Unexpected Dramatic Evolution of Placenta Increta: Case Report and Literature Review
Abstract
1. Introduction
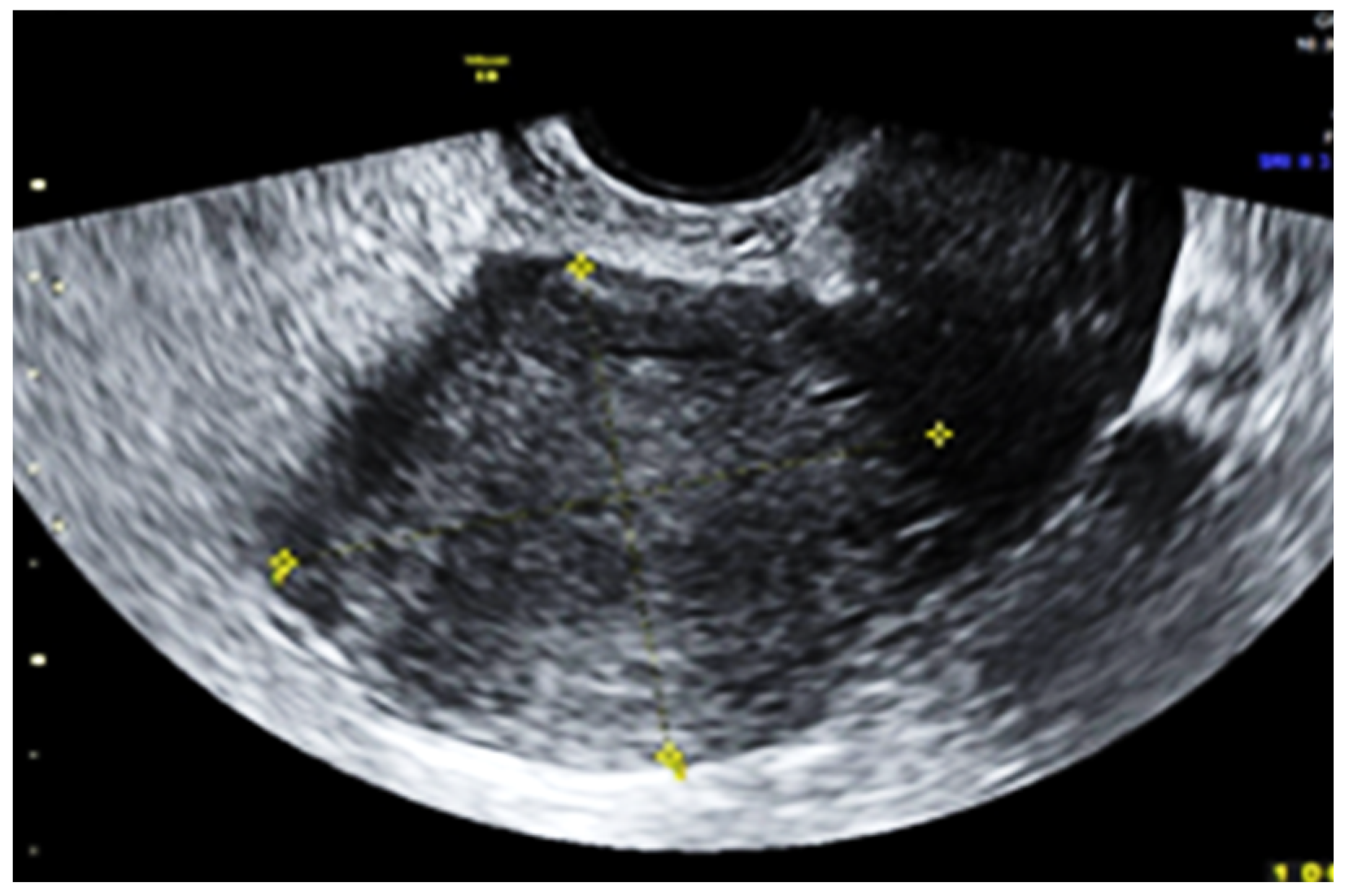
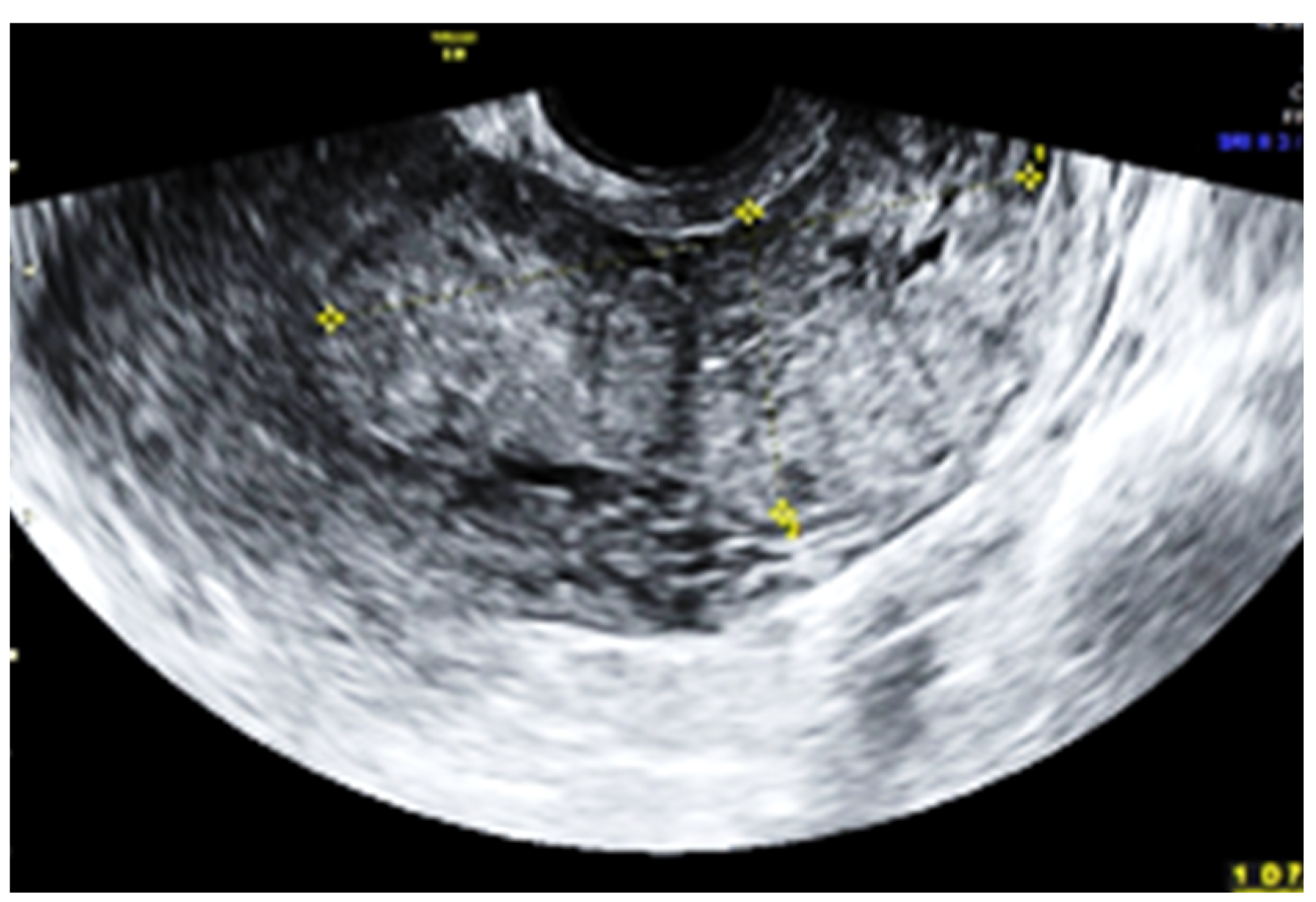
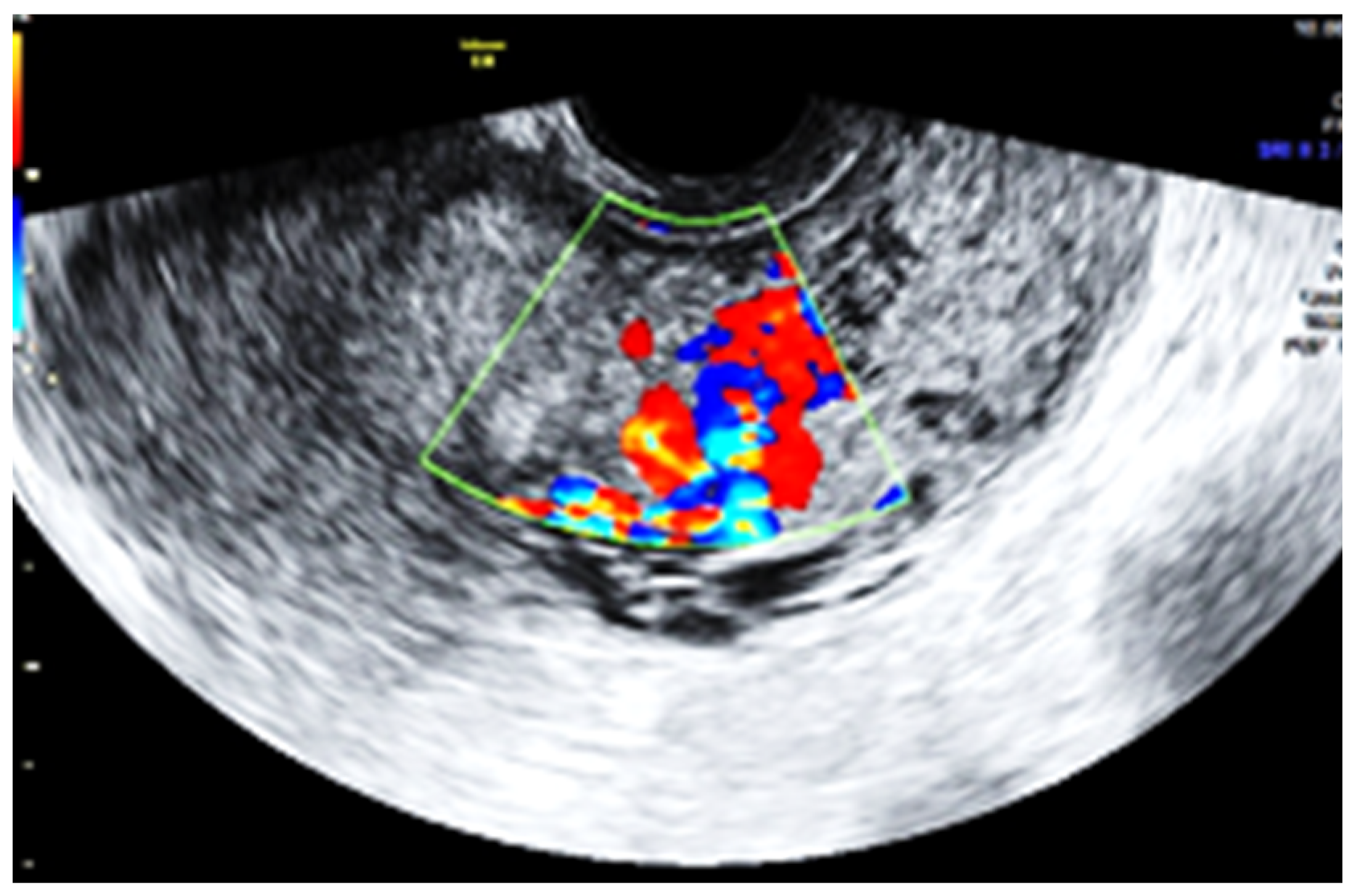
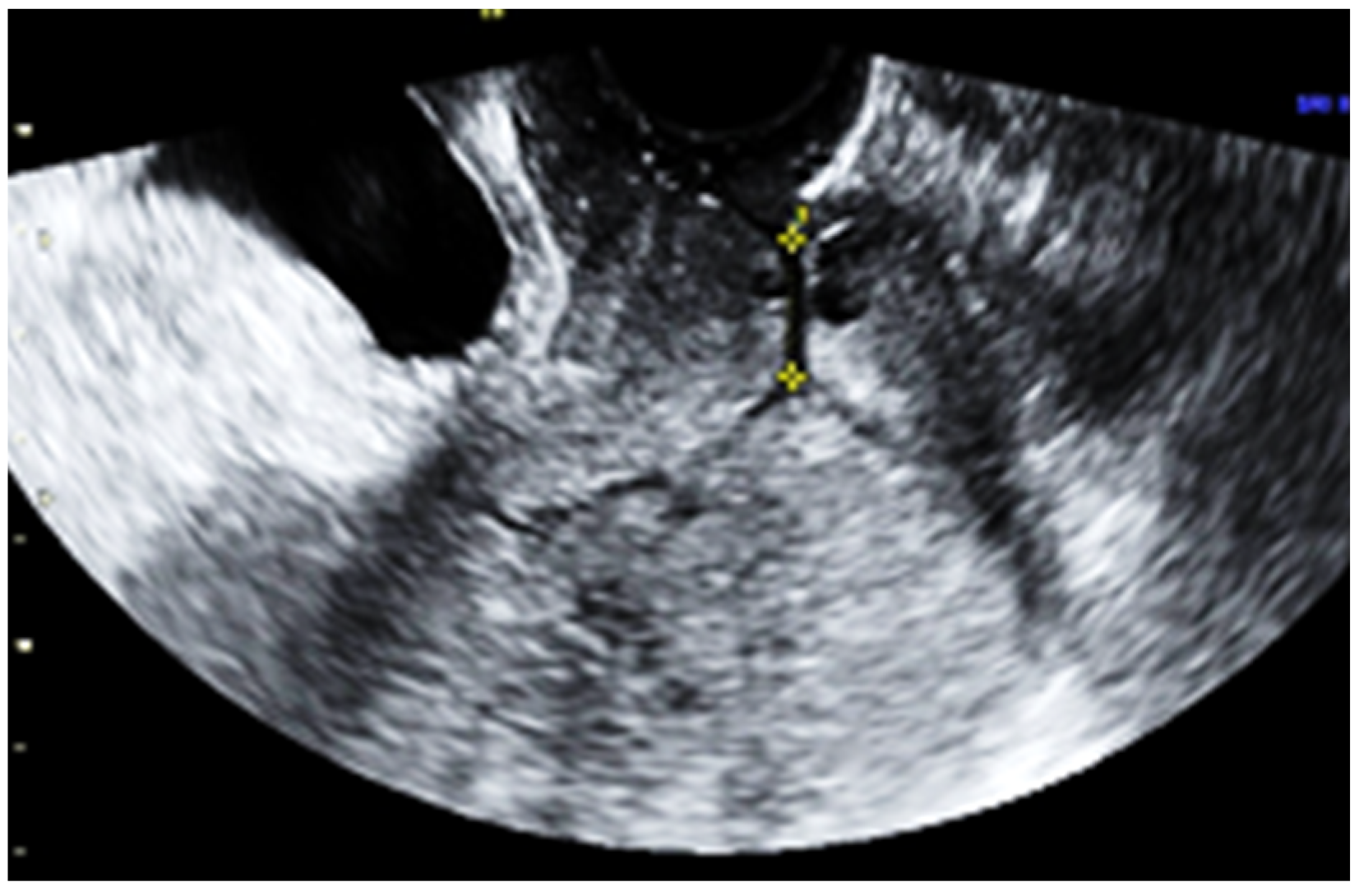
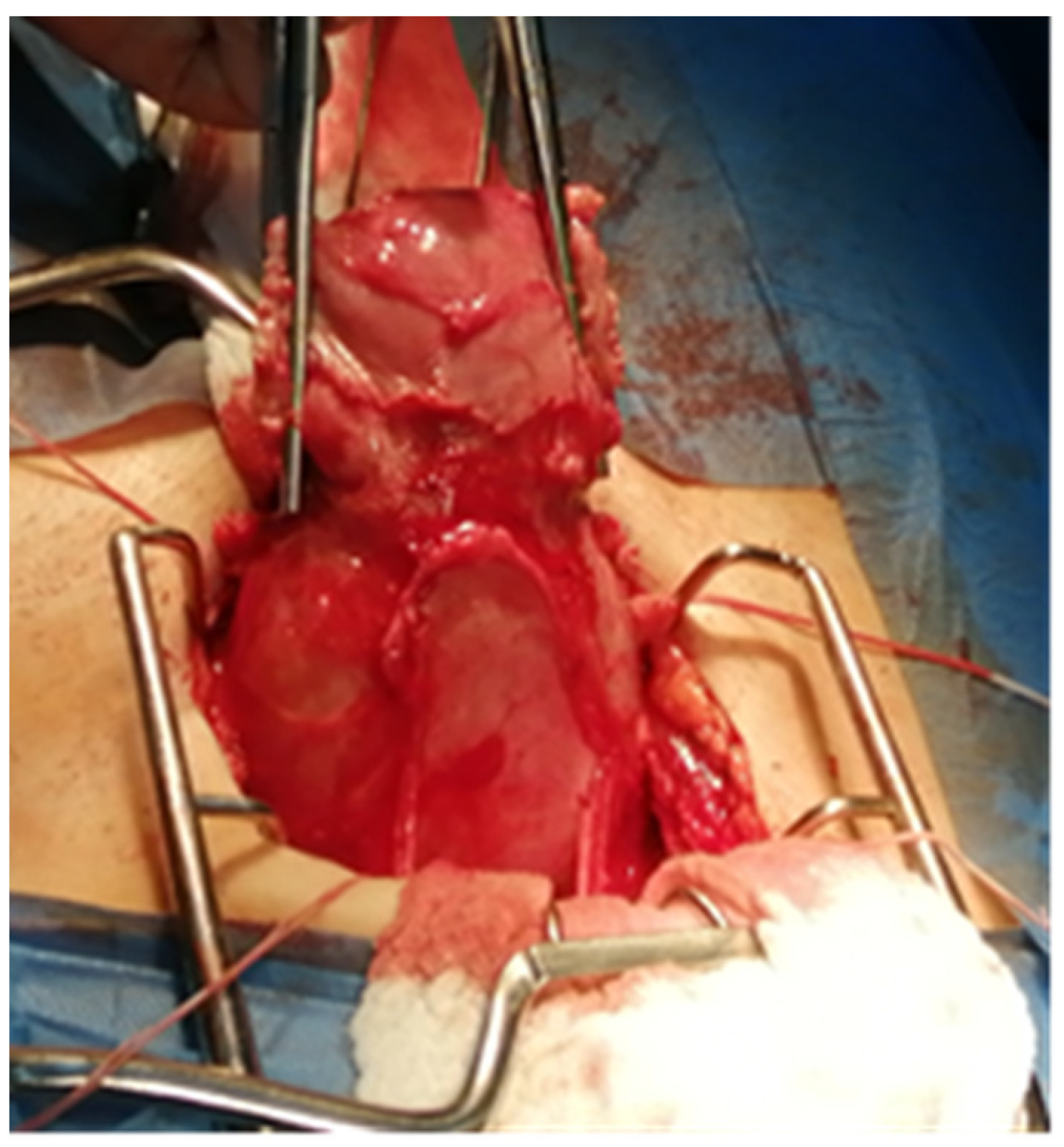
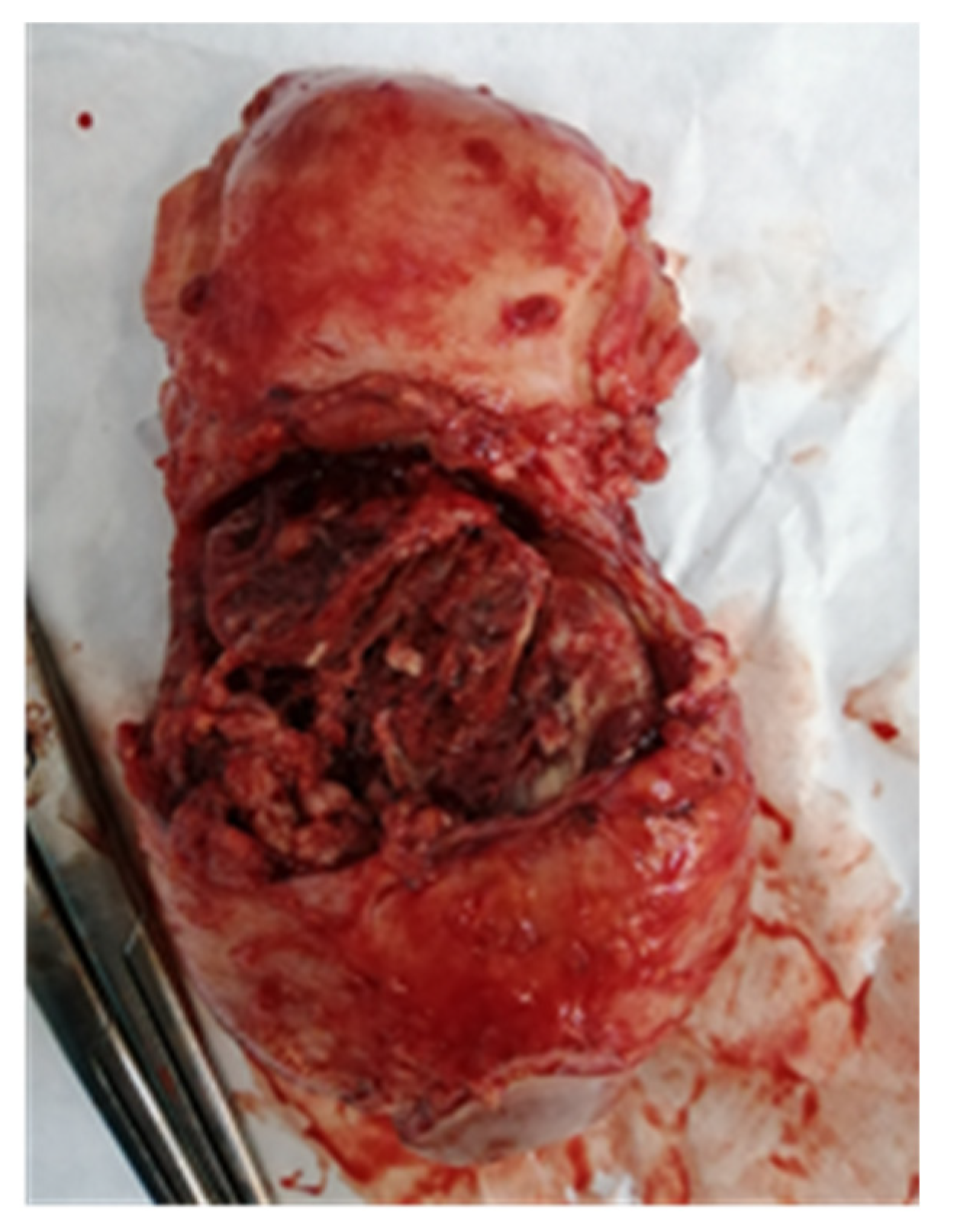
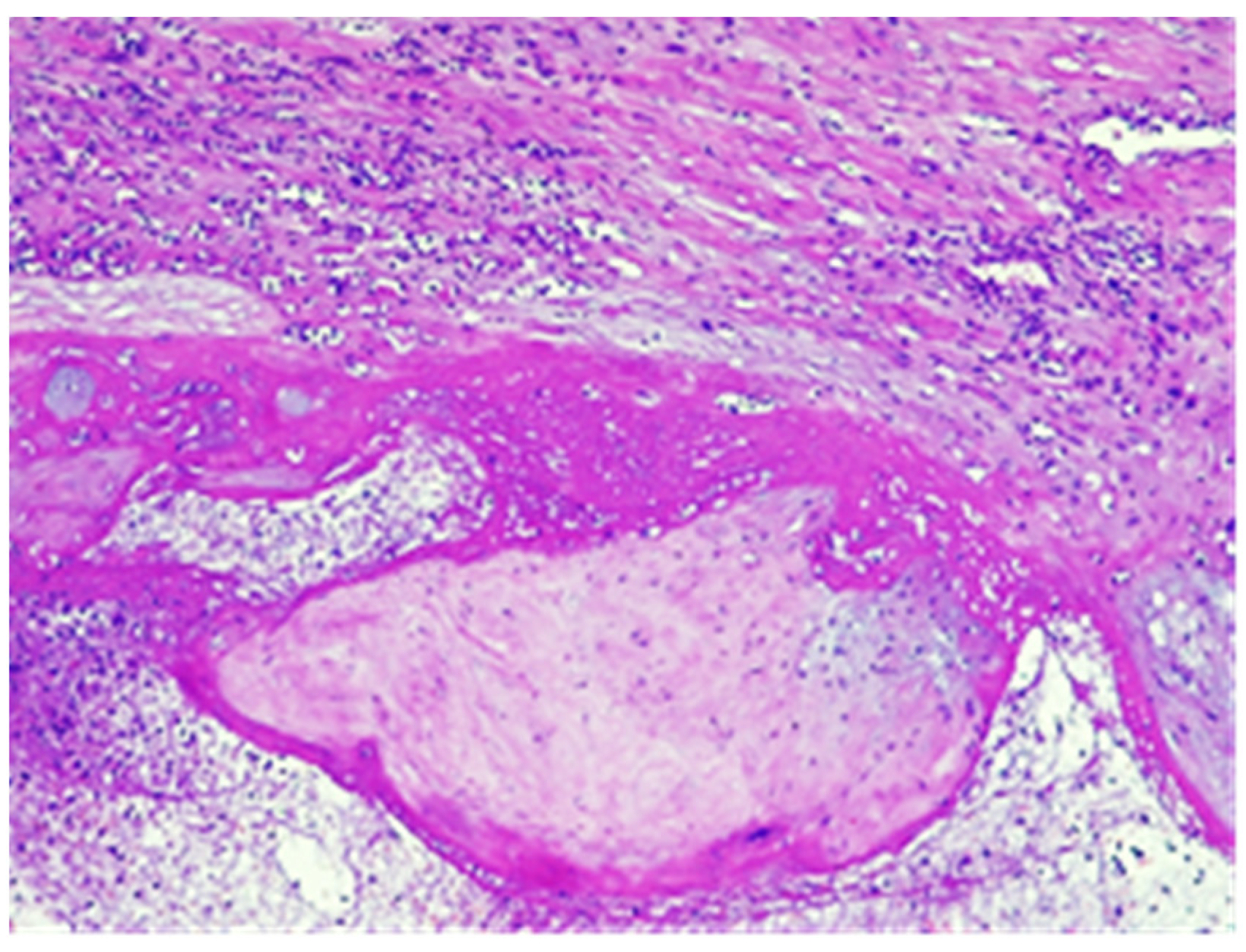
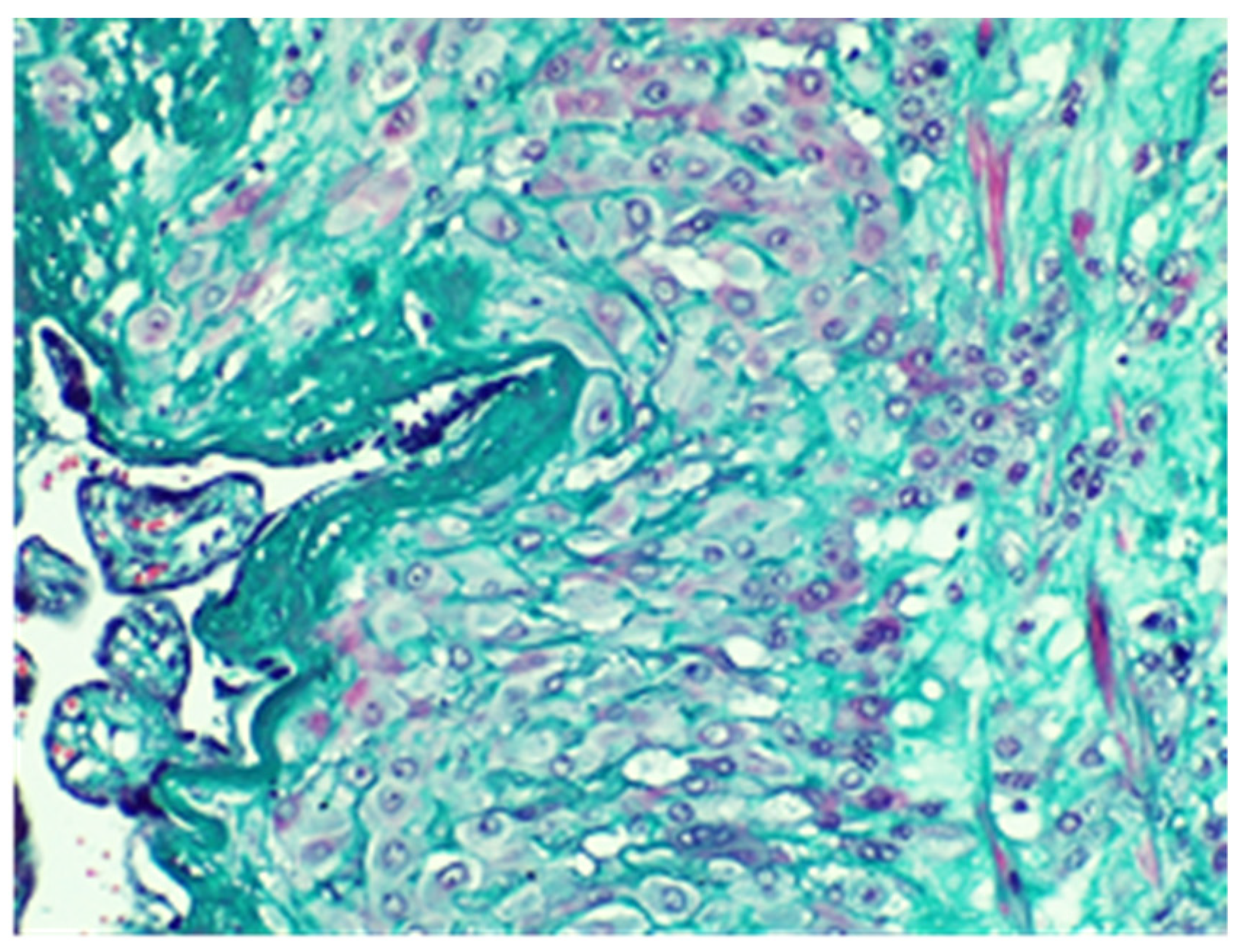
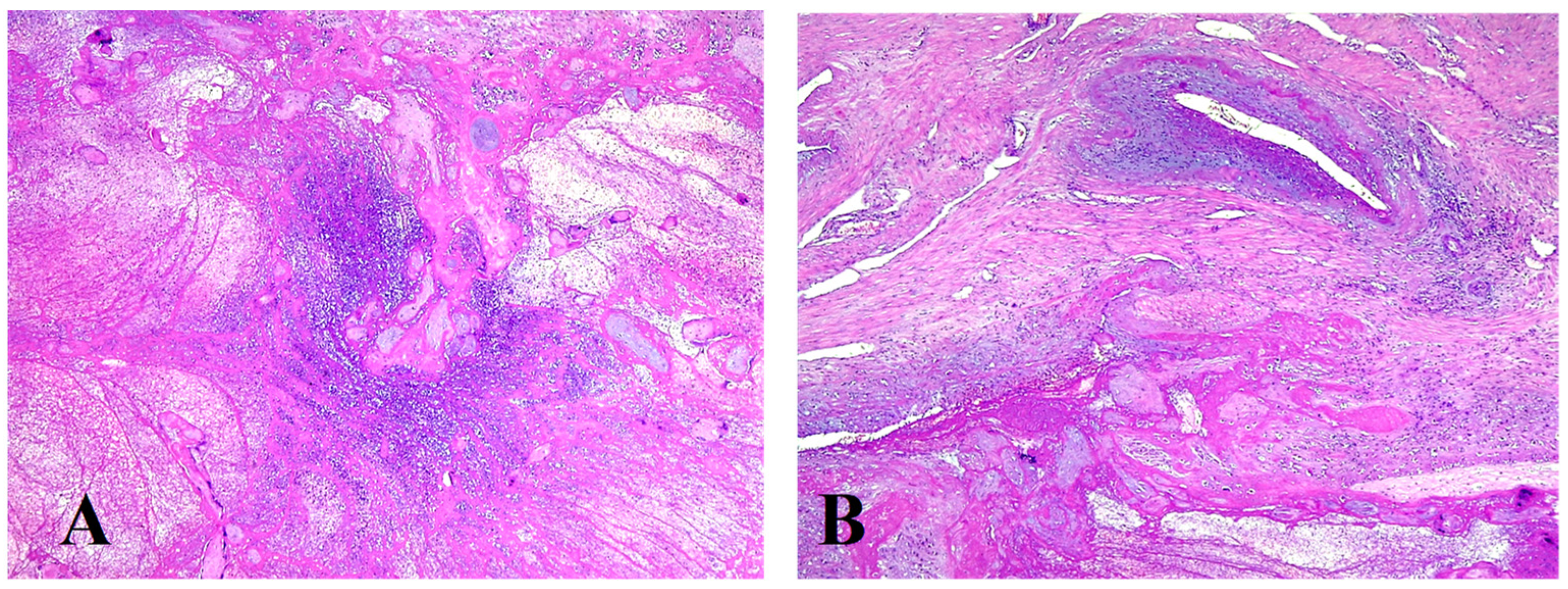
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathbun, K.M.; Hildebrand, J.P. Placenta Abnormalities. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Țarcă, V.; Țarcă, E.; Luca, F.-A. The Impact of the Main Negative Socio-Economic Factors on Female Fertility. Healthcare 2022, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.-A.; Ciobanu, C.-I.; Andrei, A.G.; Horodnic, A.V. Raising Awareness on Health Impact of the Chemicals Used in Consumer Products: Empirical Evidence from East-Central Europe. Sustainability 2018, 10, 209. [Google Scholar] [CrossRef]
- Mappa, I.; Patrizi, L.; Maruotti, G.M.; Carbone, L.; D’Antonio, F.; Rizzo, G. The role of ultrasound in the diagnosis and management of postpartum hemorrhage. J. Clin. Ultrasound 2023, 51, 362–372. [Google Scholar] [CrossRef]
- Weeks, A.D. The retained placenta. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 2, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Giouleka, S.M.; Tsakiridis, I.; Kalogiannidis, I.; Mamopoulos, A.; Tentas, I.M.; Athanasiadis, A.; Dagklis, T. Postpartum Hemorrhage: A Comprehensive Review of Guidelines. Obstet. Gynecol. Surv. 2022, 77, 665–682. [Google Scholar] [CrossRef]
- Jauniaux, E.; Chantraine, F.; Silver, R.M.; Langhoff-Roos, J. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int. J. Gynecol. Obstet. 2018, 140, 265–273. [Google Scholar] [CrossRef]
- Read, J.A.; Cotton, D.B.; Miller, F.C. Placenta accreta: Changing clinical aspects and outcome. Obstet. Gynecol. 1980, 56, 31–34. [Google Scholar]
- Rouholamin, S.; Behnamfar, F.; Zafarbakhsh, A. Placenta increta as an important cause of uterine mass after first-trimester Curettage (case report). Adv. Biomed. Res. 2014, 3, 240. [Google Scholar] [CrossRef]
- Flo, K.; Widnes, C.; Vårtun, Å.; Acharya, G. Blood flow to the scarred gravid uterus at 22–24 weeks of gestation. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 210–215. [Google Scholar] [CrossRef]
- Jauniaux, E.; Collins, S.; Burton, G.J. Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 2018, 218, 75–87. [Google Scholar] [CrossRef]
- Arredondo-Soberón, F.; Sabella, V.; Garza-Leal, J.; Valente, P.T. Placenta accreta during the first trimester of pregnancy. A case report. Ginecol. Obstet. Mex. 1995, 63, 279–281. [Google Scholar] [PubMed]
- Licon, E.; Matsuzaki, S.; Opara, K.N.; Ng, A.J.; Bender, N.M.; Grubbs, B.H.; Lee, R.H.; Ouzounian, J.G.; Pham, H.Q.; Brunette, L.L.; et al. Implementation of multidisciplinary practice change to improve outcomes for women with placenta accreta spectrum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Del Negro, V.; Aleksa, N.; Galli, C.; Ciminello, E.; Derme, M.; Vena, F.; Muzii, L.; Piccioni, M.G. Ultrasonographic Diagnosis of Placenta Accreta Spectrum (PAS) Disorder: Ideation of an Ultrasonographic Score and Correlation with Surgical and Neonatal Outcomes. Diagnostics 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.; Hatem, F.; Heller, D.S. Placenta Increta Presenting as Retained Placenta: A Report of 3 Cases. Fetal Pediatr. Pathol. 2019, 38, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, C.; Abele, H.; Hoopmann, M. Placenta Previa et Percreta: A Potentially Life-Threatening Condition. Diagnostics 2023, 13, 539. [Google Scholar] [CrossRef]
- Hecht, J.L.; Baergen, R.; Ernst, L.M.; Katzman, P.J.; Jacques, S.M.; Jauniaux, E.; Khong, T.Y.; Metlay, L.A.; Poder, L.; Qureshi, F.; et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: Recommendations from an expert panel. Mod. Pathol. 2020, 33, 2382–2396. [Google Scholar] [CrossRef]
- Heena, A.B.; Kumari, G. Retrospective study of placenta accreta, placenta increta and placenta percreta in Peripartum hysterectomy specimens. Indian J. Pathol. Microbiol. 2020, 63, 87–90. [Google Scholar] [CrossRef]
- Jin, T.; Kyozuka, H.; Fujimori, M.; Nomura, S.; Hakozaki, Y.; Suzuki, D.; Nomura, Y. Unexpected placenta accreta spectrum after the use of assisted reproductive technology in women with adenomyomectomy. Fukushima J. Med. Sci. 2021, 67, 45–48. [Google Scholar] [CrossRef]
- Jauniaux, E.; Ayres-De-Campos, D.; Langhoff-Roos, J.; Fox, K.A.; Collins, S. FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynecol. Obstet. 2019, 146, 20–24. [Google Scholar] [CrossRef]
- Létourneau, I.; Hobson, S.R.; Moretti, F.; Kingdom, J.C.; Murji, A.; Windrim, R.C.; Allen, L.M.; Werlang, A.; Vachon-Marceau, C.; Singh, S.S. Placenta Accreta Spectrum Disorders: A National Survey. J. Obstet. Gynaecol. Can. 2023, 45, 102167. [Google Scholar] [CrossRef]
- Komiya, K.; Saitou, K.; Inoue, S.; Igarashi, T.; Hirabayashi, Y.; Seo, N. Massive hemorrhage associated with undiagnosed placenta percreta in a second-trimester pregnancy receiving abortion procedure. Masui 2009, 58, 1036–1038. [Google Scholar] [PubMed]
- Lee, Y.T.; Lee, M.H.; Moon, H.; Hwang, Y.Y. A case of placenta increta which was found about 50 days after induced abortion at 1st trimester. Korean J. Obstet. Gynecol. 2000, 43, 1298–1301. [Google Scholar]
- Son, G.; Kwon, J.; Cho, H.; Kim, S.; Yoon, B.; Nam, E.; Kim, J.; Kim, Y.; Kim, J.; Cho, N.; et al. A Case of Placenta Increta Presenting as Delayed Postabortal Intraperitoneal Bleeding in the First Trimester. J. Korean Med. Sci. 2007, 22, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Kim, S.C. Placenta increta after first-trimester dilatation and curettage manifesting as an unusual uterine mass: Magnetic resonance findings. Acta Radiol. 2007, 48, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Ha, S.-Y.; Lee, K.-B.; Lee, J.-S. Retained placenta accreta after a first-trimester abortion manifesting as an uterine mass. Obstet. Gynecol. Sci. 2013, 56, 205–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartels, H.C.; Postle, J.D.; Downey, P.; Brennan, D.J. Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Dis. Markers 2018, 2018, 1507674. [Google Scholar] [CrossRef]
- Tseng, J.-J.; Chou, M.-M. Differential Expression of Growth-, Angiogenesis- and Invasion-Related Factors in The Development of Placenta Accreta. Taiwan. J. Obstet. Gynecol. 2006, 45, 100–106. [Google Scholar] [CrossRef]
- Chen, J.; Samson, S.-L.; Bentley, J.; Chen, Y. Persistent mild increase of human chorionic gonadotropin levels in a 31-year-old woman after spontaneous abortion. CMAJ 2016, 188, E504–E508. [Google Scholar] [CrossRef][Green Version]
- Lurain, J.R. Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am. J. Obstet. Gynecol. 2010, 203, 531–539. [Google Scholar] [CrossRef]
- Al Riyami, N.; Gowri, V.; Kakaria, A.K.; Lakhtakia, R. A Case of Postpartum Choriocarcinoma: Delay in Diagnosis and Lessons Learnt. J. Obstet. Gynecol. India 2014, 64 (Suppl. S1), 29–31. [Google Scholar] [CrossRef]
- Moscalu, M.; Moscalu, R.; Dascălu, C.G.; Țarcă, V.; Cojocaru, E.; Costin, I.M.; Țarcă, E.; Șerban, I.L. Histopathological Images Analysis and Predictive Modeling Implemented in Digital Pathology—Current Affairs and Perspectives. Diagnostics 2023, 13, 2379. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J. Placental site trophoblastic tumour. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, S.; Hasani, S.S.; Mousavi, A.; Gilani, M.M.; Akhavan, S.; Vakili, M.R. Placenta Site Trophoblastic Tumor and Choriocarcinoma from Previous Cesarean Section Scar: Case Reports. Iran J. Med. Sci. 2018, 43, 426–431. [Google Scholar]
- Horowitz, N.S.; Goldstein, D.P.; Berkowitz, R.S. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: Biology, natural history, and treatment modalities. Gynecol. Oncol. 2017, 144, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.; Cackovic, M. Placenta Accreta Spectrum and Postpartum Hemorrhage. Clin. Obstet. Gynecol. 2023, 66, 399–407. [Google Scholar] [CrossRef]
- Savukyne, E.; Liubiniene, L.; Strelcoviene, Z.; Nadisauskiene, R.J.; Vaboliene, E.; Machtejeviene, E.; Kaupas, R.; Lauzikiene, D. Experience of Managing Suspected Placenta Accreta Spectrum with or without Internal Iliac Artery Balloon Occlusion in Two Lithuanian University Hospitals. Medicina 2021, 57, 345. [Google Scholar] [CrossRef]
- Faralli, I.; Del Negro, V.; Chinè, A.; Aleksa, N.; Ciminello, E.; Piccioni, M.G. Placenta Accreta Spectrum (PAS) Disorder: Ultrasound versus Magnetic Resonance Imaging. Diagnostics 2022, 12, 2769. [Google Scholar] [CrossRef]
- Elmaraghy, A.M.; Fayed, S.T.; Ali, M.A.E.; Hassanien, M.A.; Mamdouh, A.M. Diagnostic Accuracy of Placental Thickness in Lower Uterine Segment Measured by Ultrasound in Prediction of Placenta Accreta Spectrum in Patients with Placenta Previa. A Diagnostic Test Accuracy Study. Int. J. Women’s Health 2023, 15, 311–320. [Google Scholar] [CrossRef]
- Tarcă, E.; Ciomaga, I.M.; Savu, B.; Mihăilă, D.; Plămădeală, P.; Aprodu, S.G. Borderline ovarian cyst treated by laparoscopic surgery: Clinical case report and literature review. Rom. J. Morphol. Embryol. 2015, 56, 1529–1534. [Google Scholar]
- Liu, X.; Fan, G.; Jin, Z.; Yang, N.; Jiang, Y.; Gai, M.; Guo, L.; Wang, Y.; Lang, J. Lower uterine segment pregnancy with placenta increta complicating first trimester induced abortion: Diagnosis and conservative management. Chin. Med. J. 2003, 116, 695–698. [Google Scholar]
- Bhide, A.; Laoreti, A.; Agten, A.K.; Papageorghiou, A.; Khalil, A.; Uprichard, J.; Thilaganathan, B.; Chandraharan, E. Lower uterine segment placental thickness in women with abnormally invasive placenta. Acta Obstet. Gynecol. Scand. 2019, 98, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Raghav, V.; Kapur, A. Medical Management of Placenta Accreta with Methotrexate: Review of Two Cases. J. South Asian Fed. Obstet. Gynaecol. 2015, 7, 86–88. [Google Scholar] [CrossRef]
- Gregoir, C.; De Becker, B.; Hauspy, J.; Vanderheyden, T.; Loquet, P. The use of methotrexate in conservative treatment of placenta accreta spectrum disorders. J. Matern.-Fetal Neonatal Med. 2022, 35, 7514–7517. [Google Scholar] [CrossRef]
- Sentilhes, L.; Kayem, G.; Silver, R.M. Conservative Management of Placenta Accreta Spectrum. Clin. Obstet. Gynecol. 2018, 61, 783–794. [Google Scholar] [CrossRef]
- Timmermans, S.; van Hof, A.C.; Duvekot, J.J. Conservative Management of Abnormally Invasive Placentation. Obstet. Gynecol. Surv. 2007, 62, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Goffinet, F.; Kayem, G. Management of placenta accreta. Acta Obstet. Gynecol. Scand. 2013, 92, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Ambroselli, C.; Kayem, G.; Provansal, M.; Fernandez, H.; Perrotin, F.; Winer, N.; Pierre, F.; Benachi, A.; Dreyfus, M.; et al. Maternal Outcome After Conservative Treatment of Placenta Accreta. Obstet. Gynecol. 2010, 115, 526–534. [Google Scholar] [CrossRef] [PubMed]

| Author | Number of Cases | Placental Pathology | Clinical Manifestation | Treatment |
|---|---|---|---|---|
| Cramer et al., 2019 [14] | 3 | placenta increta | retained placenta; delayed postpartum hemorrhage | hysterectomy for menorrhagia |
| Rouholamin et al., 2014 [15] | 2 | placenta increta | prolonged vaginal bleeding | curettage followed by hysterectomy; total abdominal hysterectomy |
| Lim et al., 2013 [25] | 1 | placenta accreta | vaginal bleeding three years after a first trimester abortion | total abdominal hysterectomy |
| Liu et al., 2003 [40] | 4 | placenta increta | severe hemorrhage in induced abortion in the first trimester | uterine artery embolization |
| Gregoir et al., 2022 [43] | 5 | placenta accreta spectrum | prolonged vaginal bleeding | methotrexate administration with or without embolization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tîrnovanu, M.C.; Tîrnovanu, V.G.; Toma, B.; Toma, L.; Țarcă, E.; Stătescu, L.; Tîrnovanu, Ș.D.; Ungureanu, C.; Trandafirescu, M.F.; Bernic, J.; et al. Unexpected Dramatic Evolution of Placenta Increta: Case Report and Literature Review. J. Pers. Med. 2023, 13, 1563. https://doi.org/10.3390/jpm13111563
Tîrnovanu MC, Tîrnovanu VG, Toma B, Toma L, Țarcă E, Stătescu L, Tîrnovanu ȘD, Ungureanu C, Trandafirescu MF, Bernic J, et al. Unexpected Dramatic Evolution of Placenta Increta: Case Report and Literature Review. Journal of Personalized Medicine. 2023; 13(11):1563. https://doi.org/10.3390/jpm13111563
Chicago/Turabian StyleTîrnovanu, Mihaela Camelia, Vlad Gabriel Tîrnovanu, Bogdan Toma, Loredana Toma, Elena Țarcă, Laura Stătescu, Ștefan Dragoș Tîrnovanu, Carmen Ungureanu, Mioara Florentina Trandafirescu, Jana Bernic, and et al. 2023. "Unexpected Dramatic Evolution of Placenta Increta: Case Report and Literature Review" Journal of Personalized Medicine 13, no. 11: 1563. https://doi.org/10.3390/jpm13111563
APA StyleTîrnovanu, M. C., Tîrnovanu, V. G., Toma, B., Toma, L., Țarcă, E., Stătescu, L., Tîrnovanu, Ș. D., Ungureanu, C., Trandafirescu, M. F., Bernic, J., & Cojocaru, E. (2023). Unexpected Dramatic Evolution of Placenta Increta: Case Report and Literature Review. Journal of Personalized Medicine, 13(11), 1563. https://doi.org/10.3390/jpm13111563






