The S/S Genotype of the 5-HTTLPR (Serotonin-Transporter-Linked Promoter Region) Variant of the SLC6A4 Gene Decreases the Risk of Pre-Eclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Criteria for Pre-Eclampsia Classification
2.3. Biological Samples
2.4. Genotyping of the 5-HTTLPR Variant of the SLC6A4 Gene by Polymerase Chain Reaction (PCR)
2.5. Statistical Analysis
3. Results
3.1. General and Clinical Characteristics of the Study Population
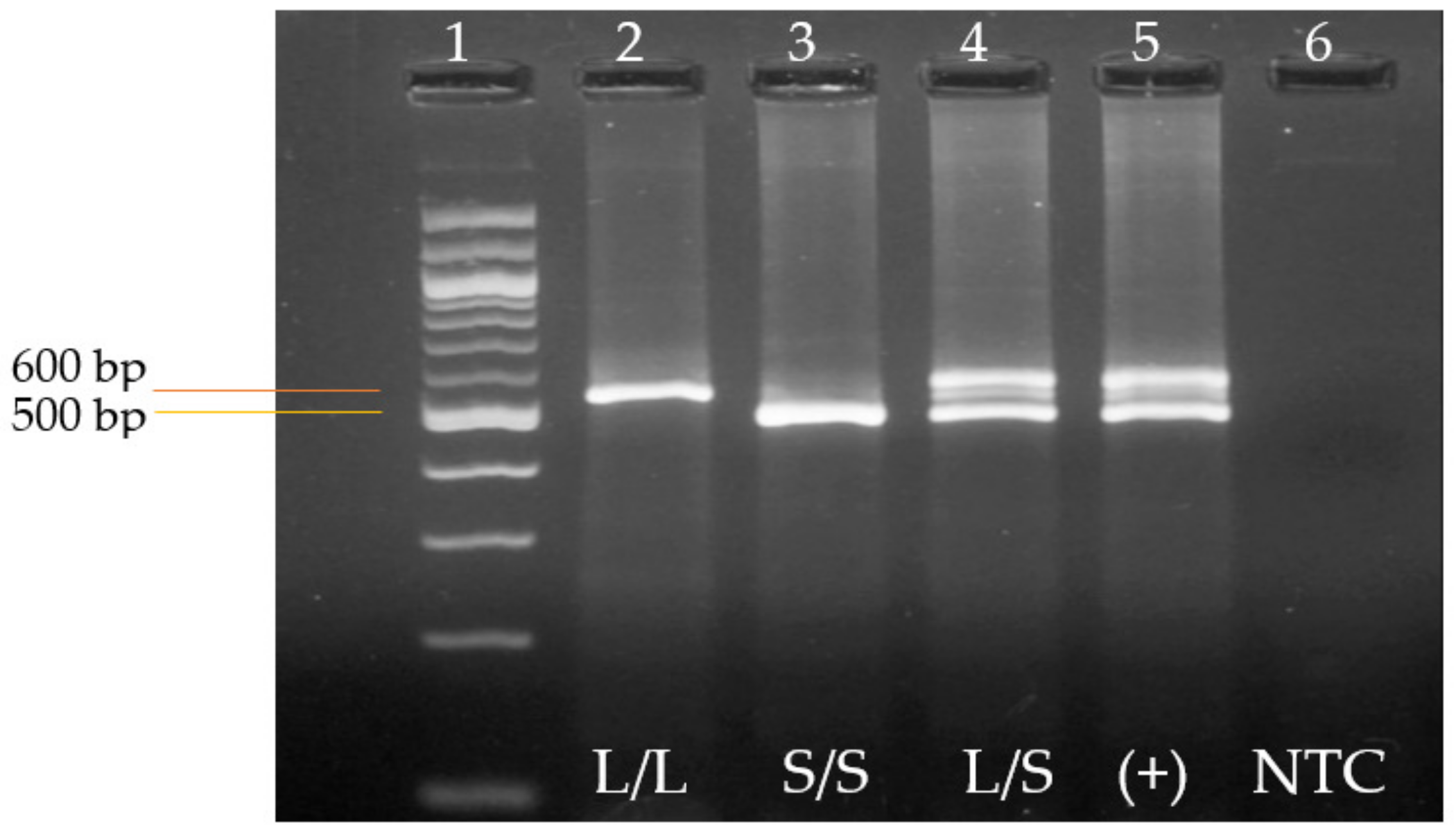
3.2. Genotyping of the 5-HTTLPR Genetic Variant by End-Point Polymerase Chain Reaction (PCR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Ma’ayeh, M.; Costantine, M.M. Prevention of preeclampsia. Semin. Fetal Neonatal Med. 2020, 25, 101123. [Google Scholar] [CrossRef] [PubMed]
- Geographic variation in the incidence of hypertension in pregnancy. World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Am. J. Obstet. Gynecol. 1988, 158, 80–83.
- ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet. Gynecol. 2002, 77, 159–167. [Google Scholar]
- Delmis, J. Hypertension in pregnancy. Lijec. Vjesn. 2006, 128, 357–368. [Google Scholar] [PubMed]
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000, 183, S1–S22. [CrossRef]
- Gaspar, P.; Cases, O.; Maroteaux, L. The developmental role of serotonin: News from mouse molecular genetics. Nat. Rev. Neurosci. 2003, 4, 1002–1012. [Google Scholar] [CrossRef]
- Ori, M.; De Lucchini, S.; Marras, G.; Nardi, I. Unraveling new roles for serotonin receptor 2B in development: Key findings from Xenopus. Int. J. Dev. Biol. 2013, 57, 707–714. [Google Scholar] [CrossRef]
- Mangos, G.J.; Spaan, J.J.; Pirabhahar, S.; Brown, M.A. Markers of cardiovascular disease risk after hypertension in pregnancy. J. Hypertens. 2012, 30, 351–358. [Google Scholar] [CrossRef]
- Magnussen, E.B.; Vatten, L.J.; Smith, G.D.; Romundstad, P.R. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet. Gynecol. 2009, 114, 961–970. [Google Scholar] [CrossRef]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, S.H.; Ho, C.H.; Liang, F.W.; Chu, C.C.; Wang, H.Y.; Lu, Y.H. End-stage renal disease after hypertensive disorders in pregnancy. Am. J. Obstet. Gynecol. 2014, 210, 147.e1–147.e8. [Google Scholar] [CrossRef] [PubMed]
- Männistö, T.; Mendola, P.; Vääräsmäki, M.; Järvelin, M.R.; Hartikainen, A.L.; Pouta, A.; Suvanto, E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013, 127, 681–690. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, B.B.; Nijdam, M.E.; Bruinse, H.W.; Roest, M.; Uiterwaal, C.S.; Grobbee, D.E.; Bots, M.L.; Franx, A. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet. Gynecol. 2013, 121, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Dunford, J.; Mehran, R.; Robson, S.; Kunadian, V. Pre-eclampsia and future cardiovascular risk among women: A review. J. Am. Coll. Cardiol. 2014, 63, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Irgens, H.U.; Reisaeter, L.; Irgens, L.M.; Lie, R.T. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ (Clin. Res. Ed.) 2001, 323, 1213–1217. [Google Scholar] [CrossRef]
- Magnussen, E.B.; Vatten, L.J.; Lund-Nilsen, T.I.; Salvesen, K.A.; Davey Smith, G.; Romundstad, P.R. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: Population based cohort study. BMJ (Clin. Res. Ed.) 2007, 335, 978. [Google Scholar] [CrossRef]
- Ray, J.G.; Vermeulen, M.J.; Schull, M.J.; Redelmeier, D.A. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet 2005, 366, 1797–1803. [Google Scholar] [CrossRef]
- Smith, G.C.; Pell, J.P.; Walsh, D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet 2001, 357, 2002–2006. [Google Scholar] [CrossRef]
- Anderson, C.M. Preeclampsia: Exposing future cardiovascular risk in mothers and their children. J. Obstet. Gynecol. Neonatal Nurs. JOGNN 2007, 36, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Palti, H.; Rothschild, E. Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum. Dev. 1989, 19, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Seidman, D.S.; Laor, A.; Gale, R.; Stevenson, D.K.; Mashiach, S.; Danon, Y.L. Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. Br. J. Obstet. Gynaecol. 1991, 98, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Paauw, N.D.; Lely, A.T. Cardiovascular Sequels During and After Preeclampsia. Adv. Exp. Med. Biol. 2018, 1065, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Q.; Hu, R. Lasting Effects of Intrauterine Exposure to Preeclampsia on Offspring and the Underlying Mechanism. AJP Rep. 2019, 9, e275–e291. [Google Scholar] [CrossRef] [PubMed]
- Wlodek, M.E.; Westcott, K.; Siebel, A.L.; Owens, J.A.; Moritz, K.M. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008, 74, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Henley, D.; Brown, S.; Pennell, C.; Lye, S.; Torpy, D.J. Evidence for central hypercortisolism and elevated blood pressure in adolescent offspring of mothers with pre-eclampsia. Clin. Endocrinol. 2016, 85, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, S.; Räikkönen, K.; Kajantie, E.; Pesonen, A.K.; Heinonen, K.; Osmond, C.; Barker, D.J.; Eriksson, J.G. Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: The Helsinki Birth Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1236–1242. [Google Scholar] [CrossRef]
- Tuovinen, S.; Aalto-Viljakainen, T.; Eriksson, J.G.; Kajantie, E.; Lahti, J.; Pesonen, A.K.; Heinonen, K.; Lahti, M.; Osmond, C.; Barker, D.J.; et al. Maternal hypertensive disorders during pregnancy: Adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1482–1491. [Google Scholar] [CrossRef]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Dildy, G.A., 3rd; Belfort, M.A.; Smulian, J.C. Preeclampsia recurrence and prevention. Semin. Perinatol. 2007, 31, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M.; El-Nazer, A.; Gonzalez-Ruiz, A. Severe preeclampsia-eclampsia in young primigravid women: Subsequent pregnancy outcome and remote prognosis. Am. J. Obstet. Gynecol. 1986, 155, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Gaugler-Senden, I.P.; Berends, A.L.; de Groot, C.J.; Steegers, E.A. Severe, very early onset preeclampsia: Subsequent pregnancies and future parental cardiovascular health. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 171–177. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, B.B.; Hoeks, L.B.; Bots, M.L.; Franx, A.; Bruinse, H.W. Outcomes of subsequent pregnancy after first pregnancy with early-onset preeclampsia. Am. J. Obstet. Gynecol. 2006, 195, 723–728. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Betrán, A.P.; Singata-Madliki, M.; Cormick, G.; Munjanja, S.P.; Fawcus, S.; Mose, S.; Hall, D.; Ciganda, A.; Seuc, A.H.; et al. Prepregnancy and early pregnancy calcium supplementation among women at high risk of pre-eclampsia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Y.; Tao, J.; Lu, L.; Zhang, Y.; Liu, J.; Zhao, M.; Guo, J.; Zhu, D.; Zhu, J.; et al. Comparison of Vascular Responses to Vasoconstrictors in Human Placenta in Preeclampsia between Preterm and Later Term. Curr. Pharm. Biotechnol. 2020, 21, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Gumusoglu, S.; Scroggins, S. The Serotonin-Immune Axis in Preeclampsia. Curr. Hypertens. Rep. 2021, 23, 37. [Google Scholar] [CrossRef]
- Bismuth-Evenzal, Y.; Gonopolsky, Y.; Gurwitz, D.; Iancu, I.; Weizman, A.; Rehavi, M. Decreased serotonin content and reduced agonist-induced aggregation in platelets of patients chronically medicated with SSRI drugs. J. Affect. Disord. 2012, 136, 99–103. [Google Scholar] [CrossRef]
- Karlsson, C.; Bodelsson, G.; Bodelsson, M.; Stjernquist, M. 5-Hydroxytryptamine contracts human uterine artery smooth muscle predominantly via 5-HT2 receptors. Hum. Reprod. 1997, 12, 361–367. [Google Scholar] [CrossRef][Green Version]
- Bolte, A.C.; van Geijn, H.P.; Dekker, G.A. Pathophysiology of preeclampsia and the role of serotonin. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 12–21. [Google Scholar] [CrossRef]
- Prasad, P.D.; Hoffmans, B.J.; Moe, A.J.; Smith, C.H.; Leibach, F.H.; Ganapathy, V. Functional expression of the plasma membrane serotonin transporter but not the vesicular monoamine transporter in human placental trophoblasts and choriocarcinoma cells. Placenta 1996, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.L.; Anacker, A.M.; Rogers, T.D.; Goeden, N.; Keller, E.H.; Forsberg, C.G.; Kerr, T.M.; Wender, C.; Anderson, G.M.; Stanwood, G.D.; et al. Impact of Maternal Serotonin Transporter Genotype on Placental Serotonin, Fetal Forebrain Serotonin, and Neurodevelopment. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Keaton, S.A.; Heilman, P.; Bryleva, E.Y.; Madaj, Z.; Krzyzanowski, S.; Grit, J.; Miller, E.S.; Jälmby, M.; Kalapotharakos, G.; Racicot, K.; et al. Altered Tryptophan Catabolism in Placentas from Women with Pre-eclampsia. Int. J. Tryptophan Res. IJTR 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Sabolovic Rudman, S.; Mustapic, M.; Kosec, V.; Pivac, N.; Rudman, F.; Muck-Seler, D. Serotonin risk factors for the development of hypertension in pregnancy. Arch. Gynecol. Obstet. 2015, 291, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Baquero Mejía, I.C. Polimorfismos Genéticos Asociados a Pre-Eclampsia. Ph.D. Thesis, Universidad de Cantabria, Santader, Spain, 2013. [Google Scholar]
- Ogilvie, A.D.; Harmar, A.J. Association between the serotonin transporter gene and affective disorder: The evidence so far. Mol. Med. 1997, 3, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Kenna, G.A. Variation in the Serotonin Transporter Gene and Alcoholism: Risk and Response to Pharmacotherapy. Alcohol Alcohol. 2016, 51, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.L.; Chen, X.F.; Ran, Y.H.; Li, X.R.; Xiong, J.; Zheng, Y.Y.; Gao, N.N.; Li, Y.F. Mouse strain differences in SSRI sensitivity correlate with serotonin transporter binding and function. Sci. Rep. 2017, 7, 8631. [Google Scholar] [CrossRef] [PubMed]
- Wankerl, M.; Wüst, S.; Otte, C. Current developments and controversies: Does the serotonin transporter gene-linked polymorphic region (5-HTTLPR) modulate the association between stress and depression? Curr. Opin. Psychiatry 2010, 23, 582–587. [Google Scholar] [CrossRef]
- Jonassen, R.; Landrø, N.I. Serotonin transporter polymorphisms (5-HTTLPR) in emotion processing: Implications from current neurobiology. Prog. Neurobiol. 2014, 117, 41–53. [Google Scholar] [CrossRef]
- Martinez-Fierro, M.L.; Garza-Veloz, I.; Castruita-Dela Rosa, C.; Ortiz-Castro, Y.; Aceves-Medina, M.C.; Vazquez-Castro, R.; Delgado-Enciso, I.; Castaneda-Lopez, M.E. Plasma cancer biomarker multiplex screening and the risk of subsequent preeclampsia. Int. J. Cardiol. 2015, 179, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Bulletins-Obstetrics, A.C.o.P. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar] [CrossRef]
- Fang, R.; Dawson, A.; Lohsoonthorn, V.; Williams, M.A. Risk Factors of Early and Late Onset Preeclampsia among Thai Women. Asian Biomed. Res. Rev. News 2009, 3, 477–486. [Google Scholar] [PubMed]
- Heils, A.; Teufel, A.; Petri, S.; Stöber, G.; Riederer, P.; Bengel, D.; Lesch, K.P. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996, 66, 2621–2624. [Google Scholar] [CrossRef] [PubMed]
- Elston, R.; Forthofer, R.J.B. Testing for Hardy-Weinberg equilibrium in small samples. Biometrics 1977, 536–542. [Google Scholar] [CrossRef]
- Tyas, B.D.; Lestari, P.; Aldika Akbar, M.I. Maternal Perinatal Outcomes Related to Advanced Maternal Age in Preeclampsia Pregnant Women. J. Fam. Reprod. Health 2019, 13, 191–200. [Google Scholar] [CrossRef]
- Shan, D.; Qiu, P.Y.; Wu, Y.X.; Chen, Q.; Li, A.L.; Ramadoss, S.; Wang, R.R.; Hu, Y.Y. Pregnancy Outcomes in Women of Advanced Maternal Age: A Retrospective Cohort Study from China. Sci. Rep. 2018, 8, 12239. [Google Scholar] [CrossRef] [PubMed]
- Lamminpaa, R.; Vehvilainen-Julkunen, K.; Gissler, M.; Heinonen, S. Preeclampsia complicated by advanced maternal age: A registry-based study on primiparous women in Finland 1997–2008. BMC Pregnancy Childbirth 2012, 12, 47. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, J.G.; Isla-Arias, M.X. Correlación entre ácido úrico y creatinina sérica en pacientes embarazadas con preeclampsia severa. Ginecol. Obstet. Mex. 2018, 86, 567–574. [Google Scholar]
- Laurent, L.; Deroy, K.; St-Pierre, J.; Côté, F.; Sanderson, J.T.; Vaillancourt, C. Human placenta expresses both peripheral and neuronal isoform of tryptophan hydroxylase. Biochimie 2017, 140, 159–165. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci. Rep. 2015, 35, e00261. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Karahoda, R. Trophoblast: The central unit of fetal growth, protection and programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Spong, C.Y.; Dashe, J.S. Williams Obstetrics, 24e; Mcgraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Aznavoorian, S.A.; Utsunomiya, T.; Krausz, M.M.; Cohn, L.H.; Shepro, D.; Hechtman, H.B. Prostacyclin inhibits 5-hydroxytryptamine release but stimulates thromboxane synthesis during cardiopulmonary bypass. Prostaglandins 1983, 25, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.C.; Cross, R.J.; Walker, R.F.; Markesbery, W.R.; Brooks, W.H.; Roszman, T.L. Influence of serotonin on the immune response. Immunology 1985, 54, 505–512. [Google Scholar] [PubMed]
- Carrasco, G.; Cruz, M.A.; Gallardo, V.; Miguel, P.; Lagos, M.; González, C. Plasma and platelet concentration and platelet uptake of serotonin in normal and pre-eclamptic pregnancies. Life Sci. 1998, 62, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Ugun-Klusek, A.; Tamang, A.; Loughna, P.; Billett, E.; Buckley, G.; Sivasubramaniam, S. Reduced placental vascular reactivity to 5-hydroxytryptamine in pre-eclampsia and the status of 5HT(2A) receptors. Vasc. Pharmacol. 2011, 55, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Canis, M.; Vaurs-Barrière, C.; Pouly, J.L.; Boespflug-Tanguy, O.; Penault-Llorca, F.; Dechelotte, P.; Dastugue, B.; Okamura, K.; Mage, G. DNA microarray analysis of gene expression profiles in deep endometriosis using laser capture microdissection. Mol. Hum. Reprod. 2004, 10, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Salmeri, N.; Viganò, P.; Cavoretto, P.; Marci, R.; Candiani, M. The kisspeptin system in and beyond reproduction: Exploring intricate pathways and potential links between endometriosis and polycystic ovary syndrome. Rev. Endocr. Metab. Disord. 2023; ahead of print. [Google Scholar] [CrossRef]
- Salmeri, N.; Farina, A.; Candiani, M.; Dolci, C.; Bonavina, G.; Poziello, C.; Viganò, P.; Cavoretto, P.I. Endometriosis and Impaired Placentation: A Prospective Cohort Study Comparing Uterine Arteries Doppler Pulsatility Index in Pregnancies of Patients with and without Moderate-Severe Disease. Diagnostics 2022, 12, 1024. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef]
- Alnoman, A.; Badeghiesh, A.M.; Baghlaf, H.A.; Dahan, M.H. Pregnancy, delivery, and neonatal outcomes among women with irritable bowel syndrome (IBS) an evaluation of over 9 million deliveries. J. Matern. Neonatal Med. 2022, 35, 5935–5942. [Google Scholar] [CrossRef]
| Characteristic | PE Cases (n = 100) | Controls (n = 100) | p-Value | OR | 95% CI |
|---|---|---|---|---|---|
| Mean maternal age in years (min–max) | 28 (23.0–32.0) | 25.0 (23.0–29.0) | 0.026 | - | - |
| Weeks of gestation at diagnosis | 34 (31.0–37.0) | 31.0 (24.7–35.0) | <0.001 | - | - |
| Primiparous, n (%) | 47 (55.95) | 37 (44.04) | 0.197 | 1.51 | 0.85–2.65 |
| Family history of PE, n (%) | 8 (8) | 9 (9) | 1 | - | - |
| Personal history of PE, n (%) | 13 (13) | 2 (2) | 0.007 | 7.32 | 1.60–33.35 |
| Personal history of HBP, n (%) | 1 (1) | 0 (0) | 0.976 | - | - |
| Smoking history, n (%) | 7 (36.8) | 12 (63.15) | 0.335 | 0.55 | 0.20–1.46 |
| History of alcohol use, n (%) | 1 (1) | 0 (0) | 0.38 | - | - |
| SBP in mm/Hg (min–max) | 150 (140–160) | 100 (100–110) | <0.001 | - | - |
| DBP in mm/Hg (min–max) | 90 (90–100) | 70 (60–70) | <0.001 | - | - |
| PE with severity criteria n (%) | 31 (31.0) | - | - | - | - |
| Early onset PE n (%) | 38 (38.0) | - | - | - | - |
| * Severe PE, n (%) | 21 (53.8) | - | - | - | - |
| Parameter | PE Cases (n = 100) | Controls (n = 100) | p-Value |
|---|---|---|---|
| Serum creatinine (mg/dL) | 0.58 (0.48–0.7) | 0.64 (0.56–0.74) | 0.022 |
| Plasma platelets (103/μL) | 214.2 (162.5–256.8) | 235.0 (194–278.3) | 0.038 |
| Serum aspartate aminotransferase (IU/L) | 29.0 (22.9–34.0) | 25.0 (16.5–29.0) | 0.208 |
| Urine protein (mg/dL) | 72 (46.7) | 3 (39.6) | 0.001 |
| Genotype/Allele | PE Cases (n = 100) | Control (n = 100) | * p-Value | OR | 95% CI |
|---|---|---|---|---|---|
| S/S, n (%) | 32 (32) | 55 (55) | Reference | - | - |
| S/L, n (%) | 53 (53) | 25 (25) | ≤0.001 | 0.27 | 0.14–0.52 |
| L/L, n (%) | 15 (15) | 20 (20) | 0.68 | 0.78 | 0.35–1.72 |
| Allele S, n (%) | 117 (58.5) | 135 (67.5) | 0.08 | 0.68 | 0.45–1.02 |
| Allele L, n (%) | 83 (41.5) | 65 (32.5) |
| Group | n | Genotype n (%) | * p-Value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| S/S | S/L + L/L | |||||
| Control | 100 | 55 (55) | 45 (45) | Reference | - | - |
| PE | 100 | 32 (32) | 68 (68) | 0.002 | 0.39 | 0.22–0.69 |
| PE without severity criteria | 69 | 22 (31.9) | 47 (68.1) | 0.005 | 0.38 | 0.21–0.73 |
| PE with severity criteria | 31 | 10 (32.3) | 21 (67.7) | 0.045 | 0.39 | 0.17–0.91 |
| Mild PE- based on proteinuria | 18 | 10 (55.6) | 8 (44.4) | 0.83 | 1.02 | 0.37–2.80 |
| Severe PE- based on proteinuria | 21 | 6 (28.6) | 15 (71.4) | 0.05 | 0.33 | 0.12–0.91 |
| Early PE | 38 | 14 (36.8) | 24 (63.3) | 0.086 | 0.48 | 0.22–1.03 |
| Late PE | 62 | 18 (29) | 44 (71) | 0.002 | 0.34 | 0.17–0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Armas, R.M.; Garza-Veloz, I.; Olivas-Chávez, J.C.; Covarrubias-Carrillo, R.M.; Martínez-Vázquez, M.C.; Monárrez-Espino, J.; Ayala-Haro, A.E.; Serrano-Amaya, C.V.; Delgado-Enciso, I.; Rodriguez-Sanchez, I.P.; et al. The S/S Genotype of the 5-HTTLPR (Serotonin-Transporter-Linked Promoter Region) Variant of the SLC6A4 Gene Decreases the Risk of Pre-Eclampsia. J. Pers. Med. 2023, 13, 1535. https://doi.org/10.3390/jpm13111535
Ramírez-Armas RM, Garza-Veloz I, Olivas-Chávez JC, Covarrubias-Carrillo RM, Martínez-Vázquez MC, Monárrez-Espino J, Ayala-Haro AE, Serrano-Amaya CV, Delgado-Enciso I, Rodriguez-Sanchez IP, et al. The S/S Genotype of the 5-HTTLPR (Serotonin-Transporter-Linked Promoter Region) Variant of the SLC6A4 Gene Decreases the Risk of Pre-Eclampsia. Journal of Personalized Medicine. 2023; 13(11):1535. https://doi.org/10.3390/jpm13111535
Chicago/Turabian StyleRamírez-Armas, Rebeca Mónica, Idalia Garza-Veloz, Juan Carlos Olivas-Chávez, Rosa Martha Covarrubias-Carrillo, Maria Calixta Martínez-Vázquez, Joel Monárrez-Espino, Anayantzin E. Ayala-Haro, Claudia Vanessa Serrano-Amaya, Ivan Delgado-Enciso, Iram Pablo Rodriguez-Sanchez, and et al. 2023. "The S/S Genotype of the 5-HTTLPR (Serotonin-Transporter-Linked Promoter Region) Variant of the SLC6A4 Gene Decreases the Risk of Pre-Eclampsia" Journal of Personalized Medicine 13, no. 11: 1535. https://doi.org/10.3390/jpm13111535
APA StyleRamírez-Armas, R. M., Garza-Veloz, I., Olivas-Chávez, J. C., Covarrubias-Carrillo, R. M., Martínez-Vázquez, M. C., Monárrez-Espino, J., Ayala-Haro, A. E., Serrano-Amaya, C. V., Delgado-Enciso, I., Rodriguez-Sanchez, I. P., & Martinez-Fierro, M. L. (2023). The S/S Genotype of the 5-HTTLPR (Serotonin-Transporter-Linked Promoter Region) Variant of the SLC6A4 Gene Decreases the Risk of Pre-Eclampsia. Journal of Personalized Medicine, 13(11), 1535. https://doi.org/10.3390/jpm13111535