The Impact of Antibiotics and Steroids on the Nasal Microbiome in Patients with Chronic Rhinosinusitis: A Systematic Review According to PICO Criteria
Abstract
:1. Introduction
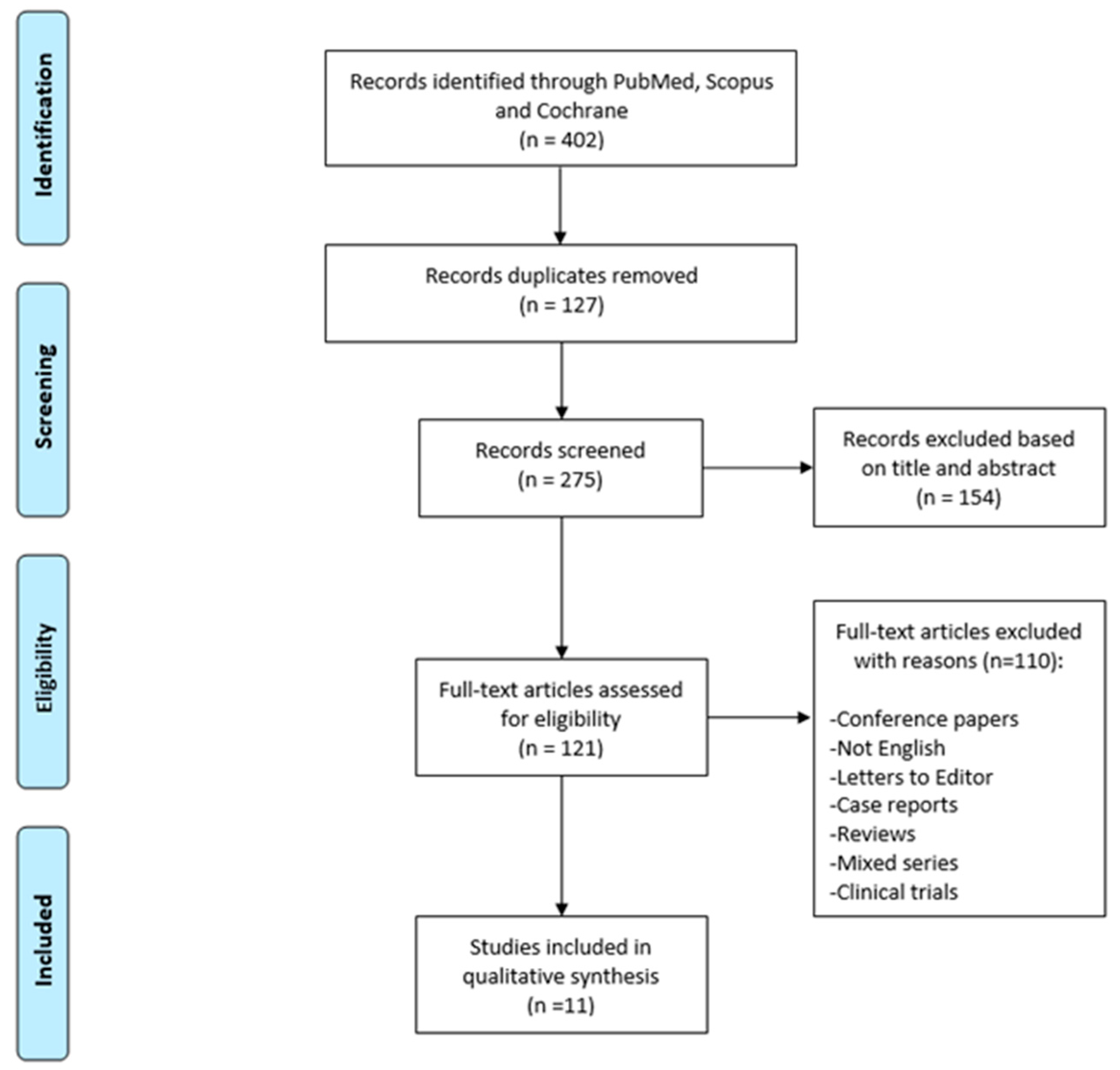
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hastan, D.; Fokkens, W.J.; Bachert, C.; Newson, R.B.; Bislimovska, J.; Bockelbrink, A.; Bousquet, P.J.; Brozek, G.; Bruno, A.; Dahlén, S.E.; et al. Chronic rhinosinusitis in Europe—An underestimated disease. A GA2LEN study. Allergy 2011, 66, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Viskens, A.S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M.; Chaker, A.; et al. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Han, M.S.; Kwak, J.; Kim, T.H. Association Between Microbiota and Nasal Mucosal Diseases in terms of Immunity. Int. J. Mol. Sci. 2021, 22, 4744. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Schenck, L.P.; Surette, M.G.; Bowdish, D.M. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016, 590, 3705–3720. [Google Scholar] [CrossRef]
- Dlugaszewska, J.; Leszczynska, M.; Lenkowski, M.; Tatarska, A.; Pastusiak, T.; Szyfter, W. The pathophysiological role of bacterial biofilms in chronic sinusitis. Eur. Arch. Otorhinolaryngol. 2016, 273, 1989–1994. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Wormald, P.J. Therapy of Sinonasal Microbiome in CRS: A Critical Approach. Curr. Allergy Asthma Rep. 2017, 17, 59. [Google Scholar] [CrossRef]
- Hopkins, C.; Williamson, E.; Morris, S.; Clarke, C.S.; Thomas, M.; Evans, H.; Little, P.; Lund, V.J.; Blackshaw, H.; Schilder, A.; et al. Antibiotic usage in chronic rhinosinusitis: Analysis of national primary care electronic health records. Rhinology 2019, 57, 420–429. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Maxfield, A.Z.; Korkmaz, H.; Gregorio, L.L.; Busaba, N.Y.; Gray, S.T.; Holbrook, E.H.; Guo, R.; Bleier, B.S. General antibiotic exposure is associated with increased risk of developing chronic rhinosinusitis. Laryngoscope 2017, 127, 296–302. [Google Scholar] [CrossRef]
- Ni, J.; Friedman, H.; Boyd, B.C.; McGurn, A.; Babinski, P.; Markossian, T.; Dugas, L.R. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr. 2019, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Mosholder, A.D.; Lee, J.Y.; Zhou, E.H.; Kang, E.M.; Ghosh, M.; Izem, R.; Major, J.M.; Graham, D.J. Long-Term Risk of Acute Myocardial Infarction, Stroke, and Death With Outpatient Use of Clarithromycin: A Retrospective Cohort Study. Am. J. Epidemiol. 2018, 187, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Schembri, S.; Williamson, P.A.; Short, P.M.; Singanayagam, A.; Akram, A.; Taylor, J.; Singanayagam, A.; Hill, A.T.; Chalmers, J.D. Cardiovascular events after clarithromycin use in lower respiratory tract infections: Analysis of two prospective cohort studies. BMJ 2013, 346, 1235. [Google Scholar] [CrossRef] [PubMed]
- Winkel, P.; Hilden, J.; Hansen, J.F.; Kastrup, J.; Kolmos, H.J.; Kjøller, E.; Jensen, G.B.; Skoog, M.; Lindschou, J.; Gluud, C.; et al. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular morbidity over 10years in the CLARICOR randomised, blinded clinical trial. Int. J. Cardiol. 2015, 182, 459–465. [Google Scholar] [CrossRef]
- Mullol, J.; Obando, A.; Pujols, L.; Alobid, I. Corticosteroid treatment in chronic rhinosinusitis: The possibilities and the limits. Immunol. Allergy Clin. N. Am. 2009, 29, 657–668. [Google Scholar] [CrossRef]
- Hox, V.; Lourijsen, E.; Jordens, A.; Aasbjerg, K.; Agache, I.; Alobid, I.; Bachert, C.; Boussery, K.; Campo, P.; Fokkens, W.; et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: An EAACI position paper. Clin. Transl. Allergy 2020, 10, 1. [Google Scholar] [CrossRef]
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Latek, M.; Lacwik, P.; Molinska, K.; Blauz, A.; Lach, J.; Rychlik, B.; Strapagiel, D.; Majak, J.; Molinska, J.; Czech, D.; et al. Effect of an Intranasal Corticosteroid on Quality of Life and Local Microbiome in Young Children With Chronic Rhinosinusitis: A Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 345–352. [Google Scholar] [CrossRef]
- Liu, C.M.; Kohanski, M.A.; Mendiola, M.; Soldanova, K.; Dwan, M.G.; Lester, R.; Nordstrom, L.; Price, L.B.; Lane, A.P. Impact of saline irrigation and topical corticosteroids on the postsurgical sinonasal microbiota. Int. Forum Allergy Rhinol. 2015, 5, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, B.; Huang, Q.; Li, C.; Wu, Y.; Huang, Z.; Li, Y.; Qu, J.; Xiao, N.; Wang, M. Efficacy and Safety of Long-Term Low-Dose Clarithromycin in Patients With Refractory Chronic Sinusitis After Endoscopic Sinus Surgery: A Prospective Clinical Trial. Ear Nose Throat J. 2021, 1455613211032020. [Google Scholar] [CrossRef] [PubMed]
- Siu, J.; Mackenzie, B.W.; Klingler, L.; Biswas, K.; Wang, Y.; Hung, C.T.; Jeong, S.H.; Barnett, D.; Tingle, M.D.; Douglas, R.G. Sinonasal and gastrointestinal bacterial composition and abundance are stable after 1 week of once-daily oral antibiotic treatment for chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2021, 11, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Lux, C.A.; Wagner Mackenzie, B.; Johnston, J.; Zoing, M.; Biswas, K.; Taylor, M.W.; Douglas, R.G. Antibiotic Treatment for Chronic Rhinosinusitis: Prescription Patterns and Associations With Patient Outcome and the Sinus Microbiota. Front. Microbiol. 2020, 11, 595555. [Google Scholar] [CrossRef] [PubMed]
- Hauser, L.J.; Ir, D.; Kingdom, T.T.; Robertson, C.E.; Frank, D.N.; Ramakrishnan, V.R. Investigation of bacterial repopulation after sinus surgery and perioperative antibiotics. Int. Forum Allergy Rhinol. 2016, 6, 34–40. [Google Scholar] [CrossRef]
- Alammar, Y.; Rousseau, S.; Desrosiers, M.; Tewfik, M.A. The Effect of Corticosteroids on Sinus Microbiota in Chronic Rhinosinusitis Patients with Nasal Polyposis. Am. J. Rhinol. Allergy 2023, 37, 638–645. [Google Scholar] [CrossRef]
- Renteria, A.E.; Maniakas, A.; Mfuna, L.E.; Asmar, M.H.; Gonzalez, E.; Desrosiers, M. Low-dose and long-term azithromycin significantly decreases Staphylococcus aureus in the microbiome of refractory CRS patients. Int. Forum Allergy Rhinol. 2021, 11, 93–105. [Google Scholar] [CrossRef]
- Cherian, L.M.; Bassiouni, A.; Cooksley, C.M.; Vreugde, S.; Wormald, P.J.; Psaltis, A.J. The clinical outcomes of medical therapies in chronic rhinosinusitis are independent of microbiomic outcomes: A double-blinded, randomised placebo-controlled trial. Rhinology 2020, 58, 559–567. [Google Scholar] [CrossRef]
- Jain, R.; Hoggard, M.; Zoing, M.; Jiang, Y.; Biswas, K.; Taylor, M.W.; Douglas, R.G. The effect of medical treatments on the bacterial microbiome in patients with chronic rhinosinusitis: A pilot study. Int. Forum Allergy Rhinol. 2018, 8, 890–899. [Google Scholar] [CrossRef]
- Liu, C.M.; Soldanova, K.; Nordstrom, L.; Dwan, M.G.; Moss, O.L.; Contente-Cuomo, T.L.; Keim, P.; Price, L.B.; Lane, A.P. Medical therapy reduces microbiota diversity and evenness in surgically recalcitrant chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2013, 3, 775–781. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sahin-Yilmaz, A.; Naclerio, R.M. Anatomy and physiology of the upper airway. Proc. Am. Thorac. Soc. 2011, 8, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Y.; Shen, Z.; Shi, L. The nasal microbiome of predicting bronchopulmonary dysplasia in preterm infants. Sci. Rep. 2022, 12, 7727. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef]
- Watson, R.L.; de Koff, E.M.; Bogaert, D. Characterising the respiratory microbiome. Eur. Respir. J. 2019, 53, 1801711. [Google Scholar] [CrossRef]
- Stearns, J.C.; Davidson, C.J.; McKeon, S.; Whelan, F.J.; Fontes, M.E.; Schryvers, A.B.; Bowdish, D.M.; Kellner, J.D.; Surette, M.G. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J. 2015, 9, 1246–1259. [Google Scholar] [CrossRef]
- Luna, P.N.; Hasegawa, K.; Ajami, N.J.; Espinola, J.A.; Henke, D.M.; Petrosino, J.F.; Piedra, P.A.; Sullivan, A.F.; Camargo, C.A., Jr.; Shaw, C.A.; et al. The association between anterior nares and nasopharyngeal microbiota in infants hospitalized for bronchiolitis. Microbiome 2018, 6, 2. [Google Scholar] [CrossRef]
- Cohen, N.A. Sinonasal mucociliary clearance in health and disease. Ann. Otol. Rhinol. Laryngol. Suppl. 2006, 196, 20–26. [Google Scholar] [CrossRef]
- Yan, M.; Pamp, S.J.; Fukuyama, J.; Hwang, P.H.; Cho, D.Y.; Holmes, S.; Relman, D.A. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 2013, 14, 631–640. [Google Scholar] [CrossRef]
- Reddy, U.D.; Dev, B. Pictorial essay: Anatomical variations of paranasal sinuses on multidetector computed tomography-How does it help FESS surgeons? Indian J. Radiol. Imaging 2012, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y. Histology of the human nasopharyngeal mucosa. J. Anat. 1965, 99, 657–672. [Google Scholar]
- van Kempen, M.J.; Rijkers, G.T.; Van Cauwenberge, P.B. The immune response in adenoids and tonsils. Int. Arch. Allergy Immunol. 2000, 122, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.M.; Relman, D.A. The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat. Cell Host Microbe 2017, 21, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Whitcroft, K.L.; Altundag, A.; Balungwe, P.; Boscolo-Rizzo, P.; Douglas, R.; Enecilla, M.L.B.; Fjaeldstad, A.W.; Fornazieri, M.A.; Frasnelli, J.; Gane, S.; et al. Position paper on olfactory dysfunction: 2023. Rhinology 2023. [Google Scholar] [CrossRef]
- Han, X.; He, X.; Zhan, X.; Yao, L.; Sun, Z.; Gao, X.; Wang, S.; Wang, Z. Disturbed microbiota-metabolites-immune interaction network is associated with olfactory dysfunction in patients with chronic rhinosinusitis. Front. Immunol. 2023, 14, 1159112. [Google Scholar] [CrossRef]
- Copeland, E.; Leonard, K.; Carney, R.; Kong, J.; Forer, M.; Naidoo, Y.; Oliver, B.G.G.; Seymour, J.R.; Woodcock, S.; Burke, C.M.; et al. Chronic Rhinosinusitis: Potential Role of Microbial Dysbiosis and Recommendations for Sampling Sites. Front. Cell. Infect. Microbiol. 2018, 8, 57. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Ancona, G.; Alagna, L.; Alteri, C.; Palomba, E.; Tonizzo, A.; Pastena, A.; Muscatello, A.; Gori, A.; Bandera, A. Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID. Front. Immunol. 2023, 14, 1080043. [Google Scholar] [CrossRef]
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Mackenzie, B.W.; Cope, E.K.; Ramakrishnan, V.R. Unraveling the role of the microbiome in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, T.J.; Campion, N.J.; Freisl, K.; Liu, D.T.; Gangl, K.; Stanek, V.; Tu, A.; Pjevac, P.; Hausmann, B.; Eckl-Dorna, J.; et al. The nasal microbiome in patients suffering from non-steroidal anti-inflammatory drugs-exacerbated respiratory disease in absence of corticosteroids. Front. Immunol. 2023, 14, 1112345. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Frank, D.N. The microbiome of the middle meatus in healthy adults. PLoS ONE 2013, 8, 85507. [Google Scholar] [CrossRef] [PubMed]
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef]
- Koskinen, K.; Pausan, M.R.; Perras, A.K.; Beck, M.; Bang, C.; Mora, M.; Schilhabel, A.; Schmitz, R.; Moissl-Eichinger, C. First Insights into the Diverse Human Archaeome: Specific Detection of Archaea in the Gastrointestinal Tract, Lung, and Nose and on Skin. mBio 2017, 8, e00824-17. [Google Scholar] [CrossRef]
- Van der Schans, C.P. Bronchial mucus transport. Respir. Care 2007, 52, 1150–1158. [Google Scholar]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef]
- Lee, J.T.; Frank, D.N.; Ramakrishnan, V. Microbiome of the paranasal sinuses: Update and literature review. Am. J. Rhinol. Allergy 2016, 30, 3–16. [Google Scholar] [CrossRef]
- Cope, E.K.; Goldberg, A.N.; Pletcher, S.D.; Lynch, S.V. Compositionally and functionally distinct sinus microbiota in chronic rhinosinusitis patients have immunological and clinically divergent consequences. Microbiome 2017, 5, 53. [Google Scholar] [CrossRef]
- Choi, E.B.; Hong, S.W.; Kim, D.K.; Jeon, S.G.; Kim, K.R.; Cho, S.H.; Gho, Y.S.; Jee, Y.K.; Kim, Y.K. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy 2014, 69, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Aurora, R.; Chatterjee, D.; Hentzleman, J.; Prasad, G.; Sindwani, R.; Sanford, T. Contrasting the microbiomes from healthy volunteers and patients with chronic rhinosinusitis. JAMA Otolaryngol. Head. Neck Surg. 2013, 139, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Chalermwatanachai, T.; Vilchez-Vargas, R.; Holtappels, G.; Lacoere, T.; Jáuregui, R.; Kerckhof, F.M.; Pieper, D.H.; Van de Wiele, T.; Vaneechoutte, M.; Van Zele, T.; et al. Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci. Rep. 2018, 8, 7926. [Google Scholar] [CrossRef] [PubMed]
- Loperfido, A.; Ciofalo, A.; Cavaliere, C.; Begvarfaj, E.; Cascone, F.; Alfonzo, G.; Cadeddu, R.; Millarelli, S.; Bellocchi, G.; Greco, A.; et al. Dupilumab’s Impact on Blood Parameters in Nasal Polyposis: 18-Month Follow-Up in Real Life. J. Immunol. Res. 2023, 2023, 4027701. [Google Scholar] [CrossRef]
- Plath, M.; Derycke, L.; Sand, M.; Van de Vyvere, D.; Delemarre, T.; Cavaliere, C.; Plinkert, P.K.; Holtappels, G.; Bachert, C. Can patient-reported outcomes and inflammatory markers define endotype 2 in chronic rhinosinusitis without nasal polyps? Ann. Allergy Asthma Immunol. 2023, 130, 485–493. [Google Scholar] [CrossRef]
- Lan, F.; Zhang, N.; Holtappels, G.; De Ruyck, N.; Krysko, O.; Van Crombruggen, K.; Braun, H.; Johnston, S.L.; Papadopoulos, N.G.; Zhang, L.; et al. Staphylococcus aureus Induces a Mucosal Type 2 Immune Response via Epithelial Cell-derived Cytokines. Am. J. Respir. Crit. Care Med. 2018, 198, 452–463. [Google Scholar] [CrossRef]
- Bachert, C.; Holtappels, G.; Merabishvili, M.; Meyer, T.; Murr, A.; Zhang, N.; Van Crombruggen, K.; Gevaert, E.; Völker, U.; Bröker, B.M.; et al. Staphylococcus aureus controls interleukin-5 release in upper airway inflammation. J. Proteom. 2018, 180, 53–60. [Google Scholar] [CrossRef]
- Shaghayegh, G.; Cooksley, C.; Ramezanpour, M.; Wormald, P.J.; Psaltis, A.J.; Vreugde, S. Chronic Rhinosinusitis, S. aureus Biofilm and Secreted Products, Inflammatory Responses, and Disease Severity. Biomedicines 2022, 10, 1362. [Google Scholar] [CrossRef]
- Lal, D.; Keim, P.; Delisle, J.; Barker, B.; Rank, M.A.; Chia, N.; Schupp, J.M.; Gillece, J.D.; Cope, E.K. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int. Forum Allergy Rhinol. 2017, 7, 561–569. [Google Scholar] [CrossRef]
- Green, B.J.; Wiriyachaiporn, S.; Grainge, C.; Rogers, G.B.; Kehagia, V.; Lau, L.; Carroll, M.P.; Bruce, K.D.; Howarth, P.H. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 2014, 9, 100645. [Google Scholar] [CrossRef]
- Huntley, K.S.; Raber, J.; Fine, L.; Bernstein, J.A. Influence of the Microbiome on Chronic Rhinosinusitis with and without Polyps: An Evolving Discussion. Front. Allergy 2021, 2, 737086. [Google Scholar] [CrossRef] [PubMed]
- Feazel, L.M.; Robertson, C.E.; Ramakrishnan, V.R.; Frank, D.N. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope 2012, 122, 467–472. [Google Scholar] [CrossRef] [PubMed]
| Author | P Population | I Intervention | C Comparison | O Outcome |
|---|---|---|---|---|
| Alammar et al. 2023 [26] | Patients with CRSwNP | Prednisone + amoxicillin clavulanate or prednisone Duration: S for 3 weeks and A for 2 weeks | Healthy controls | Corynebacterium genera increases in CRSwNP-SA, Staphylococcus and Gram-negative genera (Pseudomonas) increase in CRSwNP-S |
| Latek et al. 2023 [20] | Children with CRS | Topical mometasone + NaCl solution Duration: 12 weeks | NaCl solution | INC increases nasopharyngeal microbiome richness |
| Chen et al. 2021 [22] | Patients with RCRS | Clarithromycin Duration: 12 weeks | Before treatment | Decrease in Streptococcus pneumoniae |
| Renteria et al. 2021 [27] | Patients with RCRS | Azithromycin + topical budesonide Durations: 16 weeks | Topical placebo + topical budesonide | Decrease in Staphylococcus aureus |
| Siu et al. 2021 [23] | Patients with CRS after surgery | Doxycycline or roxithromycin Duration: 1 week | No antibiotics treatment | No significant bacterial community shifts or changes |
| Cherian et al. 2020 [28] | Patients with CRS | Prednisolone or topical budesonide or doxycycline Duration: 3 weeks | Placebo | Increase of bacterial diversity in topical budesonide group |
| Lux et al. 2020 [24] | Patients with CRS | Amoxicillin clavulanate or doxycycline | Healthy controls | Increase of bacterial community dispersion in CRS patients |
| Jain et al. 2018 [29] | Patients with CRS | Doxycycline or prednisone Duration: 1 week | Patients with CRS | Variable and unpredictable changes of bacterial communities |
| Hauser et al. 2016 [25] | Patients with CRS after surgery | Amoxicillin clavulanate or clarithromycin and saline rinses Duration: 2 weeks | n.a. | No significant changes |
| Liu et al. 2015 [21] | Patients with RCRSwNP | Saline rinses ± topical budesonide | No active RS | No significant changes |
| Liu et al. 2013 [30] | Patients with RCRS | S ± A ± saline rinses ± INC | n.a. | Decrease in microbiota diversity and evenness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loperfido, A.; Cavaliere, C.; Begvarfaj, E.; Ciofalo, A.; D’Erme, G.; De Vincentiis, M.; Greco, A.; Millarelli, S.; Bellocchi, G.; Masieri, S. The Impact of Antibiotics and Steroids on the Nasal Microbiome in Patients with Chronic Rhinosinusitis: A Systematic Review According to PICO Criteria. J. Pers. Med. 2023, 13, 1583. https://doi.org/10.3390/jpm13111583
Loperfido A, Cavaliere C, Begvarfaj E, Ciofalo A, D’Erme G, De Vincentiis M, Greco A, Millarelli S, Bellocchi G, Masieri S. The Impact of Antibiotics and Steroids on the Nasal Microbiome in Patients with Chronic Rhinosinusitis: A Systematic Review According to PICO Criteria. Journal of Personalized Medicine. 2023; 13(11):1583. https://doi.org/10.3390/jpm13111583
Chicago/Turabian StyleLoperfido, Antonella, Carlo Cavaliere, Elona Begvarfaj, Andrea Ciofalo, Giovanni D’Erme, Marco De Vincentiis, Antonio Greco, Stefano Millarelli, Gianluca Bellocchi, and Simonetta Masieri. 2023. "The Impact of Antibiotics and Steroids on the Nasal Microbiome in Patients with Chronic Rhinosinusitis: A Systematic Review According to PICO Criteria" Journal of Personalized Medicine 13, no. 11: 1583. https://doi.org/10.3390/jpm13111583
APA StyleLoperfido, A., Cavaliere, C., Begvarfaj, E., Ciofalo, A., D’Erme, G., De Vincentiis, M., Greco, A., Millarelli, S., Bellocchi, G., & Masieri, S. (2023). The Impact of Antibiotics and Steroids on the Nasal Microbiome in Patients with Chronic Rhinosinusitis: A Systematic Review According to PICO Criteria. Journal of Personalized Medicine, 13(11), 1583. https://doi.org/10.3390/jpm13111583







