Analysis of New Colposcopy Techniques in the Diagnosis and Evolution of SIL/CIN: Comparison of Colposcopy with the DSI System (COLPO-DSI Study)
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer; World Health Organization. Available online: https://gco.iarc.fr/today/home (accessed on 24 March 2021).
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012, 30 (Suppl. S5), F88–F99. [Google Scholar] [CrossRef] [PubMed]
- Segnan, N.; Anttila, A.; Karsa, L.; Ronco, G.; Törnberg, S.; Patnick, J.; De Vuyst, H.; Dillner, J.; Franceschi, S.; Arbyn, M.; et al. (Eds.) European Guidelines for Quality Assurance in Cervical Cancer Screening, 2nd ed.; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar] [CrossRef]
- Torné, A.; Andía, D.; Castro, M.; de la Fuente, J.; Hernández, J.J.; López, J.A.; Martínez, J.C.; Medina, N.; Quílez, J.C.; Ramírez Mena, M.; et al. AEPCC-Guideline: Colposcopy Guidelines. Standards of Quality; AEPCC Publications: Valencia, Spain, 2018; pp. 1–80. [Google Scholar]
- WHO. Control Integral del Cáncer Cervicouterino: Guía de Prácticas Esenciales, 2nd ed.; WHO: Washington, DC, USA, 2016; ISBN 978-92-4-354700-8. [Google Scholar]
- Pruski, D.; Przybylski, M.; Millert-Kalinska, S.; Zmaczynski, A.; Jach, R. Histopathological discrepancies between colposcopy-directed biopsy and LEEP-conization observed during SARS-CoV-2 pandemic. Ginekol. Pol. 2023, 94, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Soutter, W.P.; Diakomanolis, E.; Lyons, D.; Ghaem-Maghami, S.; Ajala, T.; Haidopoulos, D.; Doumplis, D.; Kalpaktsoglou, C.; Sakellaropoulos, G.; Soliman, S.; et al. Dynamic spectral imaging: Improving colposcopy. Clin. Cancer Res. 2009, 15, 1814–1820. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Budithi, S.; Peevor, R.; Pugh, D.; Papagiannakis, E.; Durman, A.; Banu, N.; Alalade, A.; Leeson, S. Evaluating colposcopy with dynamic spectral imaging during routine practice at five colposcopy clinics in Wales: Clinical performance. Gynecol. Obstet. Investig. 2018, 83, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Coronado, P.J.; Fasero, M. Correlating the accuracy of colposcopy to practitioner experience using the Dynamic Spectral Imaging System (DySIS). Gynecol. Obstet. Investig. 2014, 78, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Roensbo, M.T.; Hammer, A.; Blaakaer, J. Can Dynamic Spectral Imaging System colposcopy replace conventional colposcopy in the detection of high-grade cervical lesions? Acta Obstet. Gynecol. Scand. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Wang, S.S.; Schiffman, M.; Solomon, D.; ASCUS LSIL Triage Study Group. Predicting absolute risk of CIN3 during post-colposcopic follow-up: Results from the ASCUS-LSIL Triage Study (ALTS). Am. J. Obstet. Gynecol. 2006, 195, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Torné Bladé, A.; del Pino Saladrigues, M.; Cusidó Gimferrer, M.; Quitllet, F.A.; Ortiz, D.A.; Piqué, X.C.; Bordoy, J.C.; Carreño, R.G.; Troyas, R.M.G.; Rubio, B.L.; et al. AEPCC-Guideline: Cervical Cancer Prevention; AEPCC Publications: Valencia, Spain, 2014. [Google Scholar]
- Torné, A.; Del Pino, M.; Andía, D.; Bruni, L.; Centeno, C.; Coronado, P.; Cruz Quílez, J.; de la Fuente, J.; de Sanjosé, S.; Ibáñez, R.; et al. AEPCC-Guía: Prevención Secundaria del Cancer de Cuello del Útero, 2022. Conducta Clínica ante Resultados Anormales de las Pruebas de Cribado; AEPCC Publications: Valencia, Spain, 2022. [Google Scholar]
- Quílez, J.; Bosch, J.M.; Serrano, J.R.; González, J.V.; Lobo, P.; López-Arregui, E.; Quesada, M.; Ramón y Cajal, J.M.; Vanrell, C. AEPCC-Guía: Métodos Anticonceptivos y VPH; Andía, D., del Pino, M., Torné, A., Eds.; Publicaciones AEPCC: Valencia, Spain, 2018; pp. 1–47. [Google Scholar]
- Louwers, J.; Zaal, A.; Kocken, M.; ter Harmsel, W.; Graziosi, G.; Spruijt, J.; Berkhof, J.; Balas, C.; Papagiannakis, E.; Snijders, P.; et al. Dynamic spectral imaging colposcopy: Higher sensitivity for detection of premalignant cervical lesions. BJOG 2011, 118, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Louwers, J.A.; Zaal, A.; Kocken, M.; Berkhof, J.; Papagiannakis, E.; Snijders, P.; Meijer, C.; Verheijen, R. The performance of Dynamic Spectral Imaging colposcopy depends on indication for referrals. Gynecol. Oncol. 2015, 139, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Coronado, P.J.; Fasero, M. Colposcopy combined with dynamic spectral imaging. A prospective clinical study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 196, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Cholkeri-Singh, A.; Lavin, P.T.; Olson, C.G.; Papagiannakis, E.; Weinberg, L. Digital colposcopy with dynamic spectral imaging for detection of cervical Intraepithelial Neoplasia 2 in low-grade referrals: The IMPROVE-COLPO study. J. Low Genit. Tract Dis. 2018, 22, 21–26. [Google Scholar] [CrossRef] [PubMed]
- DeNardis, S.A.; Lavin, P.T.; Livingston, J.; Salter, W.R.; James-Patrick, N.; Papagiannakis, E.; Olson, C.G.; Weinberg, L. Increased detection of precancerous cervical lesions with adjunctive dynamic spectral imaging. Int. J. Womens Health 2017, 9, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zaal, A.; Louwers, J.A.; Berkhof, J.; Kocken, M.; TerHarmsel, W.A.; Graziosi, G.C.; Spruijt, J.; Balas, C.; Papagiannakis, E.; Snijders, P.; et al. Agreement between colposcopic impression and histological diagnosis among human papillomavirus type 16-positive women: A clinical trial using dynamic spectral imaging colposcopy. BJOG 2012, 119, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.B.; Petersen, L.K.; Blaakaer, J.; Johansen, T.; Mertz, H.; Dahl, K.; Bor, P. Can the dynamic spectral imaging (DSI) color map improve colposcopy examination for precancerous cervical lesions? A prospective evaluation of the DSI color map in a multi-biopsy clinical setting. BMC Womens Health 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.E.; Lavin, P.T.; Akin, M.D.; Papagiannakis, E.; Denardis, S. Rate of detecting CIN3+ among patients with ASC-US using digital colposcopy and dynamic spectral imaging. Oncol. Lett. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Piccoli, R.; Lavitola, G.; Di Spiezio Sardo, A.; Spinelli, M.; Cavallaro, A.; Nappi, C. Endocervicoscopy: A new technique for the diagnostic work-up of cervical intraepithelial neoplasia allowing a tailored excisional therapy in young fertile women. Fertil. Steril. 2010, 94, 2726–2731. [Google Scholar] [CrossRef] [PubMed]
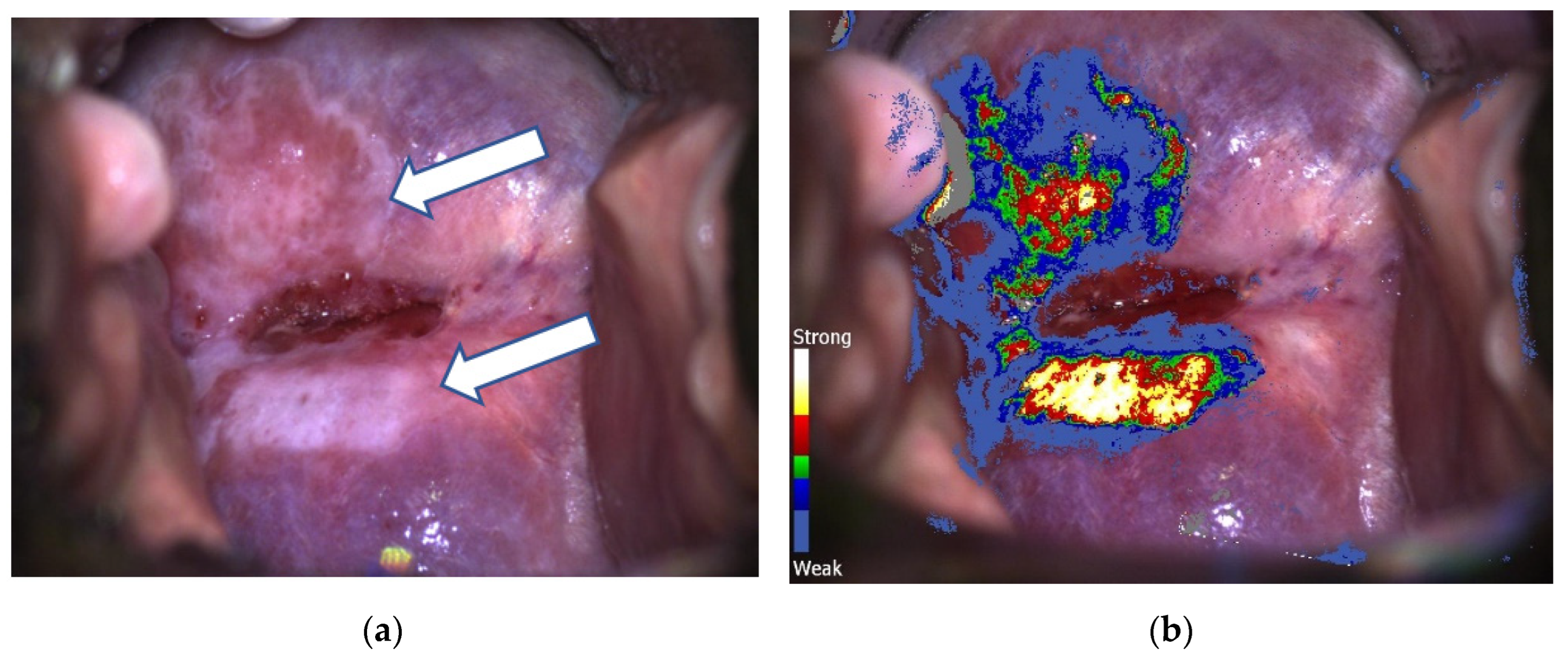
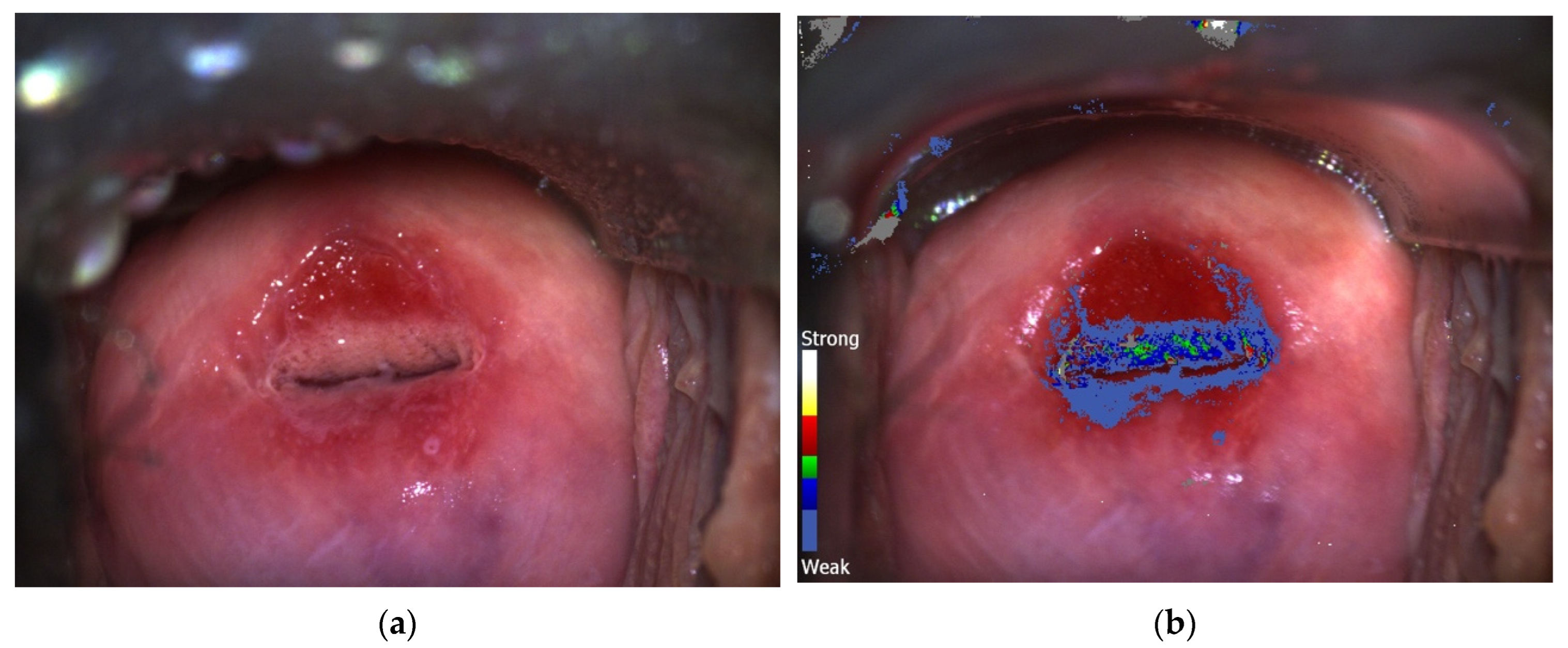
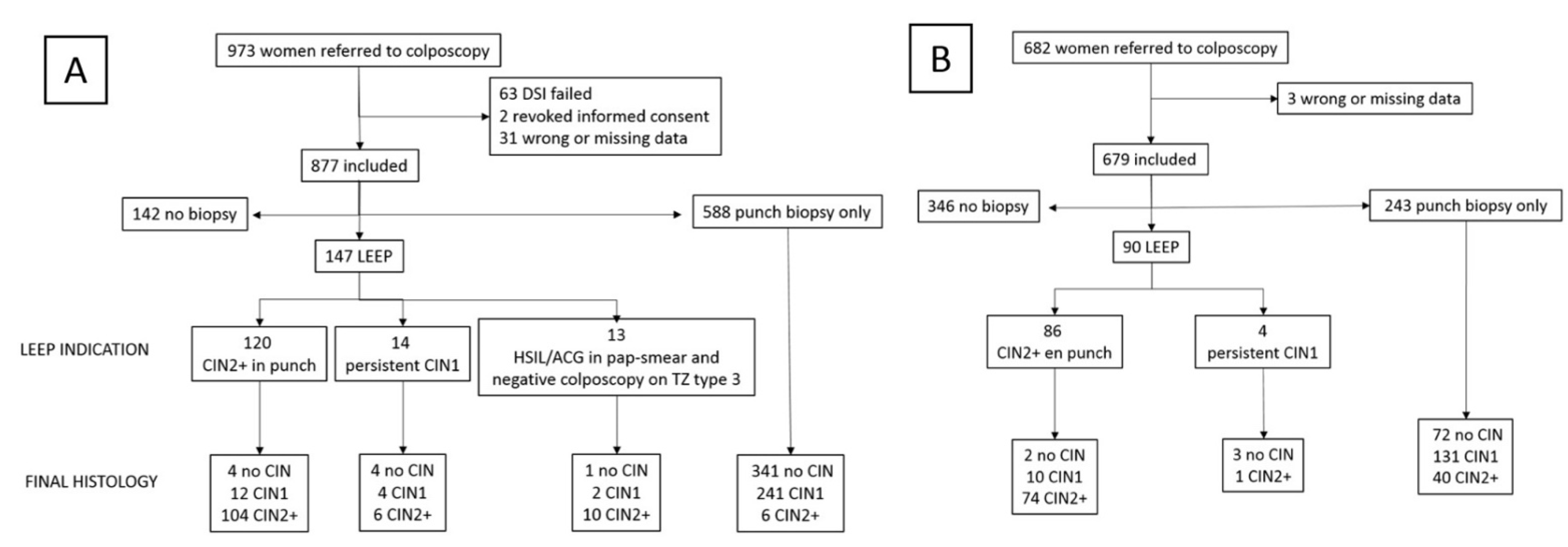
| C-DSI Group N (%)/Mean (SD) N = 973 | CC Group N (%)/Mean (SD) N = 682 | |
|---|---|---|
| Age (years) | 35.16 (10.8) | 35.7 (11.1%) |
| Age at first sexual intercourse | 18.04 (3.0) | 18.7 (9.6%) |
| Anal sex | 138 (25.5%) | 150 (22.1%) |
| Current Smoker | 197 (20.2%) | 154 (22.7%) |
| Pap smear | ||
| Normal/ASCUS/LSIL | 762 (83.4%) | 566 (88.0%) |
| ASC-H/AGC/HSIL/Cancer | 149 (16.4%) | 77 (12.0%) |
| CC/C-DSI result | ||
| Normal/Metaplasia | 359 (36.9%) | 284 (41.8%) |
| Low-grade abnormal changes | 477 (49%) | 306 (45.1%) |
| High-grade abnormal changes | 130 (13.4%) | 84 (12.4%) |
| Cancer | 7 (0.7%) | 5 (0.7%) |
| HPV | ||
| Genotype 16/18 | 137 (14.1%) | 113 (16.6%) |
| High-risk HPV no 16/18 | 199 (20.5%) | 141 (20.8%) |
| Low-risk HPV | 88 (9%) | 73 (10.8%) |
| Negative | 178 (18.3%) | 70 (10.3%) |
| Undetermined | 371 (38.1%) | 282 (41.5%) |
| Immunocompromised | ||
| HIV+ | 17 (1.8%) | 1 (0.2%) |
| Other | 25 (2.6%) | 19 (2.9%) |
| Patients who required LEEP | 162 (16.6%) | 75 (10.9%) |
| Normal/Low grade | 52 (5.3%) | 35 (5.1%) |
| High grade | 110 (11.3%) | 40 (5.8%) |
| A. Detection of HSIL/CIN2+ and HSIL/CIN3+ in Colposcopy in Combination with DySIS versus Conventional | ||||||
|---|---|---|---|---|---|---|
| Detection of HSIL/CIN2+ | ||||||
| Sensitivity *** | Specificity *** | PPV | PNV | LHR+ | LHR− | |
| C-DSI (N = 856) | 76.8 (69.4–84.2) | 82.59 (79.8–85.4) | 46.0 (39.3–52.5) | 94.9 (93.1–96.7) | 4.4 (3.7–5.3) | 00.28 (0.2–0.4) |
| CC (N = 565) | 54.2 (43.7– 64.7) | 94.0 (91.7–96.3) | 65.8 (54.7–76.9) | 90.6 (87.9–93.4) | 9.1 (6.0–13.7) | 0.5 (0.4–0.6) |
| Detection of HSIL/CIN3+ | ||||||
| Sensitivity *** | Specificity *** | PPV | PNV | LHR+ | LHR− | |
| C-DSI (N = 856) | 86.8 (78.9–94.6) | 79.4 (76.5–82.3) | 31.2 (25.0–37.4) | 98.2 (97.1–99.4) | 4.2 (3.6–5.0) | 0.2 (0.1–0.3) |
| CC (N = 547) | 72.2 (59.4–85.1) | 91.9 (89.4–94.4) | 49.3 (37.7–61.0) | 96.8 (95.1–98.5) | 8.9 (6.3–12.5) | 0.3 (0.2–0.5) |
| B. Detection of HSIL/CIN2+ based on the previous Pap-smear result and HPV presence. | ||||||
| Negative Pap smear | ||||||
| Sensitivity *** | Specificity ** | PPV | PNV | LHR+ | LHR− | |
| C-DSI (N = 74) | 66.7 (0.0–100.0) | 85.9 (77.1–94.7) | 16.7 (0.0–41.9) | 98.4 (94.5–100.0) | 10.3 (1.0–105.1) | 0.9 (0.7–1.1) |
| CC (N = 214) | 21.4 (0.0–46.5) | 97.9 (95.6–100.0) | 42.9 (0.0–86.7) | 94.4 (91.0- 97.9) | 10.2 (2.5–41.3) | 0.8 (0.6–11.1) |
| ASCUS/LSIL | ||||||
| Sensitivity ** | Specificity ** | PPV | PNV | LHR+ | LHR− | |
| C-DSI (N = 640) | 61.5 (45.0–78.1) | 82.8 (79.8–86.0) | 18.9 (11.7–26.1) | 97.1 (95.5–98.6) | 3.6 (2.7–4.9) | 0.4 (0.3–0.7) |
| CC (N = 274) | 33.3 (17.3–49.4) | 93.6 (90.3–97.0) | 46.4 (26.2–67.0) | 89.4 (85.4–93.5) | 5.22 (2.7–10.1) | 0.7 (0.6–0.9) |
| ASC-H/HSIL/ACG/Cancer | ||||||
| Sensitivity | Specificity | PPV | PNV | LHR+ | LHR− | |
| C-DSI (N = 139) | 83.3 (75.4–91.3) | 72.1 (57.5–86.7) | 87.0 (79.5–94.4) | 66.0 (51.4–80.6) | 3.0 (1.8–4.9) | 0.2 (0.1–0.4) |
| CC (N = 68) | 83.7 (71.5–95.9) | 66.7 (45.7–87.6) | 81.8 (69.3–94.4) | 69.6 (48.6–90.6) | 2.5 (1.4–4.5) | 0.2 (0.1–0.5) |
| HPV 16/18 | ||||||
| Sensitivity | Specificity * | PPV | PNV | LHR+ | LHR− | |
| C-DSI (N = 117) | 73.5 (57.2–89.8) | 78.3 (68.8–87.8) | 58.14 (42.2–74.1) | 87.84 (79.7–96.0) | 3.39 (2.2–5.4) | 0.34 (0.2–0.6) |
| CC (N = 31) | 56.67 (37.3–76.1) | 94.12 (87.8–100.0) | 81.0 (61.8–100.0) | 83.1 (74.1–92.1) | 9.6 (3.5–26.2) | 0.5 (0.3–0.7) |
| HR-HPV | ||||||
| Sensitivity | Specificity | PPV | PNV | LHR+ | LHR− | |
| C-DSI (N = 176) | 68.8 (42.9–94.6) | 81.3 (74.9–87.6) | 26.8 (12.1–41.6) | 96.3 (92.7–99.9) | 3.7 (2.3–5.8) | 0.4 (0.2–0.8) |
| CC (N = 122) | 6.3 (0.0–21.2) | 93.4 (88.2–98.6) | 12.5 (0.0–41.7) | 86.8 (80.2–93.5) | 1.0 (0.1–7.2) | 1.0 (0.88–1.2) |
| Progression to HSIL/CIN2+ | ||||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | PNV | LHR+ | LHR− | |
| 6 months | 30 (0–63.4) | 92.8 (83.8–100) | 50 (1.7–98.3) | 84.8 (73.3–96.3) | 4.2 (0.9–17.8) | 0.7 (0.5–1.1) |
| 12 months | 17.6 (0- 38.7) | 74.3 (63.7–84.9) | 13.6 (0–30.3) | 79.7 (69.5–89.9) | 0.7 (0.2–2.0) | 1.11 (0.8–1.4) |
| 24 months | 35.7 (7.0–64.4) | 75.5 (57.2–89.9) | 35.7 (7.0–64.4) | 75.5 (57.2–89.8) | 1.4 (0.6–3.3) | 0.9 (0.6–1.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González González, V.; Ramírez Mena, M.d.M.; Calvo Torres, J.; Herráiz Martínez, M.Á.; Serrano García, I.; Coronado, P. Analysis of New Colposcopy Techniques in the Diagnosis and Evolution of SIL/CIN: Comparison of Colposcopy with the DSI System (COLPO-DSI Study). J. Pers. Med. 2023, 13, 1605. https://doi.org/10.3390/jpm13111605
González González V, Ramírez Mena MdM, Calvo Torres J, Herráiz Martínez MÁ, Serrano García I, Coronado P. Analysis of New Colposcopy Techniques in the Diagnosis and Evolution of SIL/CIN: Comparison of Colposcopy with the DSI System (COLPO-DSI Study). Journal of Personalized Medicine. 2023; 13(11):1605. https://doi.org/10.3390/jpm13111605
Chicago/Turabian StyleGonzález González, Virginia, María del Mar Ramírez Mena, Javier Calvo Torres, Miguel Ángel Herráiz Martínez, Irene Serrano García, and Pluvio Coronado. 2023. "Analysis of New Colposcopy Techniques in the Diagnosis and Evolution of SIL/CIN: Comparison of Colposcopy with the DSI System (COLPO-DSI Study)" Journal of Personalized Medicine 13, no. 11: 1605. https://doi.org/10.3390/jpm13111605
APA StyleGonzález González, V., Ramírez Mena, M. d. M., Calvo Torres, J., Herráiz Martínez, M. Á., Serrano García, I., & Coronado, P. (2023). Analysis of New Colposcopy Techniques in the Diagnosis and Evolution of SIL/CIN: Comparison of Colposcopy with the DSI System (COLPO-DSI Study). Journal of Personalized Medicine, 13(11), 1605. https://doi.org/10.3390/jpm13111605





