Probiotics in Postoperative Pain Management
Abstract
1. Introduction
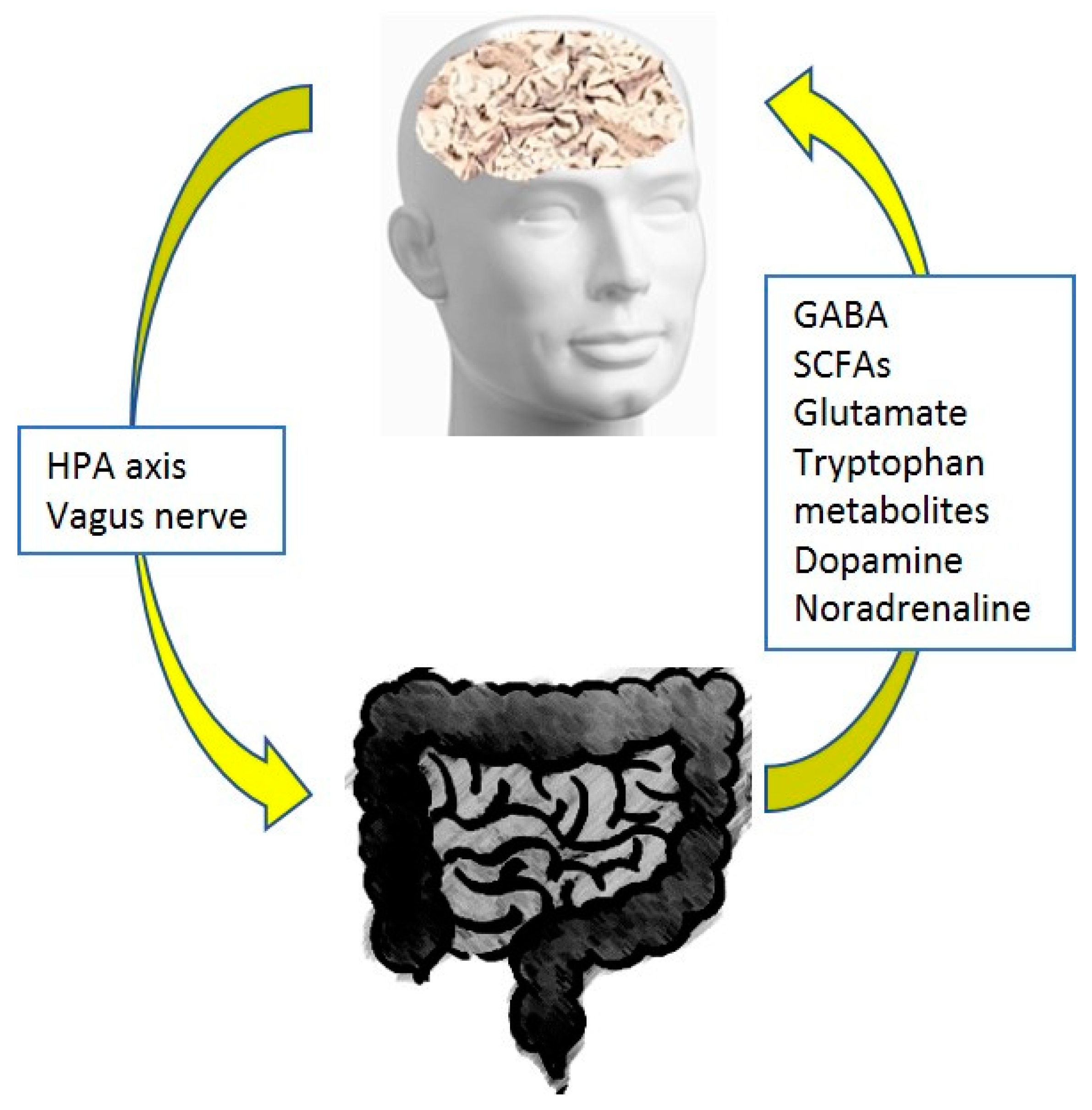
2. Pain and the Gut Microbiome
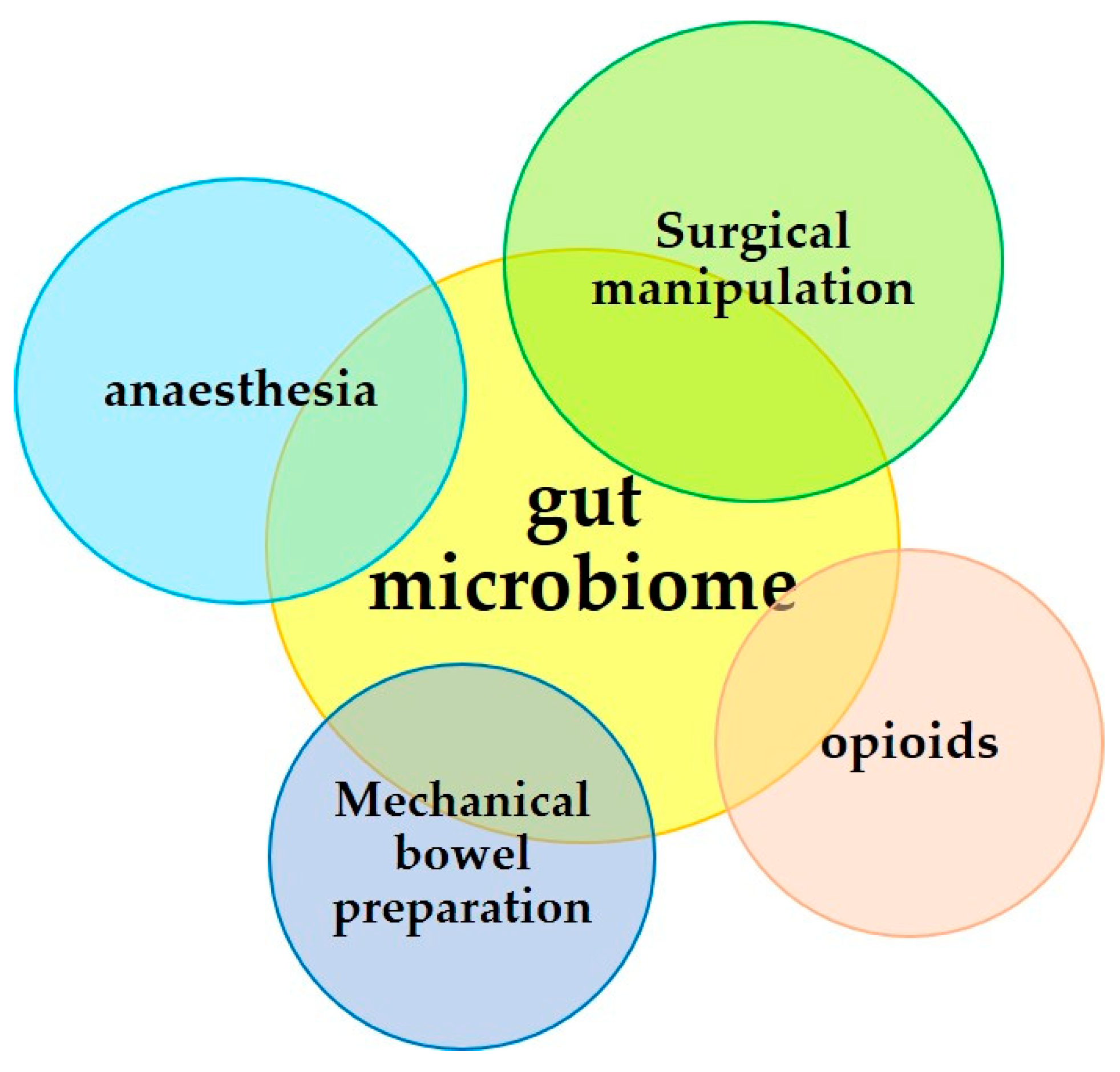
3. Surgical Operation and the Microbiome
4. Probiotics in Postoperative Pain Management
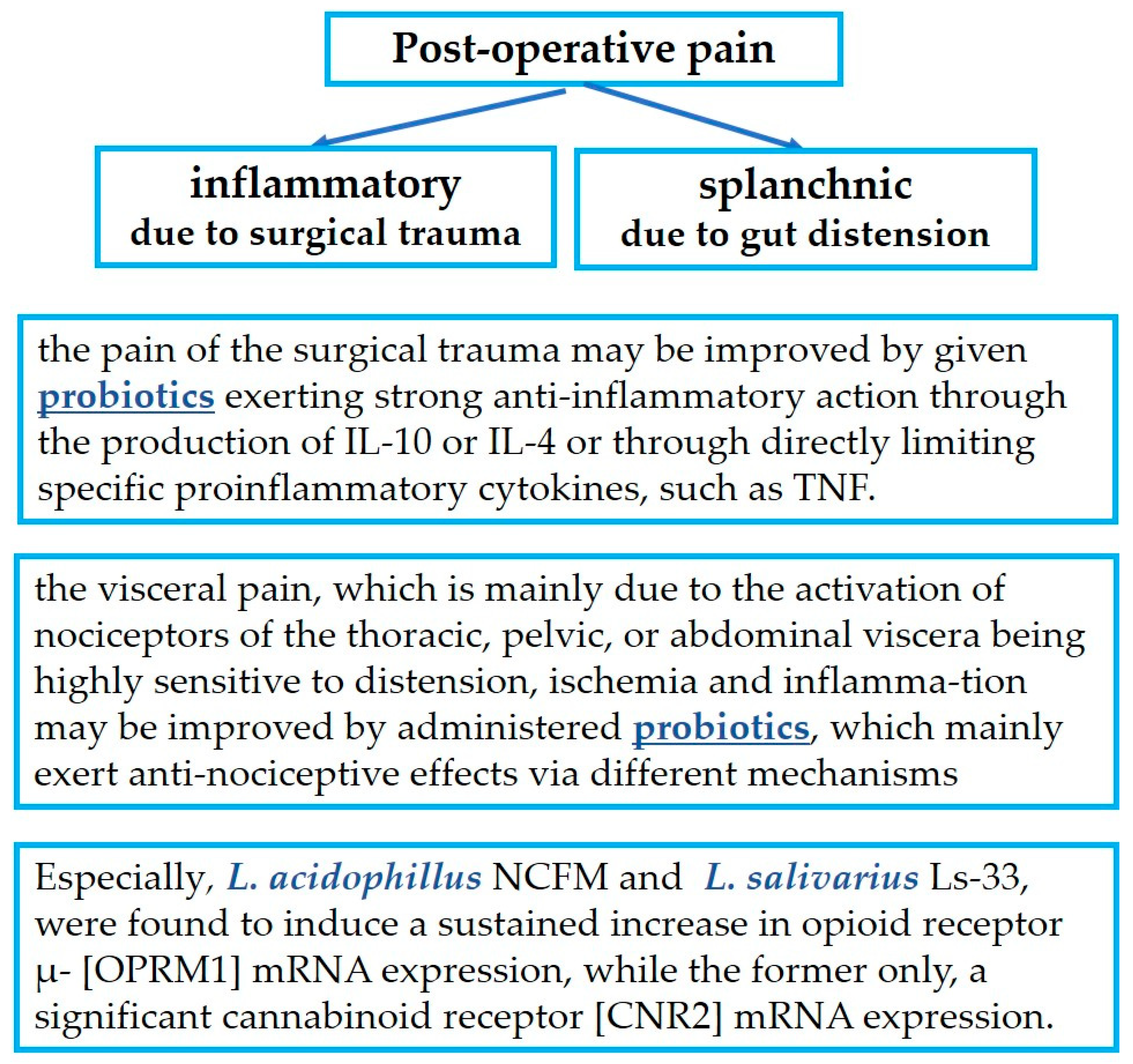
4.1. Probiotics in Relation to the Inflammation-Induced Pain of Surgical Trauma
4.2. Probiotics in Relation to Gut Distension-Induced Visceral Pain
4.2.1. Experimental Studies
4.2.2. Clinical Studies
5. Discussion-Conclusions
- The pain of surgical trauma on the abdominal wall which is of an inflammatory etiology may be improved by giving probiotics that exert strong anti-inflammatory action through the production of IL-10 or IL-4 or through directly limiting specific proinflammatory cytokines, such as TNF. Such benefits have been recognized after treatments mainly with Lactiplantibacillus plantarum, and to a lesser extent with L. acidophilus LA-5, L. rhamnosus GG ATCC 53103 and UBLR-58, L. fermentum SGL10, L. brevis GQ4237768, SGL 12, and CECT7480, L. paracasei SGL04, and MSMC39, B. longum UBBL-64 and Reuter, and L. casei Shirota. Additionally, Lactobacillus spp., B. dentium, and Bifidobacterium spp. are able to modify pain signaling by producing GABA, the most important inhibitory neurotransmitter.
- Visceral pain, which is mainly due to the activation of nociceptors of the thoracic, pelvic, or abdominal organs that are extremely sensitive to distension, tissue ischemia, and inflammation, may be improved by administered probiotics, which mainly exert antinociceptive effects via different mechanisms: L. plantarum PS128, L. acidophilus NCFM, L. rhamnosus GG ATCC53103, L. reuteri DSM 17938, L. paracasei, B. infantis 35624, B. longum and L. helveticus in combination, Bifidobacterium lactis CNCM I-2494 and Lactococcus lactis CNCM I-1631 in combination, and the less known L. farciminis, Roseburia hominis, a species of the butyrate-producing Lachnospiraceae family, and Faecalibacterium prausnitzii.
- Finally, particular mention must be made of the extraordinary action of L. acidophillus NCFM and of L. salivarius Ls-33, which induce a sustained increase in opioid receptor μ- (OPRM1) mRNA expression, while only the former also induces significant cannabinoid receptor (CNR2) mRNA expression.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Astyrakaki, E.; Papaioannou, A.; Askitopoulou, H. References to anesthesia, pain, and analgesia in the Hippocratic Collection. Anesth. Analg. 2010, 110, 188–194. [Google Scholar] [CrossRef][Green Version]
- Available online: https://www.wikidoc.org/index.php/Pain_historical_perspective (accessed on 11 August 2023).
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Sommer, F.; Backhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mazmanian, S.K. Microbiota-brain axis: Context and causality. Science 2022, 376, 938–939. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr. Med. Chem. 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Felice, V.D.; O’Mahony, S.M. The microbiome and disorders of the central nervous system. Pharmacol. Biochem. Behav. 2017, 160, 1–13. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Alizadeh, N.; Naderi, G.; Kahrizi, M.S.; Haghgouei, T.; Mobed, A.; Shah-Abadi, M.E. Microbiota-Pain Association; Recent Discoveries and Research Progress. Curr. Microbiol. 2022, 80, 29. [Google Scholar] [CrossRef]
- Bistoletti, M.; Bosi, A.; Banfi, D.; Giaroni, C.; Baj, A. The microbiota-gut-brain axis: Focus on the fundamental communication pathways. Prog. Mol. Biol. Transl. Sci. 2020, 176, 43–110. [Google Scholar] [CrossRef]
- Lozano, J.; Fernández-Ciganda, S.; González Revello, Á.; Hirigoyen, D.; Martínez, M.; Scorza, C.; Zunino, P. Probiotic potential of GABA-producing lactobacilli isolated from Uruguayan artisanal cheese starter cultures. J. Appl. Microbiol. 2022, 133, 1610–1619. [Google Scholar] [CrossRef]
- Attar, N. Good for the gut, good for the brain. Nat. Rev. Microbiol. 2016, 14, 269. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef]
- Lagomarsino, V.N.; Kostic, A.D.; Chiu, I.M. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol. Pain 2021, 9, 100056. [Google Scholar] [CrossRef]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, L.; Gao, J.; Zhu, M.; Zhang, Y.; Zhang, H.L. Gut Microbial Metabolites in Parkinson’s Disease: Implications of Mitochondrial Dysfunction in the Pathogenesis and Treatment. Mol. Neurobiol. 2021, 58, 3745–3758. [Google Scholar] [CrossRef]
- Guo, C.; Huo, Y.J.; Li, Y.; Han, Y.; Zhou, D. Gut-brain axis: Focus on gut metabolites short-chain fatty acids. World J. Clin. Cases 2022, 10, 1754–1763. [Google Scholar] [CrossRef]
- Varela, R.B.; Valvassori, S.S.; Lopes-Borges, J.; Mariot, E.; Dal-Pont, G.C.; Amboni, R.T.; Bianchini, G.; Quevedo, J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J. Psychiatr. Res. 2015, 61, 114–121. [Google Scholar] [CrossRef]
- Ustianowska, K.; Ustianowski, L.; Machaj, F.; Goracy, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int. J. Mol. Sci. 2022, 23, 13267. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Jorge, J.M.P.; Perez-Garcia, F.; Sgobba, E. Updates on industrial production of amino acids using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2016, 32, 105. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hashimoto, K.I.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum mechanosensitive channels: Towards unpuzzling “glutamate efflux” for amino acid production. Biophys. Rev. 2018, 10, 1359–1369. [Google Scholar] [CrossRef]
- Zareian, M.; Ebrahimpour, A.; Bakar, F.A.; Mohamed, A.K.S.; Forghani, B.; Ab-Kadir, M.S.B.; Saari, N. A glutamic acid-producing lactic acid bacteria isolated from Malaysian fermented foods. Int. J. Mol. Sci. 2012, 13, 5482–5497. [Google Scholar] [CrossRef]
- Neunlist, M.; Michel, K.; Reiche, D.; Dobreva, G.; Huber, K.; Schemann, M. Glycine activates myenteric neurones in adult guinea-pigs. J. Physiol. 2001, 536, 727–739. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M.; Paccagnella, N.; De Martin, S.; Montopoli, M.; Dall’Acqua, S.; et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br. J. Pharmacol. 2017, 174, 3623–3639. [Google Scholar] [CrossRef]
- Gronier, B.; Savignac, H.M.; Di Miceli, M.; Idriss, S.M.; Tzortzis, G.; Anthony, D.; Burnet, P.W.J. Increased cortical neuronal responses to NMDA and improved attentional set-shifting performance in rats following prebiotic (B-GOS((R))) ingestion. Eur. Neuropsychopharmacol. 2018, 28, 211–224. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, W.J.; Salton, S.R. Role of a VGF/BDNF/TrkB Autoregulatory Feedback Loop in Rapid-Acting Antidepressant Efficacy. J. Mol. Neurosci. 2019, 68, 504–509. [Google Scholar] [CrossRef]
- Minerbi, A.; Shen, S. Gut Microbiome in Anesthesiology and Pain Medicine. Anesthesiology 2022, 137, 93–108. [Google Scholar] [CrossRef]
- Shock, T.; Badang, L.; Ferguson, B.; Martinez-Guryn, K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 2021, 95, 108631. [Google Scholar] [CrossRef]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. The gut microbiota as a key regulator of visceral pain. Pain 2017, 158 (Suppl. 1), S19–S28. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Moloney, R.D.; Johnson, A.C.; Vicario, M. Mechanisms of Stress-Induced Visceral Pain: Implications in Irritable Bowel Syndrome. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef]
- Tap, J.; Derrien, M.; Tornblom, H.; Brazeilles, R.; Cools-Portier, S.; Dore, J.; Storsrud, S.; Le Neve, B.; Ohman, L.; Simren, M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e118. [Google Scholar] [CrossRef]
- Mars, R.A.T.; Yang, Y.; Ward, T.; Houtti, M.; Priya, S.; Lekatz, H.R.; Tang, X.; Sun, Z.; Kalari, K.R.; Korem, T.; et al. Longitudinal Multi-omics Reveals Subset-Specific Mechanisms Underlying Irritable Bowel Syndrome. Cell 2020, 182, 1460–1473.e17. [Google Scholar] [CrossRef]
- Morreale, C.; Bresesti, I.; Bosi, A.; Baj, A.; Giaroni, C.; Agosti, M.; Salvatore, S. Microbiota and Pain: Save Your Gut Feeling. Cells 2022, 11, 971. [Google Scholar] [CrossRef]
- Brenner, D.; Shorten, G.D.; O’Mahony, S.M. Postoperative pain and the gut microbiome. Neurobiol. Pain 2021, 10, 100070. [Google Scholar] [CrossRef]
- Chen, O.; Donnelly, C.R.; Ji, R.R. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr. Opin. Neurobiol. 2020, 62, 17–25. [Google Scholar] [CrossRef]
- Maeda, T.; Kishioka, S. PPAR and Pain. Int. Rev. Neurobiol. 2009, 85, 165–177. [Google Scholar]
- Galiazzo, G.; De Silva, M.; Giancola, F.; Rinnovati, R.; Peli, A.; Chiocchetti, R. Cellular distribution of cannabinoid-related receptors TRPV1, PPAR-gamma, GPR55 and GPR3 in the equine cervical dorsal root ganglia. Equine Vet. J. 2021, 54, 788–798. [Google Scholar] [CrossRef]
- Ghaderi, F.; Sotoodehnejadnematalahi, F.; Hajebrahimi, Z.; Fateh, A.; Siadat, S.D. Effects of active, inactive, and derivatives of Akkermansia muciniphila on the expression of the endocannabinoid system and PPARs genes. Sci. Rep. 2022, 12, 10031. [Google Scholar] [CrossRef]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Wang, H.; Polackwich, A.S.; Tucky, B.; Altemus, J.; Eng, C. Analysis of Gut Microbiome Reveals Significant Differences between Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Controls. J. Urol. 2016, 196, 435–441. [Google Scholar] [CrossRef]
- Brenner, D.; Cherry, P.; Switzer, T.; Butt, I.; Stanton, C.; Murphy, K.; McNamara, B.; Iohom, G.; O’Mahony, S.M.; Shorten, G. Pain after upper limb surgery under peripheral nerve block is associated with gut microbiome composition and diversity. Neurobiol. Pain 2021, 10, 100072. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; Van Praet, L.; Glorieus, E.; Van den Bosch, F.; De Vos, M.; Raes, J.; Elewaut, D. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol. 2017, 69, 114–121. [Google Scholar] [CrossRef]
- Khan, J.; Puchimada, B.; Kadouri, D.; Zusman, T.; Javed, F.; Eliav, E. The anti-nociceptive effects of Porphyromonas gingivalis lipopolysaccharide. Arch. Oral Biol. 2019, 102, 193–198. [Google Scholar] [CrossRef]
- Cruz-Aguliar, R.M.; Wantia, N.; Clavel, T.; Vehreschild, M.J.G.T.; Buch, T.; Bajbouj, M.; Haller, D.; Busch, D.; Schmid, R.M.; Stein-Thoeringer, C.K. An Open-Labeled Study on Fecal Microbiota Transfer in Irritable Bowel Syndrome Patients Reveals Improvement in Abdominal Pain Associated with the Relative Abundance of Akkermansia Muciniphila. Digestion 2019, 100, 127–138. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Kolios, G.; Manousou, P.; Kazamias, P.; Paramythiotis, D.; Papavramidis, T.; Heliadis, S.; Kouroumalis, E.; Eleftheriadis, E. Oxidative stress due to anesthesia and surgical trauma: Importance of early enteral nutrition. Mol. Nutr. Food Res. 2009, 53, 770–779. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Bolton, L. Feed Abdominal Surgery Patients to Improve Outcomes. Wounds 2021, 33, 158–160. [Google Scholar] [CrossRef]
- Banerjee, S.; Sindberg, G.; Wang, F.; Meng, J.; Sharma, U.; Zhang, L.; Dauer, P.; Chen, C.; Dalluge, J.; Johnson, T.; et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 2016, 9, 1418–1428. [Google Scholar] [CrossRef]
- Williamson, A.J.; Alverdy, J.C. Influence of the Microbiome on Anastomotic Leak. Clin. Colon. Rectal Surg. 2021, 34, 439–446. [Google Scholar] [CrossRef]
- Jalanka, J.; Salonen, A.; Salojarvi, J.; Ritari, J.; Immonen, O.; Marciani, L.; Gowland, P.; Hoad, C.; Garsed, K.; Lam, C.; et al. Effects of bowel cleansing on the intestinal microbiota. Gut 2015, 64, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, G.; Kotzampassi, K. Gut microbiome, surgical complications and probiotics. Ann. Gastroenterol. 2017, 30, 45–53. [Google Scholar] [CrossRef]
- Gorkiewicz, G.; Thallinger, G.G.; Trajanoski, S.; Lackner, S.; Stocker, G.; Hinterleitner, T.; Gully, C.; Hogenauer, C. Alterations in the colonic microbiota in response to osmotic diarrhea. PLoS ONE 2013, 8, e55817. [Google Scholar] [CrossRef] [PubMed]
- Harrell, L.; Wang, Y.; Antonopoulos, D.; Young, V.; Lichtenstein, L.; Huang, Y.; Hanauer, S.; Chang, E. Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS ONE 2012, 7, e32545. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Murakami, M.; Nakao, K.; Asahara, T.; Nomoto, K.; Tsunoda, A. Randomized clinical trial of the influence of mechanical bowel preparation on faecal microflora in patients undergoing colonic cancer resection. Br. J. Surg. 2010, 97, 1791–1797. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; De Grandi, R.; Casini, V.; Pace, F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur. J. Gastroenterol. Hepatol. 2016, 28, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Valentina, C.; Fabio, P. Gut microbiota, dysbiosis and colon lavage. Dig. Liver Dis. 2019, 51, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shang, L.; Jin, D.; Wu, X.; Long, B. General anesthesia bullies the gut: A toxic relationship with dysbiosis and cognitive dysfunction. Psychopharmacology 2022, 239, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Serrano, M.; Gerónimo-Pardo, M.; Martínez-Monsalve, A.; Crespo-Sánchez, M.D. Antibacterial effect of sevoflurane and isoflurane. Rev. Esp. Quim. 2017, 30, 84–89. [Google Scholar]
- Koutsogiannaki, S.; Schaefers, M.M.; Okuno, T.; Ohba, M.; Yokomizo, T.; Priebe, G.P.; DiNardo, J.A.; Sulpicio, S.G.; Yuki, K. From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis. Toxicol. Sci. 2017, 156, 402–411. [Google Scholar] [CrossRef]
- Chamberlain, M.; Koutsogiannaki, S.; Schaefers, M.; Babazada, H.; Liu, R.; Yuki, K. The Differential Effects of Anesthetics on Bacterial Behaviors. PLoS ONE 2017, 12, e0170089. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef]
- Mazzotta, E.; Villalobos-Hernandez, E.C.; Fiorda-Diaz, J.; Harzman, A.; Christofi, F.L. Postoperative Ileus and Postoperative Gastrointestinal Tract Dysfunction: Pathogenic Mechanisms and Novel Treatment Strategies Beyond Colorectal Enhanced Recovery After Surgery Protocols. Front. Pharmacol. 2020, 11, 583422. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ding, Y.; Wang, L.; Xiao, Y. Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res. Bull. 2020, 164, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, X.; Wu, H. Juvenile Rats Show Altered Gut Microbiota After Exposure to Isoflurane as Neonates. Neurochem. Res. 2019, 44, 776–786. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Liu, T.; Yang, N.; Li, Y.; He, J.; Qian, M.; Kuang, Z.; Zhang, W.; Ni, C.; et al. Prebiotics Regulation of Intestinal Microbiota Attenuates Cognitive Dysfunction Induced by Surgery Stimulation in APP/PS1 Mice. Aging Dis. 2020, 11, 1029–1045. [Google Scholar] [CrossRef]
- Han, C.; Zhang, Z.; Guo, N.; Li, X.; Yang, M.; Peng, Y.; Ma, X.; Yu, K.; Wang, C. Effects of Sevoflurane Inhalation Anesthesia on the Intestinal Microbiome in Mice. Front. Cell Infect. Microbiol. 2021, 11, 633527. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, Z.; Han, C.; Chen, L.; Zheng, X.; Yu, K.; Zhang, Z.; Wang, C. Effects of continuous intravenous infusion of propofol on intestinal flora in rats. Biomed. Pharmacother. 2021, 134, 111080. [Google Scholar] [CrossRef]
- Liufu, N.; Liu, L.; Shen, S.; Jiang, Z.; Dong, Y.; Wang, Y.; Culley, D.; Crosby, G.; Cao, M.; Shen, Y.; et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging 2020, 12, 1965–1986. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H.; et al. Anesthesia and Surgery Impair Blood-Brain Barrier and Cognitive Function in Mice. Front. Immunol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Peng, M.; Dong, Y.; Zhang, Y.; Chen, M.; Yin, N.; Marcantonio, E.R.; Xie, Z. Surgery plus anesthesia induces loss of attention in mice. Front. Cell Neurosci. 2015, 9, 346. [Google Scholar] [CrossRef]
- Miao, H.; Dong, Y.; Zhang, Y.; Zheng, H.; Shen, Y.; Crosby, G.; Culley, D.J.; Marcantonio, E.R.; Xie, Z. Anesthetic Isoflurane or Desflurane Plus Surgery Differently Affects Cognitive Function in Alzheimer’s Disease Transgenic Mice. Mol. Neurobiol. 2018, 55, 5623–5638. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Zhang, C.; Dong, Y.; Zhang, Y.; Nakazawa, H.; Kaneki, M.; Zheng, H.; Shen, Y.; Marcantonio, E.R.; Xie, Z. Battery of behavioral tests in mice to study postoperative delirium. Sci. Rep. 2016, 6, 29874. [Google Scholar] [CrossRef] [PubMed]
- Rodino-Janeiro, B.K.; Alonso-Cotoner, C.; Pigrau, M.; Lobo, B.; Vicario, M.; Santos, J. Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. J. Neurogastroenterol. Motil. 2015, 21, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Matzaras, R.; Anagnostou, N.; Nikopoulou, A.; Tsiakas, I.; Christaki, E. The Role of Probiotics in Inflammation Associated with Major Surgery: A Narrative Review. Nutrients 2023, 15, 1331. [Google Scholar] [CrossRef]
- Agnes, A.; Puccioni, C.; D’Ugo, D.; Gasbarrini, A.; Biondi, A.; Persiani, R. The gut microbiota and colorectal surgery outcomes: Facts or hype? A narrative review. BMC Surg. 2021, 21, 83. [Google Scholar] [CrossRef]
- Yang, T.; Velagapudi, R.; Terrando, N. Neuroinflammation after surgery: From mechanisms to therapeutic targets. Nat. Immunol. 2020, 21, 1319–1326. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: A novel regulator of pain. J. Neural Transm. (Vienna) 2020, 127, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, M.J.; Babrowski, T.; Carlisle, E.M.; Olivas, A.; Romanowski, K.S.; Seal, J.B.; Liu, D.C.; Alverdy, J.C. The human microbiome and surgical disease. Ann. Surg. 2011, 253, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Kotzampassi, K. What Surgeon Should Know about Probiotics. Nutrients 2022, 14, 4374. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, T.; Nomoto, K.; Onodera, H. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J. Gastrointest. Surg. 2013, 17, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, N.; Dang, Q.; Liu, L.; Wang, L.; Li, H.; Han, X. Exploring the roles of intestinal flora in enhanced recovery after surgery. iScience 2023, 26, 105959. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Miyoshi, M.; Kanbara, Y.; Aoyama, M.; Sakaki, H.; Shuno, K.; Hirata, K.; Takahashi, M.; Ueno, K.; Tabata, S.; et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J. Parenter. Enter. Nutr. 2011, 35, 317–328. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—A double-blind study. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef]
- Lu, H.; He, J.; Wu, Z.; Xu, W.; Zhang, H.; Ye, P.; Yang, J.; Zhen, S.; Li, L. Assessment of microbiome variation during the perioperative period in liver transplant patients: A retrospective analysis. Microb. Ecol. 2013, 65, 781–791. [Google Scholar] [CrossRef]
- Shogan, B.D.; Smith, D.P.; Christley, S.; Gilbert, J.A.; Zaborina, O.; Alverdy, J.C. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome. 2014, 3, 35. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Wang, C.; Tang, C.; Li, J. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS ONE 2012, 7, e42027. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, E.; Kotzampassi, K.; Botsios, D.; Tzartinoglou, E.; Farmakis, H.; Dadoukis, J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg. Endosc. 1996, 10, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, E.; Kotzampassi, K.; Papanotas, K.; Heliadis, N.; Sarris, K. Gut ischemia, oxidative stress, and bacterial translocation in elevated abdominal pressure in rats. World J. Surg. 1996, 20, 11–16. [Google Scholar] [CrossRef]
- Ioannidis, A.; Arvanitidis, K.; Filidou, E.; Valatas, V.; Stavrou, G.; Michalopoulos, A.; Kolios, G.; Kotzampassi, K. The Length of Surgical Skin Incision in Postoperative Inflammatory Reaction. JSLS 2018, 22, e2018.00045. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.C.; Keller, D.S.; Baldini, G.; Bordeianou, L.; Weiss, E.; Lee, L.; Boutros, M.; McClane, J.; Feldman, L.S.; Steele, S.R. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery From the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis. Colon. Rectum 2017, 60, 761–784. [Google Scholar] [CrossRef]
- Burton, D.; Nicholson, G.; Hall, G. Endocrine and metabolic response to surgery. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 144–147. [Google Scholar] [CrossRef]
- Schricker, T.; Lattermann, R. Perioperative catabolism. Can. J. Anaesth. 2015, 62, 182–193. [Google Scholar] [CrossRef]
- Hughes, D.T.; Sperandio, V. Inter-kingdom signalling: Communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar] [CrossRef]
- Kendall, M.M.; Sperandio, V. What a Dinner Party! Mechanisms and Functions of Interkingdom Signaling in Host-Pathogen Associations. mBio 2016, 7, e01748. [Google Scholar] [CrossRef]
- Biaggini, K.; Barbey, C.; Borrel, V.; Feuilloley, M.; Déchelotte, P.; Connil, N. The pathogenic potential of Pseudomonas fluorescens MFN1032 on enterocytes can be modulated by serotonin, substance P and epinephrine. Arch. Microbiol. 2015, 197, 983–990. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, J.; Ban, Y.; Jalodia, R.; Chupikova, I.; Fernandez, I.; Brito, N.; Sharma, U.; Abreu, M.T.; Ramakrishnan, S.; et al. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 13523–13532. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Menni, A.; Moysidis, M.; Tzikos, G.; Stavrou, G.; Tsetis, J.K.; Shrewsbury, A.D.; Filidou, E.; Kotzampassi, K. Looking for the Ideal Probiotic Healing Regime. Nutrients 2023, 15, 3055. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Xiang, Q.; Tang, X.; Zhang, Q.; Liu, X.; Zhao, J.; Cui, S.; Zhang, H. Lactobacillus reuteri CCFM1175 and Lactobacillus paracasei CCFM1176 Could Prevent Capsaicin-Induced Ileal and Colonic Injuries. Probiotics Antimicrob. Proteins 2023, 15, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, S.; Santhanakumar, R. The role of probiotics as wound healers: An overall view. J. Wound Care 2023, 32, 318–328. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Ioannidis, O.; Chatzakis, C.; Tirta, M.; Anestiadou, E.; Zapsalis, K.; Symeonidis, S.; Bitsianis, S.; Kotidis, E.; Pramateftakis, M.G.; Mantzoros, I.; et al. The Efficacy of Probiotics, Prebiotics, and Synbiotics in Patients Who Have Undergone Abdominal Operation, in Terms of Bowel Function Post-Operatively: A Network Meta-Analysis. J. Clin. Med. 2023, 12, 4150. [Google Scholar] [CrossRef]
- Tang, G.; Huang, W.; Tao, J.; Wei, Z. Prophylactic effects of probiotics or synbiotics on postoperative ileus after gastrointestinal cancer surgery: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0264759. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Li, J.; Feng, M.; Zhang, Y.; Yao, W.; Zhang, C.; Wan, L. Celastrol attenuates incision-induced inflammation and pain associated with inhibition of the NF-κB signalling pathway via SARM. Life Sci. 2018, 205, 136–144. [Google Scholar] [CrossRef]
- Kim, M.K.; Kang, H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C.; Choi, G.J.; Shin, H.Y.; Kim, K.S. Antinociceptive and anti-inflammatory effects of ginsenoside Rf in a rat model of incisional pain. J. Ginseng Res. 2018, 42, 183–191. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Brennan, T.J. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain 2009, 144, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Takeuchi, H.; Fujii, K.; Shiraishi, N.; Adachi, Y.; Kitano, S. Length of laparotomy incision and surgical stress assessed by serum IL-6 level. Injury 2006, 37, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of inflammatory mediators with pain perception. Biomed. Pharmacother. 2017, 96, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Penzkover, K.R.; Soderquist, R.G.; Mahoney, M.J. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012, 15, 520–526; discussion 526. [Google Scholar] [CrossRef] [PubMed]
- Tanga, F.Y.; Nutile-McMenemy, N.; DeLeo, J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. USA 2005, 102, 5856–5861. [Google Scholar] [CrossRef]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef]
- Dubey, A.K.; Podia, M.; Priyanka; Raut, S.; Singh, S.; Pinnaka, A.K.; Khatri, N. Insight Into the Beneficial Role of Lactiplantibacillus plantarum Supernatant Against Bacterial Infections, Oxidative Stress, and Wound Healing in A549 Cells and BALB/c Mice. Front. Pharmacol. 2021, 12, 728614. [Google Scholar] [CrossRef]
- Panagiotou, D.; Filidou, E.; Gaitanidou, M.; Tarapatzi, G.; Spathakis, M.; Kandilogiannakis, L.; Stavrou, G.; Arvanitidis, K.; Tsetis, J.K.; Gionga, P.; et al. Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin. Nutrients 2023, 15, 1822. [Google Scholar] [CrossRef] [PubMed]
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Spathakis, M.; Gaitanidou, M.; Arvanitidis, K.; Drygiannakis, I.; Valatas, V.; Kotzampassi, K.; Manolopoulos, V.G.; et al. The Probiotic Strains Bifidomicronbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii Regulate Wound Healing and Chemokine Responses in Human Intestinal Subepithelial Myofibroblasts. Pharmaceuticals 2022, 15, 1293. [Google Scholar] [CrossRef]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Thibeault, S.L. Role of tumor necrosis factor-alpha in wound repair in human vocal fold fibroblasts. Laryngoscope 2010, 120, 1819–1825. [Google Scholar] [CrossRef]
- Gueniche, A.; Bastien, P.; Ovigne, J.M.; Kermici, M.; Courchay, G.; Chevalier, V.; Breton, L.; Castiel-Higounenc, I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 2010, 19, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, C.; Wang, J.; Guo, Q.; Zou, W. Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain Behav. 2019, 9, e01260. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes. 2010, 1, 148–163. [Google Scholar] [CrossRef]
- Vale, G.C.; Mota, B.I.S.; Ando-Suguimoto, E.S.; Mayer, M.P.A. Effect of Probiotics Lactobacillus acidophilus and Lacticaseibacillus rhamnosus on Antibacterial Response Gene Transcription of Human Peripheral Monocytes. Probiotics Antimicrob. Proteins 2023, 15, 264–274. [Google Scholar] [CrossRef]
- Tao, Y.; Drabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1018–C1030. [Google Scholar] [CrossRef]
- Du, X.; Hao, H.; Yang, Y.; Huang, S.; Wang, C.; Gigout, S.; Ramli, R.; Li, X.; Jaworska, E.; Edwards, I.; et al. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J. Clin. Investig. 2017, 127, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Banjonjit, S.; Taweechotipatr, M.; Rungsiyanont, S. Effect of probiotic Lactobacillus paracasei on tumor necrosis factor-alpha level in gingival crevicular fluid of patients undergoing impacted third molar removal. J. Oral Sci. 2022, 64, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ferrés-Amat, E.; Espadaler-Mazo, J.; Calvo-Guirado, J.L.; Ferrés-Amat, E.; Mareque-Bueno, J.; Salavert, A.; Aguiló-García, M.; Moreno-Centeno, J.; Ferrés-Padró, E. Probiotics diminish the post-operatory pain following mandibular third molar extraction: A randomised double-blind controlled trial (pilot study). Benef. Microbes 2020, 11, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Guo, C.; Wang, Y.; Hua, L.; Xue, S.; Yu, D.; Zhang, C.; Wang, D. Oral administration of probiotic Lactobacillus casei Shirota relieves pain after single rib fracture: A randomized double-blind, placebo-controlled clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef]
- Agostini, S.; Goubern, M.; Tondereau, V.; Salvador-Cartier, C.; Bezirard, V.; Lévèque, M.; Keränen, H.; Theodorou, V.; Bourdu-Naturel, S.; Goupil-Feuillerat, N.; et al. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol. Motil. 2012, 24, 376-e172. [Google Scholar] [CrossRef]
- Darbaky, Y.; Evrard, B.; Patrier, S.; Falenta, J.; Garcin, S.; Tridon, A.; Dapoigny, M.; Silberberg, C.; Nivoliez, A.; Diop, L. Oral probiotic treatment of Lactobacillus rhamnosus Lcr35® prevents visceral hypersensitivity to a colonic inflammation and an acute psychological stress. J. Appl. Microbiol. 2017, 122, 188–200. [Google Scholar] [CrossRef]
- Kannampalli, P.; Pochiraju, S.; Chichlowski, M.; Berg, B.M.; Rudolph, C.; Bruckert, M.; Miranda, A.; Sengupta, J.N. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol. Motil. 2014, 26, 1694–1704. [Google Scholar] [CrossRef]
- Roman, P.; Abalo, R.; Marco, E.M.; Cardona, D. Probiotics in digestive, emotional, and pain-related disorders. Behav. Pharmacol. 2018, 29, 103–119. [Google Scholar] [CrossRef]
- McVey Neufeld, K.A.; Strain, C.R.; Pusceddu, M.M.; Waworuntu, R.V.; Manurung, S.; Gross, G.; Moloney, G.M.; Hoban, A.E.; Murphy, K.; Stanton, C.; et al. Lactobacillus rhamnosus GG soluble mediators ameliorate early life stress-induced visceral hypersensitivity and changes in spinal cord gene expression. Neuronal Signal 2020, 4, NS20200007. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Theodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Eutamene, H.; Houdeau, E.; Bueno, L.; Fioramonti, J.; Theodorou, V. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol. Motil. 2009, 21, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Han, W.; Lamine, F.; Eutamene, H.; Fioramonti, J.; Bueno, L.; Theodorou, V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: A possible action through interaction with epithelial cell cytoskeleton contraction. Gut 2006, 55, 1090–1094. [Google Scholar] [CrossRef]
- Liu, Y.W.; Wang, Y.P.; Yen, H.F.; Liu, P.Y.; Tzeng, W.J.; Tsai, C.F.; Lin, H.C.; Lee, F.Y.; Jeng, O.J.; Lu, C.L.; et al. Lactobacillus plantarum PS128 Ameliorated Visceral Hypersensitivity in Rats Through the Gut-Brain Axis. Probiotics Antimicrob. Proteins 2020, 12, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Lashermes, A.; Gillet, M.; Meleine, M.; Gelot, A.; Eschalier, A.; Ardid, D.; Bermudez-Humaran, L.G.; Sokol, H.; et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016, 6, 19399. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; van Goor, H.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Mucus Microbiome of Anastomotic Tissue During Surgery Has Predictive Value for Colorectal Anastomotic Leakage. Ann. Surg. 2019, 269, 911–916. [Google Scholar] [CrossRef]
- Miquel, S.; Leclerc, M.; Martin, R.; Chain, F.; Lenoir, M.; Raguideau, S.; Hudault, S.; Bridonneau, C.; Northen, T.; Bowen, B.; et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio 2015, 6, e00300-15. [Google Scholar] [CrossRef]
- Martin, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermudez-Humaran, L.G.; Langella, P. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm. Bowel Dis. 2014, 20, 417–430. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, A.V.; Söderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Laval, L.; Martin, R.; Natividad, J.N.; Chain, F.; Miquel, S.; Desclee de Maredsous, C.; Capronnier, S.; Sokol, H.; Verdu, E.F.; van Hylckama Vlieg, J.E.; et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. TRPV1: A new target for treatment of visceral pain in IBS? Gut 2008, 57, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.; Shenoy, M.; Medley, D.; Naniwadekar, A.; Pasricha, P.J. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 2007, 132, 615–627. [Google Scholar] [CrossRef]
- Boesmans, W.; Owsianik, G.; Tack, J.; Voets, T.; Vanden Berghe, P. TRP channels in neurogastroenterology: Opportunities for therapeutic intervention. Br. J. Pharmacol. 2011, 162, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Vay, L.; Gu, C.; McNaughton, P.A. The thermo-TRP ion channel family: Properties and therapeutic implications. Br. J. Pharmacol. 2012, 165, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burgos, A.; Wang, L.; McVey Neufeld, K.A.; Mao, Y.K.; Ahmadzai, M.; Janssen, L.J.; Stanisz, A.M.; Bienenstock, J.; Kunze, W.A. The TRPV1 channel in rodents is a major target for antinociceptive effect of the probiotic Lactobacillus reuteri DSM 17938. J. Physiol. 2015, 593, 3943–3957. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.V.; Dryn, D.O.; Melnyk, M.I. General anaesthesia-related complications of gut motility with a focus on cholinergic mechanisms, TRP channels and visceral pain. Front. Physiol. 2023, 14, 1174655. [Google Scholar] [CrossRef]
- Melnyk, M.I.; Dryn, D.O.; Al Kury, L.T.; Dziuba, D.O.; Zholos, A.V. Suppression of mI(CAT) in Mouse Small Intestinal Myocytes by General Anaesthetic Ketamine and its Recovery by TRPC4 Agonist (-)-englerin A. Front. Pharmacol. 2020, 11, 594882. [Google Scholar] [CrossRef]
- Verdú, E.F.; Bercík, P.; Bergonzelli, G.E.; Huang, X.X.; Blennerhasset, P.; Rochat, F.; Fiaux, M.; Mansourian, R.; Corthésy-Theulaz, I.; Collins, S.M. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology 2004, 127, 826–837. [Google Scholar] [CrossRef]
- Guarino, M.P.; Altomare, A.; Stasi, E.; Marignani, M.; Severi, C.; Alloni, R.; Dicuonzo, G.; Morelli, L.; Coppola, R.; Cicala, M. Effect of acute mucosal exposure to Lactobacillus rhamnosus GG on human colonic smooth muscle cells. J. Clin. Gastroenterol. 2008, 42, S185–S190. [Google Scholar] [CrossRef]
- Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; Tougas, G.; Bienenstock, J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 2006, 55, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Duncker, S.C.; Kamiya, T.; Wang, L.; Yang, P.; Bienenstock, J. Probiotic Lactobacillus reuteri alleviates the response to gastric distension in rats. J. Nutr. 2011, 141, 1813–1818. [Google Scholar] [CrossRef]
- McKernan, D.P.; Fitzgerald, P.; Dinan, T.G.; Cryan, J.F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 2010, 22, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Goldsmith, J.R.; Carroll, I.M.; Barros, S.P.; Palsson, O.; Jobin, C.; Ringel, Y. Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain—A randomised clinical study. Aliment. Pharmacol. Ther. 2014, 40, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutvin, S.A.; Troost, F.J.; Kilkens, T.O.; Lindsey, P.J.; Hamer, H.M.; Jonkers, D.M.; Venema, K.; Brummer, R.J. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 2009, 21, 952-e76. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Savino, F.; Cordisco, L.; Tarasco, V.; Palumeri, E.; Calabrese, R.; Oggero, R.; Roos, S.; Matteuzzi, D. Lactobacillus reuteri DSM 17938 in infantile colic: A randomized, double-blind, placebo-controlled trial. Pediatrics 2010, 126, e526–e533. [Google Scholar] [CrossRef]
- Savino, F.; Cresi, F.; Pautasso, S.; Palumeri, E.; Tullio, V.; Roana, J.; Silvestro, L.; Oggero, R. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr. 2004, 93, 825–829. [Google Scholar] [CrossRef]
- de Weerth, C.; Fuentes, S.; de Vos, W.M. Crying in infants: On the possible role of intestinal microbiota in the development of colic. Gut Microbes 2013, 4, 416–421. [Google Scholar] [CrossRef]
- Savino, F.; Garro, M.; Montanari, P.; Galliano, I.; Bergallo, M. Crying Time and RORgamma/FOXP3 Expression in Lactobacillus reuteri DSM17938-Treated Infants with Colic: A Randomized Trial. J. Pediatr. 2018, 192, 171–177.e1. [Google Scholar] [CrossRef] [PubMed]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef]
| Probiotics | Action | Type of Operation |
|---|---|---|
| Experimental Studies | ||
| L. plantarum [131] | ↑ IL-10 and TGF1 ↓ TNF and IL-1 | Skin wound healing |
| L. plantarum MTCC 2621 [132] | ↑ IL-10, earlier re-epithelization, reduction in leukocyte infiltration, increased fibroblasts and collagen | Staphylococcus aureus-infected excisional wounds |
| Lactiplantibacillus plantarum UBLP-40 [133,134] | ↑ IL-10 and TGF1 ↓ TNF and IL-1 Earlier re-epithelization, reduction in leukocyte infiltration, increased fibroblasts and collagen | Skin wound healing in rats |
| L. rhamnosus UBLR-58 L. acidophilus LA-5 L. fermentum SGL10 L. brevis GQ4237768 L. brevis SGL 12 L. paracasei SGL 04 B. longum UBBL-64 [133,134,135] | ↑ IL-10 and TGF1 ↓ TNF and IL-1 Earlier re-epithelization, reduction in leukocyte infiltration, increased fibroblasts and collagen Similar mechanisms—less intensity in relation to L. plantarum | Skin wound healing in rats |
| B. longum reuter [137] | Inhibits capsaicin-induced CGRP release | Ex vivo human explant model |
| L. rhamnosus GG ATCC 53103 [140,141] | ↑ MAPKs | Epithelial cell culture |
| L. acidophilus LA-5 [140,141] | ↑ MAPKs | Epithelial cell culture |
| Lactobacillus spp. B. dentium Bifidobacterium spp. [142] | Produce GABA through enzymatic decarboxylation of glutamate | Skin wound healing in rats |
| Clinical Studies | ||
| L. paracasei MSMC 39 [143] | ↓ TNF | Mandibular 3rd molar excision |
| L. brevis CECT7480 L. plantarum CECT7481 [144] | ↓ Pain, eating difficulty | Mandibular 3rd molar excision |
| L. casei Shirota [145] | ↓ Pain related to max inspiration | Single rib fracture |
| Probiotics | Action | Type of Operation |
|---|---|---|
| Experimental Studies | ||
| L. acidophillus NCFM [146] | Increases μ-opioid receptor and cannabinoid receptor R2 expression | Human epithelial cells Mice and rats |
| L. salivarius Ls-33 [143] | Increases μ- opioid receptor expression | Human epithelial cells Mice and rats |
| L. acidophillus NCFM [143] | Pain reduction | Colorectal distension in rats |
| Akkermansia muciniphila [47] | Affects the cannabinoid R1 1 and R2 expression | Caco-2 and Hep-G2 cell lines |
| Bifidobacterium lactis CNCM I-2494 [147] | Reduces visceral hypersensitivity and pain | Colorectal distension model |
| Lactococcus lactis CNCM I-1631 [147] | Reduces visceral hypersensitivity and pain | Colorectal distension model |
| L. rhamnosus Lcr35 [148] | IL-13/Th 17 activation Increases IL-13 | Colorectal distension model |
| L. rhamnosus GG ATCC53103 [149,150] | Alters neurotransmitters | Visceral hypersensitivity model |
| Lactobacillus rhamnosus GG [151] | Decreases splanchnic sensitivity | Maternal separation plus restraint stress |
| Roseburia hominis [152] | Reduction in visceral pain and hypersensitivity via butyrate | Stress |
| L. acidophilus NCFM [146] | Increases colorectal distension threshold | Visceral hypersensitivity model |
| Bifidobacterium longum [153] | Reduces visceral hypersensitivity Regulates glucocorticoid negative feedback on the HPA axis | Water avoidance stress |
| Lactobacillus helveticus [153] | Reduces visceral hypersensitivity Regulates glucocorticoid negative feedback on the HPA axis | Water avoidance stress |
| Lactobacillus farciminis [154,155] | Fos downregulation Reduce visceral hypersensitivity | Colorectal distension model |
| L. plantarum PS128 [156] | Inhibits 5-HTP-induced visceral hypersensitivity Modulates gut–brain HPA axis | Colorectal distension model |
| Faecalibacterium prausnitzii A2-165 [157,160,161] | Anti-inflammatory properties Antinociceptive properties | Neonatal maternal separation plus colorectal distension |
| Partial restraint stress plus colorectal distension model | ||
| Lactobacillus reuteri DSM 17938 [168] | Reduces the jejunal spinal nerve firing via the TRPV1 channel antagonist | Colorectal distension model |
| Inhibits capsaicin-induced intracellular calcium in DRGs | Jejunal mesenteric nerve bundles | |
| Lactobacillus reuteri DSM 17938 [168] | Inhibits bradycardia induced after gastric distension | Gastric distension in rats |
| Lactobacillus paracasei [171] | Decreases T-helper 2 response Decreases TGF-1, COX-2, and PGE2 levels in muscles | Muscle hypercontractility induced by Trichinella spiralis infection in mice |
| Lactobacillus rhamnosus GG [172] | Reduce smooth muscle cell contraction via acetylcholine | Ex vivo colonic mucosa |
| L.rhamnosus JB-1 [173,174] | Inhibits pain perception by altering signaling in DRG fibers | Colorectal distension model |
| L. reuteri [174] | Inhibits the mechanosensitive response | Gastric distension |
| Bifidobacterium infantis 35624 [175] | Reduces the pain behavior and increases the threshold pressure | Stress plus colorectal distension model |
| Probiotics | Action | Type of Operation |
|---|---|---|
| Clinical studies | ||
| L. acidophilus NCFM [176] | Increases μ-opioid receptor and cannabinoid receptor R2 expression | Females, mild to mode-rate abdominal pain |
| Butyrate enemas [177] | Reduction in pain perception and discomfort | Healthy volunteers |
| Lactobacillus reuteri DSM 17938 [179] | Reduces the crying time | Infantile colic |
| Lactobacillus reuteri DSM 17938 [182] | Increases the FOXP3 concentration and decreases RORγ/FOXP3 ratio Modulates T-cell response to microbes | 30 days treatment |
| B. infantis 35624 [183,184] | Alleviates symptoms of pain/discomfort, bloating/distension, and bowel movement difficulty | Irritable bowel syndrome patients |
| L. plantarum 299v (DSM 9843) [185] | Reduction in pain episodes Reduction in pain and bloating severity | Irritable bowel syndrome patients |
| Akkermansia muciniphila [54] | Patients with fecal microbiota profiles clustered closely to the donor’s experience less pain, and feces found enriched with A. muciniphila, like donor’s | Fecal microbial transfer Preclinical study Irritable bowel syndrome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fyntanidou, B.; Amaniti, A.; Soulioti, E.; Zagalioti, S.-C.; Gkarmiri, S.; Chorti, A.; Loukipoudi, L.; Ioannidis, A.; Dalakakis, I.; Menni, A.-E.; et al. Probiotics in Postoperative Pain Management. J. Pers. Med. 2023, 13, 1645. https://doi.org/10.3390/jpm13121645
Fyntanidou B, Amaniti A, Soulioti E, Zagalioti S-C, Gkarmiri S, Chorti A, Loukipoudi L, Ioannidis A, Dalakakis I, Menni A-E, et al. Probiotics in Postoperative Pain Management. Journal of Personalized Medicine. 2023; 13(12):1645. https://doi.org/10.3390/jpm13121645
Chicago/Turabian StyleFyntanidou, Barbara, Aikaterini Amaniti, Eleftheria Soulioti, Sofia-Chrysovalantou Zagalioti, Sofia Gkarmiri, Angeliki Chorti, Lamprini Loukipoudi, Aris Ioannidis, Ioannis Dalakakis, Alexandra-Eleftheria Menni, and et al. 2023. "Probiotics in Postoperative Pain Management" Journal of Personalized Medicine 13, no. 12: 1645. https://doi.org/10.3390/jpm13121645
APA StyleFyntanidou, B., Amaniti, A., Soulioti, E., Zagalioti, S.-C., Gkarmiri, S., Chorti, A., Loukipoudi, L., Ioannidis, A., Dalakakis, I., Menni, A.-E., Shrewsbury, A. D., & Kotzampassi, K. (2023). Probiotics in Postoperative Pain Management. Journal of Personalized Medicine, 13(12), 1645. https://doi.org/10.3390/jpm13121645







