The Effect of Comorbidities and Complications on COVID-19 Mortality: A Detailed Retrospective Study in Western Romania
Abstract
:1. Introduction
2. Materials and Methods
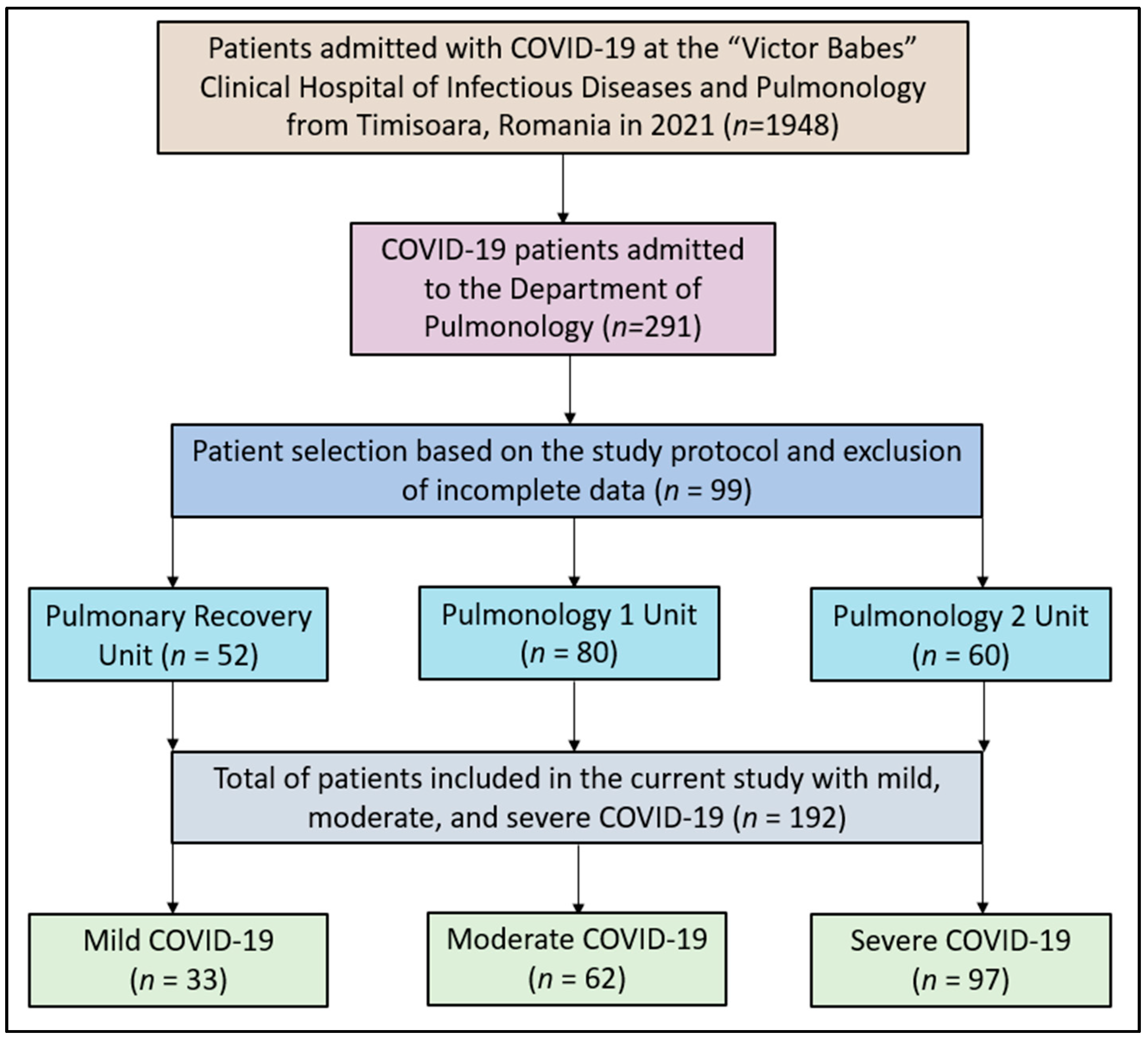
2.1. Studied Patients
2.2. Study Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Literature Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, S.R.; Navas-Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.L.; Shindo, N.; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: What is next for public health? Lancet 2020, 395, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.; Heymann, D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020, 18, 57. [Google Scholar]
- WHO/Europe|Home. Available online: https://www.who.int/europe (accessed on 24 January 2023).
- Guan, Y.; Zheng, B.; He, Y.; Liu, X.; Zhuang, Z.; Cheung, C.; Luo, S.; Li, P.H.; Zhang, L.; Guan, Y. Isolation and Characterization of Viruses Related to the SARS Coronavirus from Animals in Southern China. Science 2003, 302, 276–278. [Google Scholar] [CrossRef]
- Peckham, R. COVID-19 and the anti-lessons of history. Lancet 2020, 395, 850–852. [Google Scholar] [CrossRef]
- Raoult, D.; Zumla, A.; Locatelli, F.; Ippolito, G.; Kroemer, G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 2020, 4, 66–75. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Teng, T.; Abdalla, A.E.; Zhu, W.; Xie, L.; Wang, Y.; Guo, X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses 2020, 12, 244. [Google Scholar] [CrossRef]
- Aberhe, W.; Mariye, T.; Hailay, A.; Zereabruk, K.; Mebrahtom, G.; Gebremedhn, G.; Haile, T.; Guesh, T. The burden and outcomes of COVID-19 among patients with co-morbid disease in Africa: Protocol for a systematic review and meta-analysis. New Microbes New Infect. 2021, 39, 100802. [Google Scholar] [CrossRef]
- Corrao, S.; Pinelli, K.; Vacca, M.; Raspanti, M.; Argano, C. Type 2 Diabetes Mellitus and COVID-19: A Narrative Re-view. Front. Endocrinol. 2021, 12, 609470. [Google Scholar] [CrossRef]
- Emami, A.; Akbari, A.; Basirat, A.; Zare, H.; Javanmardi, F.; Falahati, F.; Rezaei, A. The role of comorbidities on mortality of COVID-19 in patients with diabetes. Obes. Med. 2021, 25, 100352. [Google Scholar] [CrossRef]
- Honardoost, M.; Janani, L.; Aghili, R.; Emami, Z.; Khamseh, M.E. The Association between Presence of Comorbidities and COVID-19 Severity: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2021, 50, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Schultz, P.; Morvan, J.B.; Fakhry, N.; Morinière, S.; Vergez, S.; Lacroix, C.; Bartier, S.; Barry, B.; Babin, E.; Couloigner, V.; et al. French consensus regarding precautions during tracheostomy and post-tracheostomy care in the context of COVID-19 pandemic. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Takhar, A.; Walker, A.; Tricklebank, S.; Wyncoll, D.; Hart, N.; Jacob, T.; Arora, A.; Skilbeck, C.; Simo, R.; Surda, P. Recommendation of a practical guideline for safe tracheostomy during the COVID-19 pandemic. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Polok, K.; Fronczek, J.; van Heerden, P.V.; Flaatten, H.; Guidet, B.; De Lange, D.W.; Fjølner, J.; Leaver, S.; Beil, M.; Sviri, S.; et al. Association between trache-ostomy timing and outcomes for older critically ill COVID-19 patients: Prospective observational study in European intensive care units. Br. J. Anaesth. 2022, 128, 482–490. [Google Scholar] [CrossRef]
- Jones, H.; Gendre, A.; Walshe, P.; Walsh, M.; Glynn, F.; Lacy, P.; Gaffney, R.; Walsh, R.M.; Mamdouh, S.; O’Rourke, J.; et al. The Royal College of surgeons multidisciplinary guidelines on elective tracheostomy insertion in COVID-19 ventilated patients. Surgeon 2021, 19, e265–e269. [Google Scholar] [CrossRef]
- Fernandez-Bussy, S.; Mahajan, B.; Folch, E.; Caviedes, I.; Guerrero, J.; Majid, A. Tracheostomy Tube Placement: Early and Late Complications. J. Bronchol. Interv. Pulmonol. 2015, 22, 357–364. [Google Scholar] [CrossRef]
- Chiesa-Estomba, C.M.; Lechien, J.R.; Calvo-Henríquez, C.; Fakhry, N.; Karkos, P.D.; Peer, S.; Sistiaga-Suarez, J.A.; Gónzalez-García, J.A.; Cammaroto, G.; Mayo-Yánez, M.; et al. Systematic review of international guidelines for tracheostomy in COVID-19 patients. Oral Oncol. 2020, 108, 104844. [Google Scholar] [CrossRef]
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.-B.; Wang, D.-C.; Mei, J.; et al. Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest, CT. Radiology 2020, 296, E46–E54. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 September 2023).
- Fabião, J.; Sassi, B.; Pedrollo, E.F.; Gerchman, F.; Kramer, C.K.; Leitão, C.B.; Pinto, L.C. Why do men have worse COVID-19-related outcomes? A systematic review and meta-analysis with sex adjusted for age. Braz. J. Med. Biol. Res. 2022, 55, e11711. [Google Scholar] [CrossRef]
- Twitchell, D.K.; Christensen, M.B.; Hackett, G.; Morgentaler, A.; Saad, F.; Pastuszak, A.W. Examining Male Predominance of Severe COVID-19 Outcomes: A Systematic Review. Androg. Clin. Res. Ther. 2022, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Napoli, R.D. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls Publishing: St. Petersburg, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/ (accessed on 24 January 2023).
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Mecham, J.C.; Thomas, O.J.; Pirgousis, P.; Janus, J.R. Utility of Tracheostomy in Patients with COVID-19 and Other Special Considerations. Laryngoscope 2020, 130, 2546–2549. [Google Scholar] [CrossRef]
- Abe, T.; Madotto, F.; Pham, T.; Nagata, I.; Uchida, M.; Tamiya, N.; Kurahashi, K.; Bellani, G.; Laffey, J.G.; LUNG-SAFE Investigators and the ESICM Trials Group. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit. Care 2018, 22, 195. [Google Scholar] [CrossRef]
- Bontempo, L.J.; Manning, S.L. Tracheostomy Emergencies. Emerg. Med. Clin. N. Am. 2019, 37, 109–119. [Google Scholar] [CrossRef]
- Durbin, C.G. Early complications of tracheostomy. Respir. Care 2005, 50, 511–515. [Google Scholar]
- Epstein, S.K. Late complications of tracheostomy. Respir. Care 2005, 50, 542–549. [Google Scholar]
- Murray, M.; Shen, C.; Massey, B.; Stadler, M.; Zenga, J. Retrospective analysis of post-tracheostomy complications. Am. J. Otolaryngol. 2022, 43, 103350. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.; Hopkins, P.; Auzinger, G.; Fan, K. Challenges of tracheostomy in COVID-19 patients in a tertiary centre in inner city London. Int. J. Oral. Maxillofac. Surg. 2020, 49, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Neagoe, O.C.; Ionica, M.; Mazilu, O. The role of pelvic lymphocele in the development of early postoperative complications. Medicine 2018, 97, e12353. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, T.; Ariizumi, Y.; Hiramatsu, M.; Kato, Y.; Endo, K.; Obata, K.; Kawashima, K.; Sakata, T.; Hirano, S.; Nakashima, T.; et al. Management of tracheostomy in COVID-19 patients: The Japanese experience. Auris Nasus Larynx 2021, 48, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, Y.; Zhu, F.; Yang, X.; Huang, C.; Hou, G.; Xu, W.; Hu, M.; Zhang, L.; Cheng, A.; et al. Tracheostomy in 80 COVID-19 Patients: A Multicenter, Retrospective, Observational Study. Front. Med. 2020, 7, 615845. [Google Scholar] [CrossRef]
- Hansson, A.; Sunnergren, O.; Hammarskjöld, A.; Alkemark, C.; Taxbro, K. Characteristics, complications, and a comparison between early and late tracheostomy: A retrospective observational study on tracheostomy in patients with COVID-19-related acute respiratory distress syndrome. Health Sci. Rep. 2022, 5, e595. [Google Scholar] [CrossRef]
- Vuu, S.K.M.; Soltani, T.; Liu, H.; DeMuro, J.; Albors, L.M.; Crimi, E.; Ang, D.N. Optimal timing and outcomes among COVID-19 patients undergoing tracheostomy. Surgery 2023, 173, 927–935. [Google Scholar] [CrossRef]
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, Ü.; Herrmann, E.; Adam, E.H. Association of mortality and early tracheostomy in patients with COVID-19: A retrospective analysis. Sci. Rep. 2022, 12, 15406. [Google Scholar] [CrossRef]
- Piazza, C.; Filauro, M.; Dikkers, F.G.; Nouraei, S.A.R.; Sandu, K.; Sittel, C.; Amin, M.R.; Campos, G.; Eckel, H.E.; Peretti, G. Long-term intubation and high rate of tracheostomy in COVID-19 patients might determine an unprecedented increase of airway stenoses: A call to action from the European Laryngological Society. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1–7. [Google Scholar] [CrossRef]
- Mishra, P.; Jedge, P.; Yadav, K.V.; Galagali, J.; Gaikwad, V.; Chethna, R.; Kaushik, M. Outcome of Tracheostomy in COVID-19 Patients. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 404–408. [Google Scholar] [CrossRef]
- Ferro, A.; Kotecha, S.; Auzinger, G.; Yeung, E.; Fan, K. Systematic review and meta-analysis of tracheostomy out-comes in COVID-19 patients. Br. J. Oral Maxillofac. Surg. 2021, 59, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Schuler, P.J.; Greve, J.; Hoffmann, T.K.; Hahn, J.; Boehm, F.; Bock, B.; Reins, J.; Ehrmann, U.; Barth, E.; Traeger, K.; et al. Surgical tracheostomy in a cohort of COVID-19 patients. HNO 2021, 69, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Soni, K.D.; Singh, A.; Choudhary, N.; Perveen, F.; Aggarwal, R.; Patel, N.; Kumar, S.; Trikha, A. Clinical characteristics of COVID-19 patients who underwent tracheostomy and its effect on outcome: A retrospective observational study. World J. Virol. 2022, 11, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Meister, K.D.; Pandian, V.; Hillel, A.T.; Walsh, B.K.; Brodsky, M.B.; Balakrishnan, K.; Best, S.R.; Chinn, S.B.; Cramer, J.D.; Graboyes, E.M.; et al. Multidisciplinary Safety Recommendations after Tracheostomy During COVID-19 Pandemic: State of the Art Review. Otolaryngol.–Head Neck Surg. 2021, 164, 984–1000. [Google Scholar] [CrossRef] [PubMed]
- Cardasis, J.J.; Rasamny, J.K.; Berzofsky, C.E.; Bello, J.A.; Multz, A.S. Outcomes after Tracheostomy for Patients with Respiratory Failure due to COVID-19. Ear Nose Throat J. 2022, 101, 354–358. [Google Scholar] [CrossRef]
- Brisinda, G.; Chiarello, M.M.; Tropeano, G.; Altieri, G.; Puccioni, C.; Fransvea, P.; Bianchi, V. SARS-CoV-2 and the pancreas: What do we know about acute pancreatitis in COVID-19 positive patients? World J. Gastroenterol. 2022, 28, 5240–5249. [Google Scholar] [CrossRef]
- Nicolescu, L.C.; Popescu, C.L.; Popescu, C.V.; Nicolescu, C.M.; Nesiu, A.; Pilat, L.; Stanciu, A.N.; Mihu, A.G. The evaluation of vitamin D deficiency as a risk factor in the case of patients with moderate COVID-19. Farmacia 2022, 70, 507–513. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- Muntean, D.; Horhat, F.-G.; Bădițoiu, L.; Dumitrașcu, V.; Bagiu, I.-C.; Horhat, D.-I.; Coșniță, D.; Krasta, A.; Dugăeşescu, D.; Licker, M. Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study of Trends in a Tertiary Healthcare Unit. Medicina 2018, 54, 92. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Hu, K.; Chopra, A. The incidence, clinical characteristics, and outcomes of pneumothorax in hospitalized COVID-19 patients: A systematic review. Heart Lung 2021, 50, 599–608. [Google Scholar] [CrossRef]
- Posso, M.; Comas, M.; Román, M.; Domingo, L.; Louro, J.; González, C.; Sala, M.; Anglès, A.; Cirera, I.; Cots, F.; et al. Comorbidities and Mortality in Patients With COVID-19 Aged 60 Years and Older in a University Hospital in Spain. Arch. Bronconeumol. 2020, 56, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Trinkmann, F.; Saur, J.; Borggrefe, M.; Akin, I. Cardiovascular Comorbidities in Chronic Obstructive Pulmonary Disease (COPD)—Current Considerations for Clinical Practice. J. Clin. Med. 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Neagoe, C.O.; Mazilu, O. Pelvic intraoperative iatrogenic oncosurgical injuries: Single-center experience. Off. J. Balk Union Oncol. 2016, 21, 498–504. [Google Scholar]
- Naranje, P.; Bhalla, A.S.; Jana, M.; Garg, M.; Nair, A.D.; Singh, S.K.; Banday, I. Imaging of Pulmonary Superinfections and Co-Infections in COVID-19. Curr. Probl. Diagn. Radiol. 2022, 51, 768–778. [Google Scholar] [CrossRef]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Stessel, B.; Dubois, J. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect. Dis. 2022, 22, 207. [Google Scholar] [CrossRef]
- Mena, G.E.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 372, eabg5298. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Charles, E.J.; Mehaffey, J.H. Socio-economic status and COVID-19-related cases and fatalities. Public Health 2020, 189, 129–134. [Google Scholar] [CrossRef]
- Mayo Clinic. COVID-19: Who’s at Higher Risk of Serious Symptoms? Available online: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-who-is-at-risk/art-20483301 (accessed on 24 January 2023).
- CDC Healthcare Workers. Centers for Disease Control and Prevention. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 24 January 2023).
- Bello-Chavolla, O.Y.; Bahena-López, J.P.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; González-Díaz, A.; Márquez-Salinas, A.; Fermín-Martínez, C.A.; Naveja, J.J.; Aguilar-Salinas, C.A. Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico. J. Clin. Endocrinol. Metab. 2020, 105, 2752–2761. [Google Scholar] [CrossRef]
- Santus, P.; Radovanovic, D.; Saderi, L.; Marino, P.; Cogliati, C.; De Filippis, G.; Rizzi, M.; Franceschi, E.; Pini, S.; Giuliani, F.; et al. Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: A prospective observational multicentre study. BMJ Open 2020, 10, e043651. [Google Scholar] [CrossRef]
- Shang, J.; Wang, Q.; Zhang, H.; Wang, X.; Wan, J.; Yan, Y.; Gao, Y.; Cheng, J.; Li, Z.; Lin, J. The relationship between diabetes mellitus and COVID-19 prognosis: A retrospective cohort study in Wuhan, China. Am. J. Med. 2021, 134, e6–e14. [Google Scholar] [CrossRef]
- Xiang, G.; Xie, L.; Chen, Z.; Hao, S.; Fu, C.; Wu, Q.; Liu, X.; Li, S. Clinical risk factors for mortality of hospitalized patients with COVID-19: Systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Bailly, L.; Fabre, R.; Courjon, J.; Carles, M.; Dellamonica, J.; Pradier, C. Obesity, diabetes, hypertension and severe outcomes among inpatients with coronavirus disease 2019: A nationwide study. Clin. Microbiol. Infect. 2022, 28, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef] [PubMed]
- Hasson, R.; Sallis, J.F.; Coleman, N.; Kaushal, N.; Nocera, V.G.; Keith, N. COVID-19: Implications for Physical Activity, Health Disparities, and Health Equity. Am. J. Lifestyle Med. 2022, 16, 420–433. [Google Scholar] [CrossRef]
- Lynch, J. Health Equity, Social Policy, and Promoting Recovery from COVID-19. J. Health Polit. Policy Law 2020, 45, 983–995. [Google Scholar] [CrossRef]

| Variables | Mild (n = 33) | Moderate (n = 62) | Severe (n = 97) | Total (n = 192) | p-Value |
|---|---|---|---|---|---|
| Age (median, IQR) | 60.4 (14.8) | 65.8 (11.5) | 62.5 (11.5) | 63.2 (12.2) | 0.085 |
| Days of hospital stay (median, IQR) | 10.2 (5.0) | 11.9 (8.3) | 12.9 (9.6) | 12.1 (8.6) | 0.288 |
| Male gender n (%) | 23 (69.7) | 38 (61.3) | 64 (66.0) | 125 (65.1) | 0.692 |
| Comorbidities | |||||
| Cardiovascular n (%) | |||||
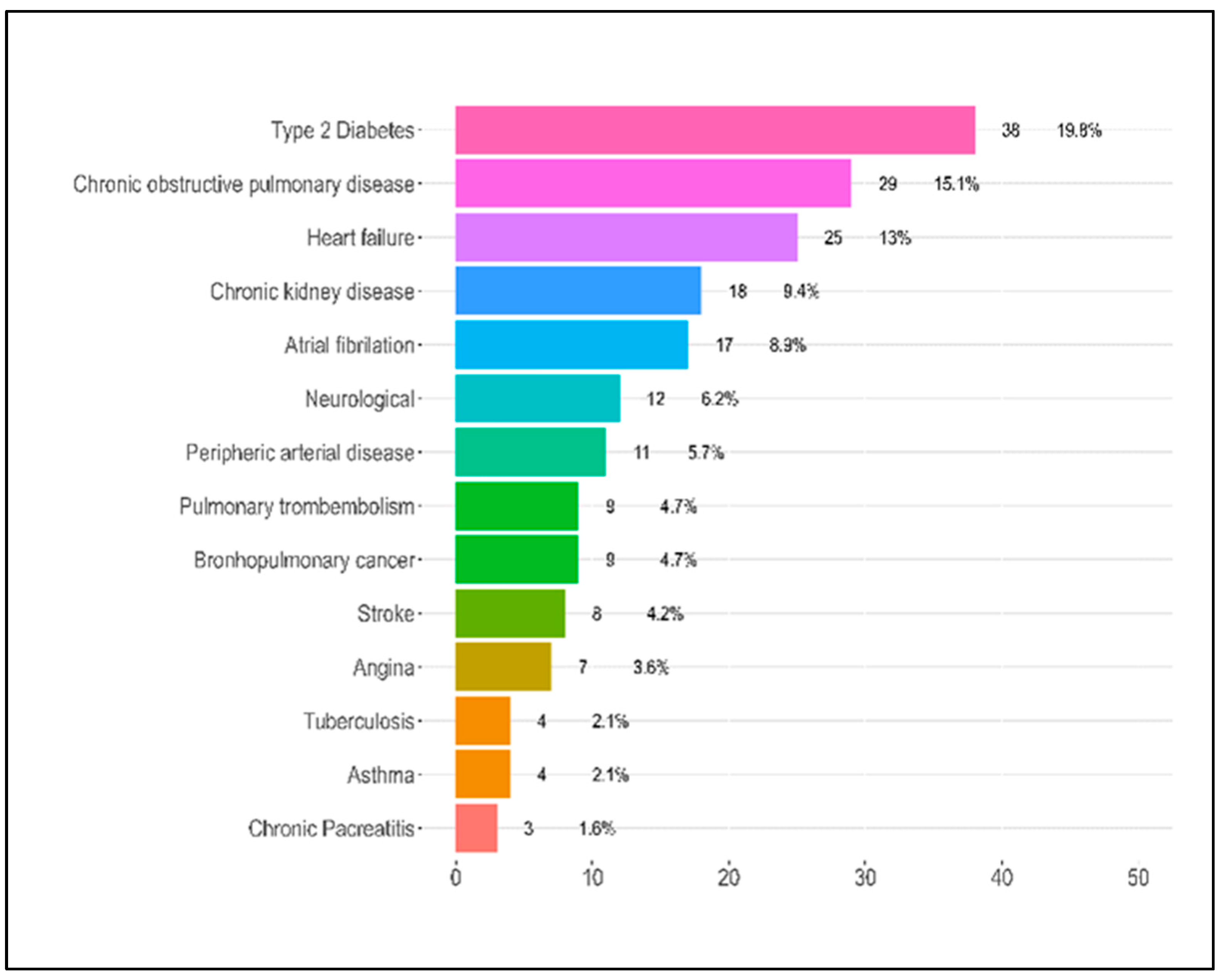
| Atrial fibrillation | 2.0 (6.1%) | 4.0 (6.5%) | 11.0 (11.3%) | 17.0 (8.9%) | 0.471 |
| CHD | 0.0 (0.0%) | 0.0 (0.0%) | 7.0 (7.2%) | 7.0 (3.6%) | 0.029 |
| Hypertension | 0.071 | ||||
| Without hypertension | 8.0 (24.2%) | 18.0 (29.0%) | 27.0 (27.8%) | 53.0 (27.6%) | |
| Hypertension grade I | 8.0 (24.2%) | 3.0 (4.8%) | 13.0 (13.4%) | 24.0 (12.5%) | |
| Hypertension grade II | 12.0 (36.4%) | 35.0 (56.5%) | 39.0 (40.2%) | 86.0 (44.8%) | |
| Hypertension grade III | 5.0 (15.2%) | 6.0 (9.7%) | 18.0 (18.6%) | 29.0 (15.1%) | |
| Heart failure | 4.0 (12.1%) | 7.0 (11.3%) | 14.0 (14.4%) | 25.0 (13.0%) | 0.836 |
| Obesity n (%) | 0.117 | ||||
| Normal weight | 27.0 (81.8%) | 44.0 (71.0%) | 67.0 (69.1%) | 138.0 (71.9%) | |
| Obesity grade I | 0.0 (0.0%) | 4.0 (6.5%) | 10.0 (10.3%) | 14.0 (7.3%) | |
| Obesity grade II | 6.0 (18.2%) | 14.0 (22.6%) | 15.0 (15.5%) | 35.0 (18.2%) | |
| Obesity grade III | 0.0 (0.0%) | 0.0 (0.0%) | 5.0 (5.2%) | 5.0 (2.6%) | |
| Peripheric arterial disease | 0.0 (0.0%) | 2.0 (3.2%) | 9.0 (9.3%) | 11.0 (5.7%) | 0.083 |
| Pulmonary n (%) | |||||
| Asthma | 0.0 (0.0%) | 1.0 (1.6%) | 3.0 (3.1%) | 4.0 (2.1%) | 0.534 |
| Lung Cancer | 2.0 (6.1%) | 3.0 (4.8%) | 4.0 (4.1%) | 9.0 (4.7%) | 0.900 |
| COPD | 5.0 (15.2%) | 16.0 (25.8%) | 8.0 (8.2%) | 29.0 (15.1%) | 0.011 |
| Tuberculosis | 0.0 (0.0%) | 0.0 (0.0%) | 4.0 (4.1%) | 4.0 (2.1%) | 0.135 |
| Thromboembolism | 0.0 (0.0%) | 2.0 (3.2%) | 7.0 (7.2%) | 9.0 (4.7%) | 0.191 |
| Others n (%) | |||||
| T2DM | 6.0 (18.2%) | 13.0 (21.0%) | 19.0 (19.6%) | 38.0 (19.8%) | 0.946 |
| CKD | 2.0 (6.1%) | 4.0 (6.5%) | 12.0 (12.4%) | 18.0 (9.4%) | 0.354 |
| Neurological | 5.0 (15.2%) | 2.0 (3.2%) | 9.0 (9.3%) | 16.0 (8.3%) | 0.120 |
| Stroke | 4.0 (12.1%) | 1.0 (1.6%) | 3.0 (3.1%) | 8.0 (4.2%) | 0.038 |
| Chronic pancreatitis | 0 (0.0) | 0 (0.0) | 3 (3.1) | 3 (1.6) | 0.225 |
| Number of comorbidities | 0.005 | ||||
| No comorbidities | 12.0 (36.4%) | 10.0 (16.1%) | 8.0 (8.2%) | 30.0 (15.6%) | |
| 1–3 comorbidities | 6.0 (18.2%) | 27.0 (43.5%) | 38.0 (39.2%) | 71.0 (37.0%) | |
| 4–5 comorbidities | 11.0 (33.3%) | 18.0 (29.0%) | 41.0 (42.3%) | 70.0 (36.5%) | |
| 6–8 comorbidities | 4.0 (12.1%) | 7.0 (11.3%) | 10.0 (10.3%) | 21.0 (10.9%) |
| Variables | Mild (n = 33) | Moderate (n = 62) | Severe (n = 97) | Total (n = 192) | p-Value |
|---|---|---|---|---|---|
| Pulmonary complications | |||||
| Emphysema | 10 (30.3) | 13 (21.0) | 16 (16.5) | 39 (20.3) | 0.232 |
| Pulmonary thromboembolism | 0 (0.0) | 2 (3.2) | 10 (10.3) | 12 (6.2) | 0.052 |
| Cystic degeneration | 0 (0.0) | 0 (0.0) | 8 (8.2) | 8 (4.2) | 0.017 |
| Pulmonary superinfection | 4 (12.1) | 9 (14.5) | 1 (1.0) | 14 (7.3) | 0.003 |
| Pneumothorax | 0 (0.0) | 2 (3.2) | 12 (12.4) | 14 (7.3) | 0.020 |
| Pneumomediastinum | 2 (6.1) | 5 (8.1) | 19 (19.6) | 26 (13.5) | 0.045 |
| Others | |||||
| Digestive hemorrhage | 2 (6.1) | 2 (3.2) | 0 (0.0) | 4 (2.1) | 0.081 |
| Pancreatic injury | 0 (0.0) | 7 (11.3) | 4 (4.1) | 11 (5.7) | 0.049 |
| Hematoma | 0 (0.0) | 7 (11.3) | 8 (8.2) | 15 (7.8) | 0.145 |
| Neurological complications | 6 (18.2) | 2 (3.2) | 4 (4.1) | 12 (6.2) | 0.008 |
| Anxiety | 10 (30.3) | 16 (25.8) | 9 (9.3) | 35 (18.2) | 0.004 |
| Tracheostomy | 0 (0.0) | 2 (3.2) | 10 (10.3) | 12 (6.2) | 0.052 |
| Sepsis | 0 (0.0) | 2 (3.2) | 10 (10.3) | 12 (6.2) | 0.052 |
| Respiratory failure | 3.0 (9.1%) | 10.0 (16.1%) | 24.0 (24.7%) | 37.0 (19.3%) | 0.108 |
| Number of complications | 0.155 | ||||
| 1 | 26.0 (78.8%) | 43.0 (69.4%) | 59.0 (60.8%) | 128.0 (66.7%) | |
| 2 | 7.0 (21.2%) | 17.0 (27.4%) | 29.0 (29.9%) | 53.0 (27.6%) | |
| 3 | 0.0 (0.0%) | 2.0 (3.2%) | 9.0 (9.3%) | 11.0 (5.7%) | |
| Mortality | 1 (3.0) | 5 (8.1) | 44 (45.4) | 50 (26.0) | <0.001 |
| Dependent: Tracheostomy | No | Yes | Total | p-Value | |
|---|---|---|---|---|---|
| Hospitalization days | Mean (SD) | 10.5 (5.4) | 36.2 (11.3) | 12.1 (8.6) | <0.001 |
| Age | Mean (SD) | 63.9 (11.9) | 52.3 (11.9) | 63.2 (12.2) | 0.001 |
| Area of lung injury on CT | Mean (SD) | 55.8 (17.5) | 71.8 (16.2) | 56.8 (17.8) | 0.002 |
| Deceased | No | 140 (77.8) | 2 (16.7) | 142 (74.0) | <0.001 |
| Yes | 40 (22.2) | 10 (83.3) | 50 (26.0) | ||
| Respiratory failure | No | 155 (86.1) | 0 (0.0) | 155 (80.7) | <0.001 |
| Yes | 25 (13.9) | 12 (100.0) | 37 (19.3) | ||
| Obesity | Normal weight | 134 (74.4) | 4 (33.3) | 138 (71.9) | <0.001 |
| Obesity grade I | 9 (5.0) | 5 (41.7) | 14 (7.3) | ||
| Obesity grade II | 34 (18.9) | 1 (8.3) | 35 (18.2) | ||
| Obesity grade III | 3 (1.7) | 2 (16.7) | 5 (2.6) | ||
| Hypertension | Without hypertension | 53 (29.4) | 0 (0.0) | 53 (27.6) | 0.110 |
| Hypertension grade I | 21 (11.7) | 3 (25.0) | 24 (12.5) | ||
| Hypertension grade II | 80 (44.4) | 6 (50.0) | 86 (44.8) | ||
| Hypertension grade III | 26 (14.4) | 3 (25.0) | 29 (15.1) | ||
| Number of complications | 1 | 128 (71.1) | 0 (0.0) | 128 (66.7) | <0.001 |
| 2 | 48 (26.7) | 5 (41.7) | 53 (27.6) | ||
| 3 | 4 (2.2) | 7 (58.3) | 11 (5.7) | ||
| Number of comorbidities | No comorbidities | 30 (16.7) | 0 (0.0) | 30 (15.6) | 0.003 |
| 1–3 comorbidities | 71 (39.4) | 0 (0.0) | 71 (37.0) | ||
| 4–5 comorbidities | 61 (33.9) | 9 (75.0) | 70 (36.5) | ||
| 6–8 comorbidities | 18 (10.0) | 3 (25.0) | 21 (10.9) | ||
| Sepsis | No | 178 (98.9) | 0 (0.0) | 178 (92.7) | <0.001 |
| Yes | 2 (1.1) | 12 (100.0) | 14 (7.3) | ||
| Dependent: Mortality | Survived | Died | OR (Univariate) | OR (Multivariate) | |
|---|---|---|---|---|---|
| Hypertension grade I | No | 120 (60.6) | 78 (39.4) | - | - |
| Yes | 20 (90.9) | 2 (9.1) | 0.15 (0.02–0.55, p = 0.013) | 0.11 (0.01–0.54, p = 0.015) | |
| Hypertension grade II | No | 82 (69.5) | 36 (30.5) | - | - |
| Yes | 58 (56.9) | 44 (43.1) | 1.73 (0.99–3.02, p = 0.053) | 1.25 (0.56–2.79, p = 0.592) | |
| Hypertension grade III | No | 122 (65.6) | 64 (34.4) | - | - |
| Yes | 18 (52.9) | 16 (47.1) | 1.69 (0.80–3.55, p = 0.161) | 1.61 (0.59–4.37, p = 0.349) | |
| Obesity grade I | No | 132 (62.9) | 78 (37.1) | - | - |
| Yes | 8 (80.0) | 2 (20.0) | 0.42 (0.06–1.74, p = 0.284) | 0.18 (0.02–1.27, p = 0.121) | |
| Obesity grade II | No | 110 (60.4) | 72 (39.6) | - | - |
| Yes | 30 (78.9) | 8 (21.1) | 0.41 (0.17–0.90, p = 0.035) | 0.29 (0.11–0.73, p = 0.012) | |
| Lung cancer | No | 136 (64.2) | 76 (35.8) | - | - |
| Yes | 4 (50.0) | 4 (50.0) | 1.79 (0.41–7.76, p = 0.420) | 2.35 (0.38–15.17, p = 0.347) | |
| Atrial fibrillation | No | 128 (65.3) | 68 (34.7) | - | - |
| Yes | 12 (50.0) | 12 (50.0) | 1.88 (0.80–4.46, p = 0.146) | 1.35 (0.44–4.04, p = 0.593) | |
| T2DM | No | 118 (69.4) | 52 (30.6) | - | - |
| Yes | 22 (44.0) | 28 (56.0) | 2.89 (1.52–5.56, p = 0.001) | 2.46 (1.13–5.42, p = 0.024) | |
| Respiratory failure | No | 124 (67.4) | 60 (32.6) | - | - |
| Yes | 16 (44.4) | 20 (55.6) | 2.58 (1.25–5.40, p = 0.010) | 2.86 (1.25–6.72, p = 0.014) | |
| CKD | No | 132 (66.7) | 66 (33.3) | - | - |
| Yes | 8 (36.4) | 14 (63.6) | 3.50 (1.43–9.16, p = 0.007) | 7.54 (1.84–36.68, p = 0.007) | |
| Heart failure | No | 124 (66.7) | 62 (33.3) | - | - |
| Yes | 16 (47.1) | 18 (52.9) | 2.25 (1.07–4.76, p = 0.032) | 3.43 (1.36–9.15, p = 0.011) | |
| COPD | No | 120 (65.2) | 64 (34.8) | - | - |
| Yes | 20 (55.6) | 16 (44.4) | 1.50 (0.72–3.09, p = 0.272) | 1.85 (0.73–4.71, p = 0.191) | |
| Stroke | No | 134 (63.8) | 76 (36.2) | - | - |
| Yes | 6 (60.0) | 4 (40.0) | 1.18 (0.29–4.24, p = 0.807) | 0.61 (0.13–2.59, p = 0.504) | |
| Peripheral arterial disease | No | 132 (63.5) | 76 (36.5) | - | - |
| Yes | 8 (66.7) | 4 (33.3) | 0.87 (0.23–2.85, p = 0.823) | 0.98 (0.18–4.63, p = 0.979) | |
| Tuberculosis | No | 138 (63.9) | 78 (36.1) | - | - |
| Yes | 2 (50.0) | 2 (50.0) | 1.77 (0.21–14.98, p = 0.572) | 0.23 (0.02–3.31, p = 0.273) | |
| Dependent: Mortality | Survived | Died | OR (Univariate) | OR (Multivariate) | |
|---|---|---|---|---|---|
| Tracheostomy | No | 140 (77.8) | 40 (22.2) | - | - |
| Yes | 2 (16.7) | 10 (83.3) | 17.50 (4.39–116.91, p < 0.001) | 9.89 (1.78–81.48, p = 0.015) | |
| Respiratory insufficiency | No | 124 (80.0) | 31 (20.0) | - | - |
| Yes | 18 (48.6) | 19 (51.4) | 4.22 (1.99–9.07, p < 0.001) | 2.10 (0.73–5.69, p = 0.154) | |
| Pneumothorax | No | 140 (78.7) | 38 (21.3) | - | - |
| Yes | 2 (14.3) | 12 (85.7) | 22.11 (5.72–146.03, p < 0.001) | 20.91 (4.84–147.17, p < 0.001) | |
| Pneumomediastinum | No | 126 (75.9) | 40 (24.1) | - | - |
| Yes | 16 (61.5) | 10 (38.5) | 1.97 (0.81–4.64, p = 0.125) | 2.38 (0.87–6.47, p = 0.088) | |
| Emphysema | No | 107 (69.9) | 46 (30.1) | - | - |
| Yes | 35 (89.7) | 4 (10.3) | 0.27 (0.08–0.71, p = 0.017) | 0.40 (0.10–1.25, p = 0.141) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, M.S.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Oancea, C.; Pescaru, C.C.; Velescu, D.; Wellmann, N.; Motofelea, A.C.; Ciuca, I.M.; et al. The Effect of Comorbidities and Complications on COVID-19 Mortality: A Detailed Retrospective Study in Western Romania. J. Pers. Med. 2023, 13, 1552. https://doi.org/10.3390/jpm13111552
Marc MS, Rosca D, Bratosin F, Fira-Mladinescu O, Oancea C, Pescaru CC, Velescu D, Wellmann N, Motofelea AC, Ciuca IM, et al. The Effect of Comorbidities and Complications on COVID-19 Mortality: A Detailed Retrospective Study in Western Romania. Journal of Personalized Medicine. 2023; 13(11):1552. https://doi.org/10.3390/jpm13111552
Chicago/Turabian StyleMarc, Monica Steluta, Daniela Rosca, Felix Bratosin, Ovidiu Fira-Mladinescu, Cristian Oancea, Camelia Corina Pescaru, Diana Velescu, Norbert Wellmann, Alexandru Catalin Motofelea, Ioana Mihaiela Ciuca, and et al. 2023. "The Effect of Comorbidities and Complications on COVID-19 Mortality: A Detailed Retrospective Study in Western Romania" Journal of Personalized Medicine 13, no. 11: 1552. https://doi.org/10.3390/jpm13111552
APA StyleMarc, M. S., Rosca, D., Bratosin, F., Fira-Mladinescu, O., Oancea, C., Pescaru, C. C., Velescu, D., Wellmann, N., Motofelea, A. C., Ciuca, I. M., Saracin, K., & Manolescu, D. (2023). The Effect of Comorbidities and Complications on COVID-19 Mortality: A Detailed Retrospective Study in Western Romania. Journal of Personalized Medicine, 13(11), 1552. https://doi.org/10.3390/jpm13111552












