Fox, I.K.; Mackinnon, S.E. Alternatives to sural nerve grafts in the upper extremity. Hand 2015, 10, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Saffari, T.M.; Bedar, M.; Hundepool, C.A.; Bishop, A.T.; Shin, A.Y. The role of vascularization in nerve regeneration of nerve graft. Neural Regen. Res. 2020, 15, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Toia, F.; Matta, D.; De Michele, F.; Pirrello, R.; Cordova, A. Animal models of vascularized nerve grafts: A systematic review. Neural Regen. Res. 2023, 18, 2615–2618. [Google Scholar] [CrossRef] [PubMed]
- Koshima, I.; Harii, K. Experimental study of vascularized nerve grafts: Morphometric study of axonal regeneration of nerves transplanted into silicone tubes. Ann. Plast. Surg. 1985, 14, 235–243. [Google Scholar] [CrossRef]
- Giacomini, P.S. Book Review: Electromyography and Neuromuscular Disorders: Clinical Electrophysiologic Correlations. McGill J. Med. 2020, 9, 20061. [Google Scholar] [CrossRef]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef]
- Cicero, L.; Licciardi, M.; Cirincione, R.; Puleio, R.; Giammona, G.; Giglia, G.; Sardo, P.; Edoardo Vigni, G.; Cioffi, A.; Sanfilippo, A.; et al. Polybutylene succinate artificial scaffold for peripheral nerve regeneration. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2022, 110, 125–134. [Google Scholar] [CrossRef]
- Stålberg, E.; van Dijk, H.; Falck, B.; Kimura, J.; Neuwirth, C.; Pitt, M.; Podnar, S.; Rubin, D.I.; Rutkove, S.; Sanders, D.B.; et al. Standards for quantification of EMG and neurography. Clin. Neurophysiol. 2019, 130, 1688–1729. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.; Barkhaus, P.E.; Nandedkar, S.D.; Swash, M. Motor unit number estimation (MUNE): Where are we now? Clin. Neurophysiol. 2018, 129, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Gordin, E.; Lee, T.S.; Ducic, Y.; Arnaoutakis, D. Facial Nerve Trauma: Evaluation and Considerations in Management. Craniomaxillofac. Trauma Reconstr. 2015, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Lazar, S.V.; Wang, A. Bioengineering approaches for nerve graft revascularization: Current concepts and future directions. Wiley Interdiscip. Rev. Syst. Biol. Med. 2023, 15, e1609. [Google Scholar] [CrossRef]
- Lee, J.Y.; Giusti, G.; Wang, H.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Functional evaluation in the rat sciatic nerve defect model: A comparison of the sciatic functional index, ankle angles, and isometric tetanic force. Plast. Reconstr. Surg. 2013, 132, 1173–1180. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Zhou, S.; Yu, Z.; Tian, Z.; Zhang, C.; Yang, W. Vascularized versus nonvascularized facial nerve grafts using a new rabbit model. Plast. Reconstr. Surg. 2015, 135, 331e–339e. [Google Scholar] [CrossRef]
- Mani, G.V.; Shurey, C.; Green, C.J. Is Early Vascularization of Nerve Grafts Necessary? J. Hand Surg. 1992, 17, 536–543. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Taleb, M.; Mira, J.C.; Calixto, J.B. Muscle fiber type reorganization and behavioral functional recovery of rat median nerve repair with vascularized or conventional nerve grafts. Restor. Neurol. Neurosci. 1996, 10, 5–12. [Google Scholar] [CrossRef]
- Donzelli, R.; Capone, C.; Sgulò, F.G.; Mariniello, G.; Maiuri, F. Vascularized nerve grafts: An experimental study. Neurol. Res. 2016, 38, 669–677. [Google Scholar] [CrossRef]
- Kemp, S.W.P.; Phua, P.D.; Stanoulis, K.N.; Wood, M.D.; Liu, E.H.; Gordon, T.; Borschel, G.H. Functional recovery following peripheral nerve injury in the transgenic Thy1 -GFP rat. J. Peripher. Nerv. Syst. 2013, 18, 220–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, N.; Cao, Y.; Wang, F.; Wang, P.; Ma, Y.; Lu, B.; Hou, G.; Fang, Z.; Liang, Z.; et al. Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording. Sci. Adv. 2019, 5, eaaw1066. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, T.; Lese, I.; Haberthür, D.; Zubler, C.; Hlushchuk, R.; Hewer, E.; Maistriaux, L.; Gianello, P.; Lengelé, B.; Rieben, R.; et al. Development of vascularized nerve scaffold using perfusion-decellularization and recellularization. Mater. Sci. Eng. C 2020, 117, 111311. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; You, Y.; Zhang, G.; Zhao, F.; Sha, Z.; Shen, Y. The use of fiber-reinforced scaffolds cocultured with schwann cells and vascular endothelial cells to repair rabbit sciatic nerve defect with vascularization. Biomed Res. Int. 2013, 2013, 362918. [Google Scholar] [CrossRef] [PubMed]
| Side | Level | Nerve Diameter (mm) | Axon Diameter (μm) | Axons/5 HPF | Fiber Diameter (μm) | Fiber/5 HPF | Neural/Connective Tissue | Axons/Fibers |
|---|---|---|---|---|---|---|---|---|
| Right | 1 | 1.01 | 58.33 | 2.53 | 8.43 | 84.33 | 3.33 | 0.59 |
| 2 | 1.45 | 61.67 | 3.27 | 8.83 | 105.33 | 3.33 | 0.61 | |
| 3 | 1.21 | 45.67 | 2.67 | 8.03 | 90.33 | 3 | 0.54 | |
| 4 | 1.13 | 28 | 2.07 | 7.53 | 57.33 | 2.67 | 0.49 | |
| 5 | 0.89 | 27.67 | 3 | 8.67 | 49.67 | 3 | 0.52 | |
| Left | 1 | 1.16 | 34 | 2.4 | 6.97 | 64.67 | 2.33 | 0.48 |
| 2 | 0.79 | 35 | 3.47 | 8.73 | 79.33 | 2 | 0.49 | |
| 3 | 0.84 | 27.67 | 2.7 | 6.9 | 55.33 | 2.33 | 0.58 | |
| 4 | 0.79 | 42.33 | 2.63 | 8.07 | 78.67 | 2.67 | 0.54 | |
| 5 | 0.65 | 19.33 | 2.1 | 7.27 | 40.67 | 2 | 0.47 | |
| p = 0.0378 | p = 0.0000 | p = 8.6465 | p = 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giglia, G.; Rosatti, F.; Giannone, A.G.; Gambino, G.; Zizzo, M.G.; Florena, A.M.; Sardo, P.; Toia, F. Vascularized versus Free Nerve Grafts: An Experimental Study on Rats. J. Pers. Med. 2023, 13, 1682. https://doi.org/10.3390/jpm13121682
Giglia G, Rosatti F, Giannone AG, Gambino G, Zizzo MG, Florena AM, Sardo P, Toia F. Vascularized versus Free Nerve Grafts: An Experimental Study on Rats. Journal of Personalized Medicine. 2023; 13(12):1682. https://doi.org/10.3390/jpm13121682
Chicago/Turabian StyleGiglia, Giuseppe, Fernando Rosatti, Antonino Giulio Giannone, Giuditta Gambino, Maria Grazia Zizzo, Ada Maria Florena, Pierangelo Sardo, and Francesca Toia. 2023. "Vascularized versus Free Nerve Grafts: An Experimental Study on Rats" Journal of Personalized Medicine 13, no. 12: 1682. https://doi.org/10.3390/jpm13121682
APA StyleGiglia, G., Rosatti, F., Giannone, A. G., Gambino, G., Zizzo, M. G., Florena, A. M., Sardo, P., & Toia, F. (2023). Vascularized versus Free Nerve Grafts: An Experimental Study on Rats. Journal of Personalized Medicine, 13(12), 1682. https://doi.org/10.3390/jpm13121682










