Abstract
Acute myeloid leukemia has a poor prognosis in older adults, and its management is often unclear due to its underrepresentation in clinical trials. Both overall survival (OS) and health-related quality-of-life (HRQoL) are key outcomes in this population, and patient-reported outcomes may contribute to patient stratification and treatment assignment. This prospective study included 138 consecutive patients treated in daily practice with the currently available non-targeted therapies (intensive chemotherapy [IC], attenuated chemotherapy [AC], hypomethylating agents [HMA], or palliative care [PC]). We evaluated patients’ condition at diagnosis (Life expectancy [Lee Index for Older Adults], Geriatric Assessment in Hematology [GAH scale], HRQoL [EQ-5D-5L questionnaire], and fatigue [fatigue items of the QLQ-C30 scale]), OS, early death (ED), treatment tolerability (TT) and change in HRQoL over 12 months follow-up. The median OS was 7.1 months (IC not reached, AC 5.9, HMA 8.8, and PC 1.0). Poor risk AML category and receiving just palliative care, as well as a higher Lee index score in the patients receiving active therapy, independently predicted a shorter OS. The Lee Index and GAH scale were not useful for predicting TT. The white blood cell count was a valid predictor for ED. Patients’ HRQoL remained stable during follow-up.
1. Introduction
Acute myeloid leukemia (AML) is the most commonly diagnosed type of acute leukemia [1] and occurs mainly in patients aged over 60 years. There are large differences between older and younger patients in clinical prognosis, with higher rates of early death (ED) and chemotherapy-related toxicities in the former [2,3]. The prognosis for older adults with AML can be estimated with the Medical Research Council/Leukemia Research Foundation (MRC/LRF), the MD Anderson Cancer Center, and the European LeukemiaNet prognostic scores [4,5,6], among others.
Despite the higher prevalence of this hematologic cancer in the elderly, its management is still unclear in this patient population [7]. Fit older adults treated by intensive chemotherapy (IC), especially if they are included in the good risk category or followed by either allogeneic hematopoietic cell transplantation (HCT) or oral azacitidine maintenance, have prolonged their overall survival (OS) [8,9,10,11]. Nevertheless, many older patients have worse health status (poor performance status, multimorbidity, polypharmacy, malnutrition, cognitive impairment, etc.) and may have difficulties in following the above-mentioned clinical pathway and have reduced survival [11,12]. The available options for old and unfit patients are hypomethylating agents (HMA), such as azacitidine or decitabine [13,14,15,16], attenuated standard chemotherapy (AC), and palliative care (PC). Recently, the combination of azacitidine and venetoclax has shown improved overall survival, as compared to azacitidine plus placebo, in newly diagnosed unfit patients [17].
It is crucial to evaluate patient characteristics and disease heterogeneity thoroughly to offer appropriate personalized therapy [18,19]. Currently, patient evaluation is mainly based on traditional factors, including age, Eastern Cooperative Oncology Group (ECOG) performance status, and comorbidity, but this approach does not fully describe these patients [20]. Patient-reported outcomes (PROs) such as health-related quality-of-life (HRQoL), fatigue perception, etc., are currently gaining relevance in treatment decision-making. Studying the impact of both traditional factors and PROs in a multidimensional evaluation will help us to understand the reasons underlying treatment assignment and guide the choice of the most appropriate therapy. A prominent unmet clinical need is the lack of straightforward tools to predict early death (ED) and treatment tolerability (TT) in patients receiving the different forms of therapy considered since most available information comes from the IC setting [3,5,12,15,21,22,23,24,25].
In light of the above, we aimed to describe the OS of older adults diagnosed with AML and the change in their HRQoL as a function of the treatment strategy: to analyze the relative prognostic value for OS of life expectancy, geriatric assessment, fatigue, and HRQoL at diagnosis, adjusted for MRC/LRF risk categories; to study in depth the impact of white blood cell count (WBC) and other covariates on ED and TT; and, lastly, to describe the transfusion burden and the need for hospitalization for each treatment strategy, as well as the hematopoietic cell transplantation (HCT) rate.
2. Materials and Methods
2.1. Study Design and Participants
SVLMA was a prospective observational study carried out by the Departments of Hematology of 40 Spanish hospitals according to the Helsinki Declaration and local regulations. It was approved by the Independent Ethics Committee of Hospital Clínico San Carlos (Madrid, Spain), and all patients gave their written informed consent.
This study included all patients meeting the selection criteria who were consecutively recruited from February 2018 to April 2019. Eligible patients were aged ≥60 years and diagnosed with AML as defined in the World Health Organization (WHO) 2016 criteria [26]. Patients with previously treated AML or acute promyelocytic leukemia were excluded.
Included patients were treated with all strategies available at the outset of the study in clinical practice: IC, AC, HMA, or PC. IC comprised any “3 + 7” scheme including cytarabine daily doses 100 mg/sqm and over, in combination with either (i) daunorubicin at a daily dose of at least 60 mg/sqm (or equivalent anthracycline dose) or (ii) fludarabine (any dose), and the FLAG-IDA scheme. Lower doses of the same drugs or shorter schedules were considered AC. Each therapy was administered at the discretion of the participating physician and according to routine clinical practice.
2.2. Assessments and Endpoints
Patients were followed up for 12 months and assessed at 5 timepoints: baseline/selection visit and four consecutive visits every 3 months (at 0, 3, 6, 9, and 12 months).
The primary study endpoint was the median OS, defined as the time from diagnosis to death from any cause. Secondary efficacy endpoints included 1-year survival rate, treatment strategies, ED, TT, and change in patient HRQoL. We also studied the potential impact of patient conditions (life expectancy, performance status, geriatric assessment, and fatigue scores) on ED and TT, as well as the potential impact of WBC at diagnosis on ED. Additionally, data about treatment response, transfusion burden, need for hospitalization, HCT rate, and the date and reason of death were also collected.
HRQoL was measured by the EuroQoL-5L-5D questionnaire [27], and fatigue was scored (FA-score) by using the 3 fatigue items of the QLQ-C30 [28]. Life expectancy (at 4 years) before AML diagnosis was calculated with the Lee Index for Older Adults [29]. Geriatric assessment was performed with the aid of the Geriatric Assessment in Hematology (GAH) scale [30,31]. Details on these scales can be consulted in the Supplementary Materials.
2.3. Statistical Methods
Median OS and 1-year survival rate were analyzed using the Kaplan-Meier method. Those patients receiving HCT were censored. A Cox regression multivariate analysis was used to determine the impact of the covariates to predict OS. Variables showing significant differences (p < 0.150) in the univariate analyses were included in a multivariate analysis.
ED was defined as the patient’s death within the first 8 weeks of treatment initiation [32].
TT was measured as the number of patients that needed either treatment termination or modification (i.e., at the patient’s request, when the patient had any adverse event that was considered severe or life-threatening by the investigator, and also when the patient experienced any deterioration of previous comorbidities or early death as a consequence of the adverse event). In addition, the physician’s subjective opinion on the observed patient tolerability on a scale of 0 (minimal) to 10 (maximal) was also collected.
Treatment response was defined following the European LeukemiaNet criteria [6].
Transfusion burden was calculated as the number of packed red blood cell concentrates (PRBC) and the number of platelet transfusion procedures received by each patient during follow-up. Need for hospitalization was analyzed as the proportion of patients requiring hospitalization (excluding hospice or palliative care stays) during follow-up, as well as the proportion of days that the patients spent at the hospital as in-patients during the observation time.
The impact of WBC on ED was analyzed by ANCOVA (accounting for time from diagnosis to treatment initiation) and a receiver operating characteristics (ROC) curve. The area under the curve (AUC) was calculated to identify the cut-off point, maximizing sensitivity and specificity. HRQoL was descriptively analyzed, including the frequency distributions of EQ-5D-5L dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and measures of central tendency and dispersion of EQ-VAS and preference indexes; the latter was also analyzed using repeated-measures linear models. The Lee index and GAH scale validity as treatment-tolerability predictive values were analyzed by a full multiple linear regression model and multiple logistic regression. Optimal cut-off points were determined by ROC analysis.
All the variables were analyzed in the overall series and adjusted by treatment. Missing data were not imputed and were left as missing. The hypothesis tests used were two-sided and with a significance level of 0.05. IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA) was used for all the analysis.
3. Results
3.1. Patient Disposition and Frontline Treatment
A total of 151 patients were enrolled in the study, 13 of whom did not meet inclusion criteria, so 138 were finally evaluable (Figure 1, panel a). All treatments were administered according to the physician’s criteria and the local clinical practice. The study flow is displayed in Figure 1, panel b. and patient characteristics are shown in Table 1. Median (interquartile range, IQR) patient age was 75.2 (68.6–80.6) years, and most were male (57.2%). Patient ECOG performance status was predominantly good (ECOG 0–1 72.6%), but 27.4% of patients had an ECOG score of 2–3 (Table 1); 35 patients out of 137 (25.5%) had a previous hematological disease, and 27 (19.6%) had previously received chemotherapy and/or radiotherapy.

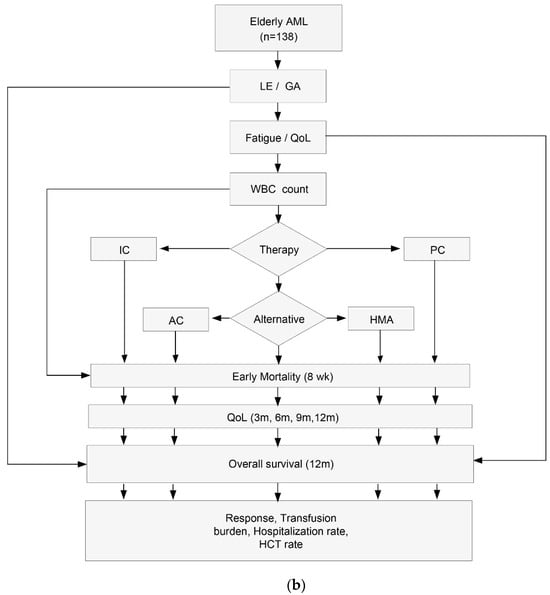
Figure 1.
(a) Patient enrollment flowchart. (b). Study flowchart. AML, acute myeloid leukemia; LE, Life expectancy; GA, Geriatric assessment; QoL, quality-of-life; WBC, white blood cell count; IC, intensive chemotherapy; AC, attenuated chemotherapy; HMA, hypomethylating agents; PC, palliative care; wk, week; m, month; HCT, hematopoietic cell transplantation.

Table 1.
Patient characteristics at diagnosis.
Fifty-five patients (39.9%) had an abnormal karyotype, and in 17 (13.2%) it was complex. In one case of the HMA group, cytogenetics was not available. Among the patients with normal cytogenetics, the prevalence of mutations in the genes NPM1, FLT3, and CEBPA was 9.3%, 18.6%, and 3.2%, respectively. Details on WHO classification are displayed in Figure 2 (genomic data available in the Supplementary Materials, Table S1).
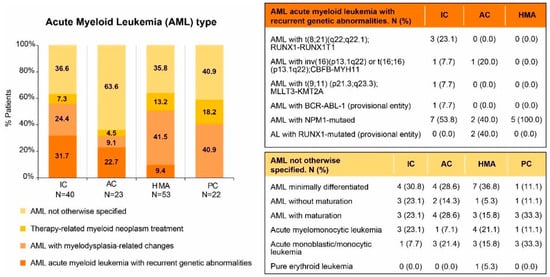
Figure 2.
AML type following WHO 2016 classification criteria. AC, attenuated chemotherapy; AML, acute myeloid leukemia; IC, intensive chemotherapy; HMA, hypomethylating agents; PC, palliative care.
Most patients (54.3%) were included in the poor MRC/LRF risk category. Good MRC/LRF risk score was predominant in the patients receiving IC, while poor risk score was predominant in the rest of the patients (Figure 3). These differences were statistically significant (p < 0.001).
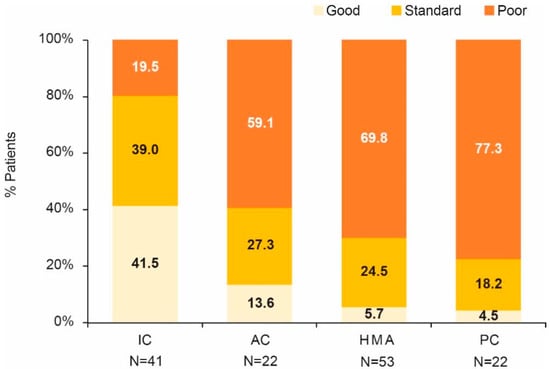
Figure 3.
MRC/LRF categories by treatment group. AC, attenuated chemotherapy; IC, intensive chemotherapy; HMA, hypomethylating agents; PC, palliative care.
The need for PRBC at diagnosis was maximal for patients who were later assigned to PC and minimal for those assigned IC (Figure S1), but the differences between groups were not statistically significant (p = 0.101).
Life expectancy prior to AML diagnosis was maximal in the IC group, minimal in the PC, and intermediate in those treated by AC and HMA (Table 1 and Figure 4). Patient condition, according to the GAH scale, was intermediate and similar across treatment groups (Table 1). Some GAH dimension subscores, more specifically, the number of drugs, difficulties in performing activities of daily life, comorbidities, and smoking habits, were statistically different between the treatment strategies (Table S2). EQ-5D-VAS and fatigue were also similar in the different groups (Table 1; details on the different domains are shown in Table S3).

Figure 4.
Lee Index for Older Adults by treatment group. (a) Distribution of Lee Index Score by quartiles. (b). Distribution of Lee Index Score by categories (score < 6 pts and score ≥ 6 pts). AC, attenuated chemotherapy; IC, intensive chemotherapy; HMA, hypomethylating agents; PC, palliative care.
One hundred and thirty-eight patients initiated frontline therapy according to standard clinical practice (Table S4). The mean time (±SD) from diagnosis to therapy was 9.8 ± 14.3 days. Forty-one patients (29.7%) received IC, 22 (15.9%) AC, 53 (38.4%) HMA, and 22 (15.9%) did not receive active therapy but just PC.
3.2. Overall Survival and Prognostic Factors
Fifty-six patients (40.6%) died during follow-up. Median OS for the overall series was 7.1 months (95%CI 4.7–9.5), with significant differences by treatment groups: median IC not reached; AC, 5.9 (95% CI 3.3–8.5); HMA, 8.8 (95% CI 6.5–11.0), and PC 1.0 (95% CI 0.2–1.8) (p < 0.001) (Figure 5).

Figure 5.
Overall survival by treatment group. AC, attenuated chemotherapy; IC, intensive chemotherapy; HMA, hypomethylating agents; PC, palliative care.
Overall, the 1-year survival rate was 31.9% (95% CI 23.1–40.7). When stratified by treatment strategy, 1-year survival was 61.7% (95% CI 43.8–79.6) for patients on IC, 29.8% (95% CI 9.0–50.6) for AC, and 29.5% (95% CI 16.5–42.6) for HMA (Figure S2). Given the absence of data at 12 months follow-up, this variable could not be calculated for patients on PC.
As regards the impact of variables predicting OS, multivariate analysis showed that patients in the MRC/LRF poor category (HR = 2.69, 95% CI = 1.66–4.37, p < 0.001) and those receiving PC (HR = 4.97, 95% CI = 2.93–8.43, p < 0.001) had a higher mortality risk. No other relevant prognostic factors for OS were identified.
3.3. Early Death: Impact of WBC at Diagnosis
Twenty-eight patients (21.5%) died before 8 weeks from diagnosis, fourteen of whom (50%) were receiving PC.
The study hypothesis set out that WBC could be a relevant predictor of ED. When we compared the mean (±SD) WBC of the patients who died early vs. the WBC of those surviving that period, we found higher values for patients with ED (55.0 ± 79.7 × 109/L vs. 19.1 ± 45.7 × 109/L; p < 0.001) and a positive correlation between ED proportion and WBC strata (p = 0.002, Figures S3 and S4). The ANCOVA analysis, performed from diagnosis to treatment onset, showed similar results (53.0 × 109/L, 95% CI 32.2–73.8 vs. 19.6 × 109/L, 95% CI 8.9–30.4; p = 0.006). These results were confirmed by a ROC analysis, demonstrating the validity of WBC for predicting ED in the overall series (AUC = 0.718, 95% CI 0.606–0.829, p < 0.001) at the expense of patients treated with alternative chemotherapy (either AC or HMA; AUC = 0.805, 95% CI = 0.698–0.911, p = 0.002), but could not be confirmed for those receiving IC (p = 0.231). The optimal cut-off was 5.8 × 109 leucocytes/L in the overall series and 9.5 × 109 leucocytes/L in those patients receiving alternative chemotherapy.
3.4. Treatment Tolerability
TT, according to the investigator, was considered intermediate: overall (mean ± SD) 6.4 ± 2.9; IC 6.0 ± 2.7; AC 5.5 ± 3.4 and HMA 6.9 ± 2.3. Objective TT was poor in 30/116 (25.9%) of the patients receiving active antileukemic treatment, with no statistically significant differences between the treatment groups (IC 24.4%, AC 22.7%, HMA 28.3%, p = 0.85). Reasons for treatment termination and types of treatment modification due to toxicity are shown in Tables S5 and S6.
3.5. Treatment Response
The response could not be evaluated in 34 out of 116 (29.3%) patients receiving active treatment, mainly because of the lack of longitudinal bone marrow data. The overall response rate was 54.3% (45/81). By treatment groups, IC-treated patients had a higher response rate than those receiving alternative chemotherapy (IC 28/34, 82.4%; AC or HMA 16/47, 34.0%; p < 0.001).
3.6. Patient Condition and Patient-Reported Outcomes
Neither Lee index/GAH scores nor fatigue score at diagnosis/EQ-VAS showed independent prognostic value on OS in the overall series (Table 2). When we restricted the OS analysis to those patients receiving active therapy (i.e., excluding PC), MRC/LRF poor category (HR = 2.02, 95% CI = 1.11–3.64, p = 0.020) and a higher Lee score (HR = 1.08, 95%CI = 1.002–1.156, p = 0.044) were the only covariates linked to a higher mortality risk.

Table 2.
Analysis of prognostic factors for overall survival (N = 138).
Lee and GAH scores were not associated with ED (p = 0.142 and p = 0.310, respectively), and as regards TT, neither of them can be considered appropriate tools for predicting TT (AUC 0.429, 95% CI 0.311–0.547, p = 0.222 and AUC 0.684, 95% CI 0.521–0.847, p = 0.140; respectively).
Most patients were allocated to the most favorable category of each EQ-5D dimension, and this status was maintained until the end of the study (Figure 6). The score obtained in the preference index was stable during the study follow-up and closer to 1 (perfect health) than to 0 (death). From month 6 onwards, data from patients on PC care were no longer available due to the high mortality rate, so they were not included in the comparative analysis. After 12 months of follow-up, EQ-VAS results indicated a significant improvement in HRQoL in the overall population (p = 0.016), and specifically in patients on HMA treatment (p = 0.040), but the preference index was not statistically different in either of them (p = 0.66, and p = 0.08, respectively; Table 3).
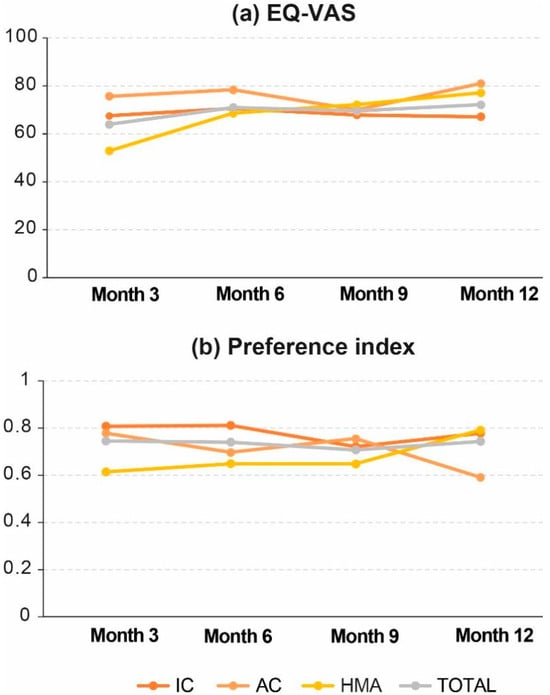
Figure 6.
Change in HRQoL during follow-up: (a) EQ-VAS and (b) Preference index. AC, attenuated chemotherapy; IC, intensive chemotherapy; HMA, hypomethylating agents; PC, palliative care.

Table 3.
HRQoL evaluation throughout study visits.
3.7. Transfusion Burden, Hospitalization and Transplantation
Most patients (71.7%) required PRBC transfusion during the study follow-up. We did not observe statistically significant differences in the number of PRBC transfused between the treatment strategies. However, for the first 3 months of study follow-up, the number of PRBC (mean ± SD) transfused was maximal in IC (15.2 ± 10.1) and AC (16.4 ± 10.4) groups, minimal in the PC subset (9.5 ± 7.6) and intermediate in those patients treated by HMA group (10.6 ± 7.3) (p = 0.045).
Concerning platelet transfusions, they were needed by 56.5% of the patients and followed the same trend observed for the PRBC (14.5 ± 11.0, 11.7 ± 9.1, 8.1 ± 8.4 and 10.1 ± 9.5, respectively, p = 0.12). The number of platelet transfusions received by patients in the different treatment groups was similar for the rest of the follow-up.
Hospital admission due to SAEs occurring during the study follow-up was required for 73 (52.9%) patients and was more frequent in IC and AC groups than in the patients on HMA or PC (65.9%, 63.6%, 50.9%, and 22.7%, respectively, p = 0.008). Nevertheless, the proportion of hospitalization days out of days under observation was similar in all treatment groups (p = 0.332).
Eleven patients (8.0% of patients recruited, 9.5% of those receiving active therapy) were transplanted (four autologous and seven allogeneic), ten had received IC (24.4% of patients receiving such therapy), and one had received AC (4.5% of patients receiving AC).
4. Discussion
The prospective observational SvLMA study described survival and HRQoL, as well as clinical characteristics and management, of patients older than 60 years treated with any of the currently available non-targeted strategies for AML in Spain (2018–2019). Our data revealed an acceptable overall patient condition and a predominantly poor MRC/LRF score, along with considerable variability between treatment groups. Median OS was 7.1 months (95% CI 4.68–9.53), and 1-year survival rate was 31.9% (95% CI 23.1–40.7). HRQoL remained stable throughout the study.
Treatment strategies ranged in our study from intensive chemotherapy (29.7%) to palliative care (15.9%) and included alternative chemotherapy (54.3%). The most common treatment option in our series was HMA (38.4%). The proportion of patients receiving IC in our study differs from other recent series [33,34] in which IC was predominant (44–54%). As regards the proportion of elderly patients with AML receiving just PC, our figure is lower than that observed in Europe (24–35%) and within the range documented in the USA (10.0–61.4%) [35]
The OS of older adults with AML reported in previous research shows wide variations as a likely consequence of differences in the studied populations and the time frame in which the treatment was administered. A population-based study carried out in the US [1] reported that 5-year OS has improved in patients aged 60–69 years from 4% in the 1980s to 24% in 2010–2017, while the improvement was very slight in those aged 70 and over (from 1% to 5% in the same time periods). The median OS of 3637 AML patients aged 60 years and over treated in Spain and reported to the PETHEMA registry between 1999 and 2013 [33] was 4.7 months, and 1-year and 5-year OS were 29% and 7%, respectively, without significant differences between treatment periods. In our study and that of PETHEMA, the median age was over 70 years (75 and 72, respectively), and the OS in the different treatment groups was short (1.0 and 1.2 months for PC; 5.9 months and 7.8 months for alternative chemotherapy; median not reached and 10.3 months for IC; respectively); 1-year OS was also similar (31.9% and 29%). HMA-treated patients in our study showed a median OS (8.8 months) similar to that obtained in a population-based study evaluating survival in decitabine and azacitidine-treated patients in the USA [36] and other European academic series [15,16].
Our data show that the MRC/LRF (which integrates cytogenetics, WBC, ECOG, age, and secondary AML) poor risk category and PC are associated with poor OS. Lee index can be used to estimate the 4-year life expectancy of any patient older than 50 years before AML diagnosis, and in our series, was associated with OS, as we also described in myelodysplastic syndromes [37], in those patients receiving active therapy. Importantly, the patients that were assigned to IC not only had a lower proportion of poor-risk MRC/LRF category than the other participating patients but also a better life expectancy prior to AML diagnosis. By contrast, we did not observe an independent association between geriatric assessment (GAH score) and OS when life expectancy was included in the model. Geriatric assessment is claimed to be a good predictor of OS in elderly AML [38], but this has only been demonstrated for some of their constitutive dimensions (i.e., cognitive function and objective physical performance) [39], and others did not find any association between physical performance and OS [40].
Fatigue score at diagnosis was not associated with OS in our patients, in contrast to what has been published in myelodysplastic syndromes [28]. The potential contribution of HRQoL for OS prediction in older adults with AML is controversial [40,41], and in our experience, EQ-5D-5L EQ-VAS at diagnosis was not associated with OS.
At present, few hematologists resort to the physician’s clinical eye (i.e., follow a gestalt approach [42]) for treatment recommendation to older adults with AML, while most use a more analytic one mainly based on age, ECOG, comorbidity burden, early death rate, etc. [12,21,24,43]. A physician’s personality and behavioral traits may also have a role in treatment assignment [44]. Our results on the Lee index score and on the selected dimensions of geriatric assessment and HRQoL evaluation agree with the published literature, showing that there is still much room for improvement in this field and that patient condition at diagnosis, including patients’ life expectancy prior to AML, should be studied thoroughly.
ED in elderly AML patients is a critical issue, and WBC is a commonly cited predictor [5,15,21,23]. In our study, we observed that WBC was significantly higher in patients who died before 8 weeks, both in the overall population and in the patients treated with HMA. The ROC analyses confirmed the validity of WBC as a predictor of ED in those populations. Nevertheless, a recent study [24] from a large cohort of patients (15–99 years old) treated with IC did not identify WBC as a predictor of ED (4 weeks). Although the new predictive scheme was also validated in a cohort of patients receiving low-intensity therapy, WBC was not directly analyzed as a predictor of ED in this patient subset. Our data suggest that the optimal cut-off may be heterogeneous for the different treatment strategies, and we also hypothesize that it might also be different for different age ranges. Even though the GAH proved reliable in assessing health status and validity to predict clinical changes [31], the results obtained in our study do not support its role as an ED predictive factor. Similarly, despite the Lee index score being independently associated with OS, it was not an appropriate predictor of ED in our series. Neither Lee index nor GAH scores were associated with TT, according to our data. Nevertheless, further research in a larger patient series should be conducted to confirm these findings before excluding the Lee index for older adults and the GAH scale as ED or TT predictors.
HRQoL evaluation is acquiring increasing importance in the decision-making process in hematologic and solid neoplasms, but there are still barriers to overcome in older adults diagnosed with AML, mainly due to the difficulty of retrieving this type of information from retrospective studies, the still anecdotal presence of HRQoL data in AML registries [45], and the lack of consensus on the best validated specific AML-HRQoL tool [46,47,48]. Fortunately, HRQoL evaluation in comparative trials on older adults with AML has become more frequent in recent times. According to our data, HRQoL improved slightly during follow-up for the overall population and, more specifically, for the HMA-treated population, but the difference was not clinically relevant since the preference values remained stable. Others have detected a functional and HRQoL decline in patients treated by IC that recovers slowly and may last 3 years or more [40,49]
Nowadays, HCT procedures have acquired a high technical level and are being used more frequently and safely in older AML adults [50]. Nevertheless, the actual proportion of older adults with AML being allotransplanted in clinical practice is extremely, small and our data (8.0%) are in agreement with other recent sources [33,51].
The SvLMA study has several limitations: (i) We have not analyzed targeted therapies because, at the outset of the study (2018), they were very infrequent in clinical practice. (ii) Genomic studies were not included in the analysis because the number of genes analyzed was very heterogeneous, and this fact precluded the use of the European LeukemiaNet prognostic scoring system. (iii) Sample size was calculated for the primary endpoints (OS and HRQoL), and consequently, the power for subgroup analysis is limited. Our study also has several strengths: its prospective and multicenter design, the comprehensive approach to the evaluation of the patient condition, and the additional evaluation of several PROs provide meaningful information about the prognosis and clinical management of older adults diagnosed with AML in Spain.
5. Conclusions
This real-world study showed a significant variation in overall survival between the different treatment groups. The MRC/LRF poor risk category and palliative treatment were associated with poor OS, while the MRC/LRF poor risk category and a higher Lee index score were associated with shorter OS in those patients receiving active therapy. The WBC successfully predicted early mortality in the overall population and in HMA-treated individuals. We could not predict treatment tolerability in any of the treatment groups using the GAH scale and the Lee index. HRQoL remained stable during follow-up, and the HCT rate was very low and limited to the patients treated by IC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13121667/s1, Figure S1. Transfusion dependence at diagnosis by treatment subgroup. Figure S2. Proportion of patients alive at 12 months by treatment group. Figure S3. Proportion of patients dying before day 56 by WBC strata. Figure S4. Comparison of white blood cell counts between patients dying and surviving at 8 weeks by treatment group. Table S1. Molecular Findings at Diagnosis. Table S2. Geriatric Assessment in Hematology (GAH) Domains. Table S3. HRQoL Domains of EQ-5D-5L Questionnaire at Diagnosis. Table S4. Frontline Treatment Strategy. Table S5. Reasons for Treatment Termination by Treatment Group. Table S6. Treatment Modifications by Treatment Group.
Author Contributions
F.R. designed the study with the assistance of M.R. and R.L.; J.S., M.L.H., M.F.-N., P.M., C.R.-M., M.B., F.I., T.B., M.T.O., M.Á.Á., M.V., T.C.-V., B.G., A.A., L.G., P.F., M.A.D. and F.R. treated patients and collected the data. F.R. reviewed data and statistical analyses and provided critical input and wrote the initial draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the article processing charges were funded by Celgene Spain (a Bristol Myers Squibb company).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Hospital Clínico San Carlos (Madrid, Spain) (protocol code: CEL-LMA-2017-01; approved on 31 July 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and Supplementary Materials. BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-andresearch/disclosure-commitment.html.
Acknowledgments
Medical writing and editorial assistance was provided by Reyes Prieto, and statistical analysis was performed by Dolores Pérez, Laura Casas, and Nuria Pajuelo from Evidenze Health España S.L. Both activities were funded by Celgene Spain (a Bristol Myers Squibb company).
Conflicts of Interest
CRM has received research grants from Astellas, speaker honorarium from BMS, Astellas, and Abbvie, and travel grants from Servier and BMS. MR is an employee and RL was an employee of Bristol Myers Squibb Company, Celgene. The rest of the authors declare no conflict of interest.
References
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef]
- Wheatley, K.; Brookes, C.L.; Howman, A.J.; Goldstone, A.H.; Milligan, D.W.; Prentice, A.G.; Moorman, A.V.; Burnett, A.K. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br. J. Haematol. 2009, 145, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; O’Brien, S.; Cortes, J.; Giles, F.; Faderl, S.; Jabbour, E.; Garcia-Manero, G.; Wierda, W.; Pierce, S.; Shan, J.; et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 2006, 106, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.H. Acute myeloid leukemia: 2021 update on risk-stratification and management. Am. J. Hematol. 2020, 95, 1368–1398. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef]
- Appelbaum, F.R. Impact of allogeneic hematopoietic cell transplantation on the outcome of older patients with acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2017, 30, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Devillier, R.; Forcade, E.; Garnier, A.; Guenounou, S.; Thepot, S.; Guillerm, G.; Ceballos, P.; Hicheri, Y.; Dumas, P.Y.; Peterlin, P.; et al. In-depth time-dependent analysis of the benefit of allo-HSCT for elderly patients with CR1 AML: A FILO study. Blood Adv. 2022, 6, 1804–1812. [Google Scholar] [CrossRef]
- Wei, A.H.; Dohner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Ravandi, F.; O’Brien, S.; Cortes, J.; Faderl, S.; Garcia-Manero, G.; Jabbour, E.; Wierda, W.; Kadia, T.; Pierce, S.; et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010, 116, 4422–4429. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Gattermann, N.; Germing, U.; Sanz, G.; List, A.F.; Gore, S.; Seymour, J.F.; et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 562–569. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.; Thepot, S.; Pleyer, L.; Maurillo, L.; Itzykson, R.; Bargay, J.; Stauder, R.; Venditti, A.; Seegers, V.; Martinez-Robles, V.; et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: Clinical use and outcome prediction. Leuk. Res. 2015, 39, 296–306. [Google Scholar] [CrossRef]
- Falantes, J.; Pleyer, L.; Thepot, S.; Almeida, A.M.; Maurillo, L.; Martinez-Robles, V.; Stauder, R.; Itzykson, R.; Pinto, R.; Venditti, A.; et al. Real life experience with frontline azacitidine in a large series of older adults with acute myeloid leukemia stratified by MRC/LRF score: Results from the expanded international E-ALMA series (E-ALMA+). Leuk. Lymphoma 2018, 59, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Recher, C.; Rollig, C.; Berard, E.; Bertoli, S.; Dumas, P.Y.; Tavitian, S.; Kramer, M.; Serve, H.; Bornhauser, M.; Platzbecker, U.; et al. Long-term survival after intensive chemotherapy or hypomethylating agents in AML patients aged 70 years and older: A large patient data set study from European registries. Leukemia 2022, 36, 913–922. [Google Scholar] [CrossRef]
- Almeida, A.M.; Ramos, F. Acute myeloid leukemia in the older adults. Leuk. Res. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Schiffer, C.A. “I am older, not elderly,” said the patient with acute myeloid leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 521–523. [Google Scholar] [CrossRef]
- Scott, J.M.; Stene, G.; Edvardsen, E.; Jones, L.W. Performance Status in Cancer: Not Broken, But Time for an Upgrade? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2824–2829. [Google Scholar] [CrossRef]
- Malfuson, J.V.; Etienne, A.; Turlure, P.; de Revel, T.; Thomas, X.; Contentin, N.; Terre, C.; Rigaudeau, S.; Bordessoule, D.; Vey, N.; et al. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica 2008, 93, 1806–1813. [Google Scholar] [CrossRef]
- Krug, U.; Rollig, C.; Koschmieder, A.; Heinecke, A.; Sauerland, M.C.; Schaich, M.; Thiede, C.; Kramer, M.; Braess, J.; Spiekermann, K.; et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: A web-based application for prediction of outcomes. Lancet 2010, 376, 2000–2008. [Google Scholar] [CrossRef]
- Walter, R.B.; Othus, M.; Borthakur, G.; Ravandi, F.; Cortes, J.E.; Pierce, S.A.; Appelbaum, F.R.; Kantarjian, H.A.; Estey, E.H. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: A novel paradigm for treatment assignment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kadia, T.; Begna, K.; DiNardo, C.D.; Borthakur, G.; Short, N.J.; Jain, N.; Daver, N.; Jabbour, E.; Garcia-Manero, G.; et al. Prediction of early (4-week) mortality in acute myeloid leukemia with intensive chemotherapy. Am. J. Hematol. 2022, 97, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.J.; Seftel, M.D.; Richardson, C.; Barbaric, D.; Barnett, M.J.; Bruyere, H.; Forrest, D.L.; Horsman, D.E.; Smith, C.; Song, K.; et al. Leukocyte count as a predictor of death during remission induction in acute myeloid leukemia. Leuk. Lymphoma 2006, 47, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Efficace, F.; Gaidano, G.; Breccia, M.; Voso, M.T.; Cottone, F.; Angelucci, E.; Caocci, G.; Stauder, R.; Selleslag, D.; Sprangers, M.; et al. Prognostic value of self-reported fatigue on overall survival in patients with myelodysplastic syndromes: A multicentre, prospective, observational, cohort study. Lancet Oncol. 2015, 16, 1506–1514. [Google Scholar] [CrossRef]
- Lee, S.J.; Lindquist, K.; Segal, M.R.; Covinsky, K.E. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 2006, 295, 801–808. [Google Scholar] [CrossRef]
- Bonanad, S.; De la Rubia, J.; Gironella, M.; Persona, E.P.; Gonzalez, B.; Lago, C.F.; Arnan, M.; Zudaire, M.; Rivas, J.A.H.; Soler, A.; et al. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: The GAH Scale. J. Geriatr. Oncol. 2015, 6, 353–361. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Gonzalez, B.; de la Rubia, J.; Rivas, J.A.H.; Soler, J.A.; Lago, C.F.; Arnao, M.; Gironella, M.; Persona, E.P.; Zudaire, M.T.; et al. Further psychometric validation of the GAH scale: Responsiveness and effect size. J. Geriatr. Oncol. 2017, 8, 211–215. [Google Scholar] [CrossRef]
- Park, J.H.; Qiao, B.; Panageas, K.S.; Schymura, M.J.; Jurcic, J.G.; Rosenblat, T.L.; Altman, J.K.; Douer, D.; Rowe, J.M.; Tallman, M.S. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood 2011, 118, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cuadron, D.; Serrano, J.; Gil, C.; Tormo, M.; Martinez-Sanchez, P.; Perez-Simon, J.A.; Garcia-Boyero, R.; Rodriguez-Medina, C.; Lopez-Pavia, M.; Benavente, C.; et al. Evolving treatment patterns and outcomes in older patients (>/=60 years) with AML: Changing everything to change nothing? Leukemia 2021, 35, 1571–1585. [Google Scholar] [CrossRef] [PubMed]
- Sharplin, K.; Wee, L.Y.A.; Singhal, D.; Edwards, S.; Danner, S.; Lewis, I.; Thomas, D.; Wei, A.; Yong, A.S.M.; Hiwase, D.K. Outcomes and health care utilization of older patients with acute myeloid leukemia. J. Geriatr. Oncol. 2021, 12, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Hubscher, E.; Sikirica, S.; Bell, T.; Brown, A.; Welch, V.; Russell-Smith, A.; D’Amico, P. Patterns of undertreatment among patients with acute myeloid leukemia (AML): Considerations for patients eligible for non-intensive chemotherapy (NIC). J. Cancer Res. Clin. Oncol. 2021, 147, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Wang, R.; Wang, X.; Shallis, R.M.; Podoltsev, N.A.; Bewersdorf, J.P.; Huntington, S.F.; Neparidze, N.; Giri, S.; Gore, S.D.; et al. Clinical outcomes of older patients with AML receiving hypomethylating agents: A large population-based study in the United States. Blood Adv. 2020, 4, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.; Robledo, C.; Pereira, A.; Pedro, C.; Benito, R.; de Paz, R.; Del Rey, M.; Insunza, A.; Tormo, M.; Diez-Campelo, M.; et al. Multidimensional assessment of patient condition and mutational analysis in peripheral blood, as tools to improve outcome prediction in myelodysplastic syndromes: A prospective study of the Spanish MDS group. Am. J. Hematol. 2017, 92, E534–E541. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.R. Personalizing therapy for older adults with acute myeloid leukemia: Role of geriatric assessment and genetic profiling. Cancer Treat. Rev. 2019, 75, 52–61. [Google Scholar] [CrossRef]
- Klepin, H.D.; Geiger, A.M.; Tooze, J.A.; Kritchevsky, S.B.; Williamson, J.D.; Pardee, T.S.; Ellis, L.R.; Powell, B.L. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013, 121, 4287–4294. [Google Scholar] [CrossRef]
- Timilshina, N.; Breunis, H.; Brandwein, J.M.; Minden, M.D.; Gupta, V.; O’Neill, S.; Tomlinson, G.A.; Buckstein, R.; Li, M.; Alibhai, S.M.H. Do quality of life or physical function at diagnosis predict short-term outcomes during intensive chemotherapy in AML? Ann. Oncol. 2014, 25, 883–888. [Google Scholar] [CrossRef]
- Oliva, E.N.; Nobile, F.; Alimena, G.; Ronco, F.; Specchia, G.; Impera, S.; Breccia, M.; Vincelli, I.; Carmosino, I.; Guglielmo, P.; et al. Quality of life in elderly patients with acute myeloid leukemia: Patients may be more accurate than physicians. Haematologica 2011, 96, 696–702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cervellin, G.; Borghi, L.; Lippi, G. Do clinicians decide relying primarily on Bayesians principles or on Gestalt perception? Some pearls and pitfalls of Gestalt perception in medicine. Intern. Emerg. Med. 2014, 9, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Barosi, G.; Venditti, A.; Angelucci, E.; Gobbi, M.; Pane, F.; Tosi, P.; Zinzani, P.; Tura, S. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: A project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 2013, 27, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, Y.N.; Zhang, Y.L.; Chen, J.; Mao, Y.Y.; Zhang, L.; Zhou, D.B.; Cao, X.X.; Li, J. Impact of Physicians’ Personalities and Behavioral Traits on Treatment-Related Decision-making for Elderly Acute Myeloid Leukemia. J. Gen. Intern. Med. 2021, 36, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Lennmyr, E.B.; Karlsson, K.; Abrahamsson, M.; Ebrahim, F.; Lubking, A.; Hoglund, M.; Juliusson, G.; Hallbook, H. Introducing patient-reported outcome in the acute leukemia quality registries in Sweden. Eur. J. Haematol. 2020, 104, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.A.; Kirtane, K.; Walter, R.B.; Lee, S.J.; Lyman, G.H. Patient-reported outcomes in acute myeloid leukemia: Where are we now? Blood Rev. 2018, 32, 81–87. [Google Scholar] [CrossRef]
- Korol, E.E.; Wang, S.; Johnston, K.; Ravandi-Kashani, F.; Levis, M.; van Nooten, F. Health-Related Quality of Life of Patients with Acute Myeloid Leukemia: A Systematic Literature Review. Oncol. Ther. 2017, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Abdallah, M.; Kumar, A.J.; Neuendorff, N.R.; Dahiya, S.; Klepin, H.D. Health-Related Quality of Life and Treatment of Older Adults with Acute Myeloid Leukemia: A Young International Society of Geriatric Oncology Review Paper. Curr. Hematol. Malig. Rep. 2019, 14, 523–535. [Google Scholar] [CrossRef]
- Timilshina, N.; Breunis, H.; Tomlinson, G.A.; Brandwein, J.M.; Buckstein, R.; Durbano, S.; Alibhai, S.M.H. Long-term recovery of quality of life and physical function over three years in adult survivors of acute myeloid leukemia after intensive chemotherapy. Leukemia 2019, 33, 15–25. [Google Scholar] [CrossRef]
- Appelbaum, F.R. Effectiveness of allogeneic hematopoietic cell transplantation for older patients with acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2021, 34, 101320. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Satram-Hoang, S.; Hurst, D.; Hoang, K.Q.; Momin, F.; Reyes, C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann. Hematol. 2015, 94, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).