The Influence of Different Irradiation Regimens on Inflammation and Vascularization in a Random-Pattern Flap Model
Abstract
1. Introduction
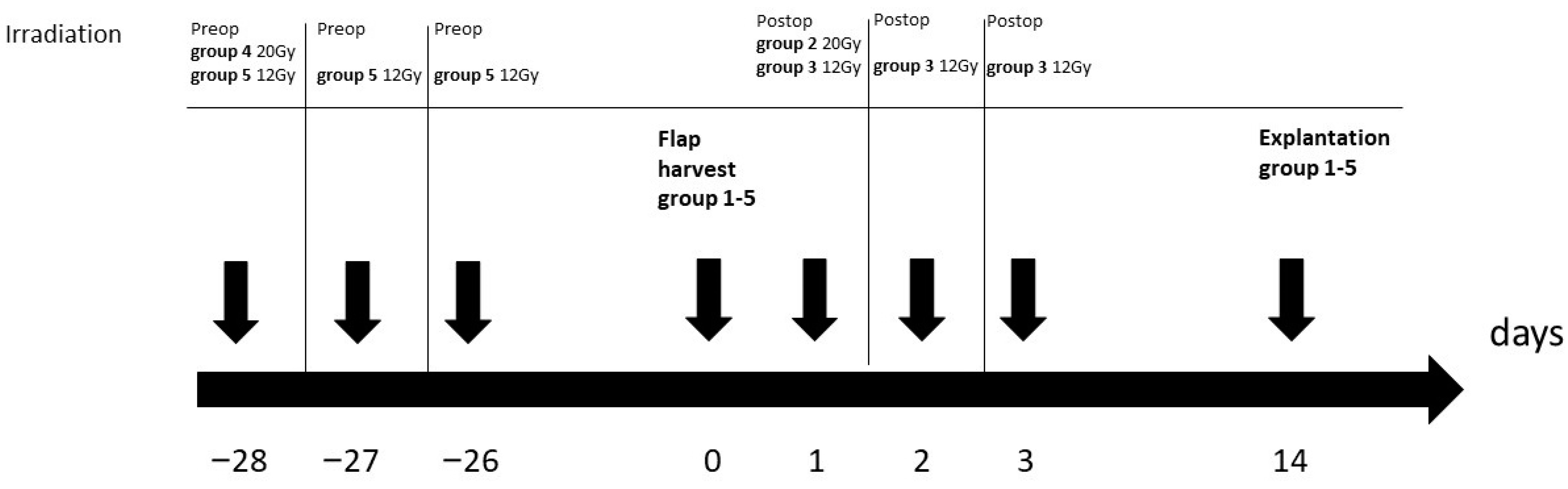
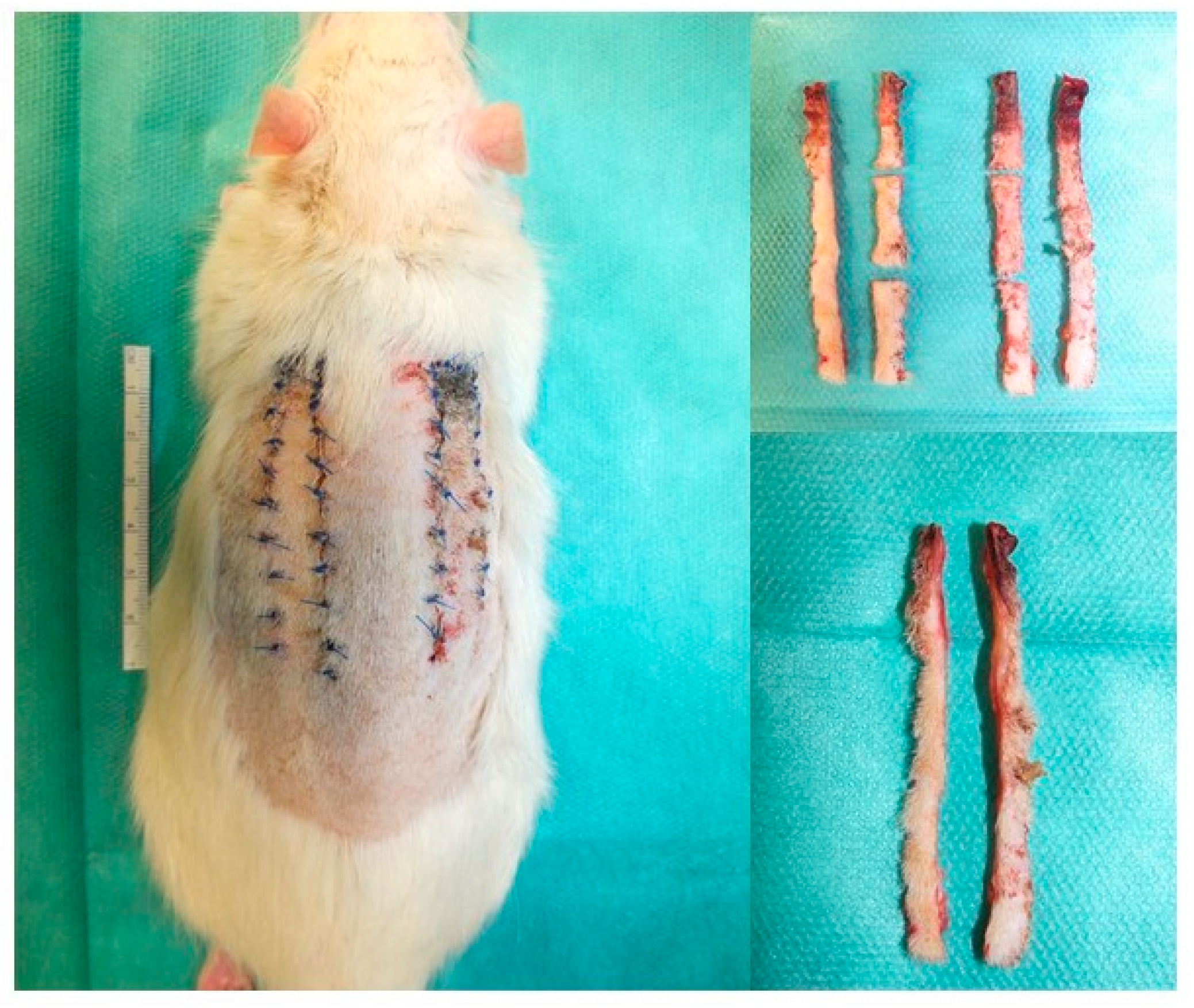
2. Materials and Methods
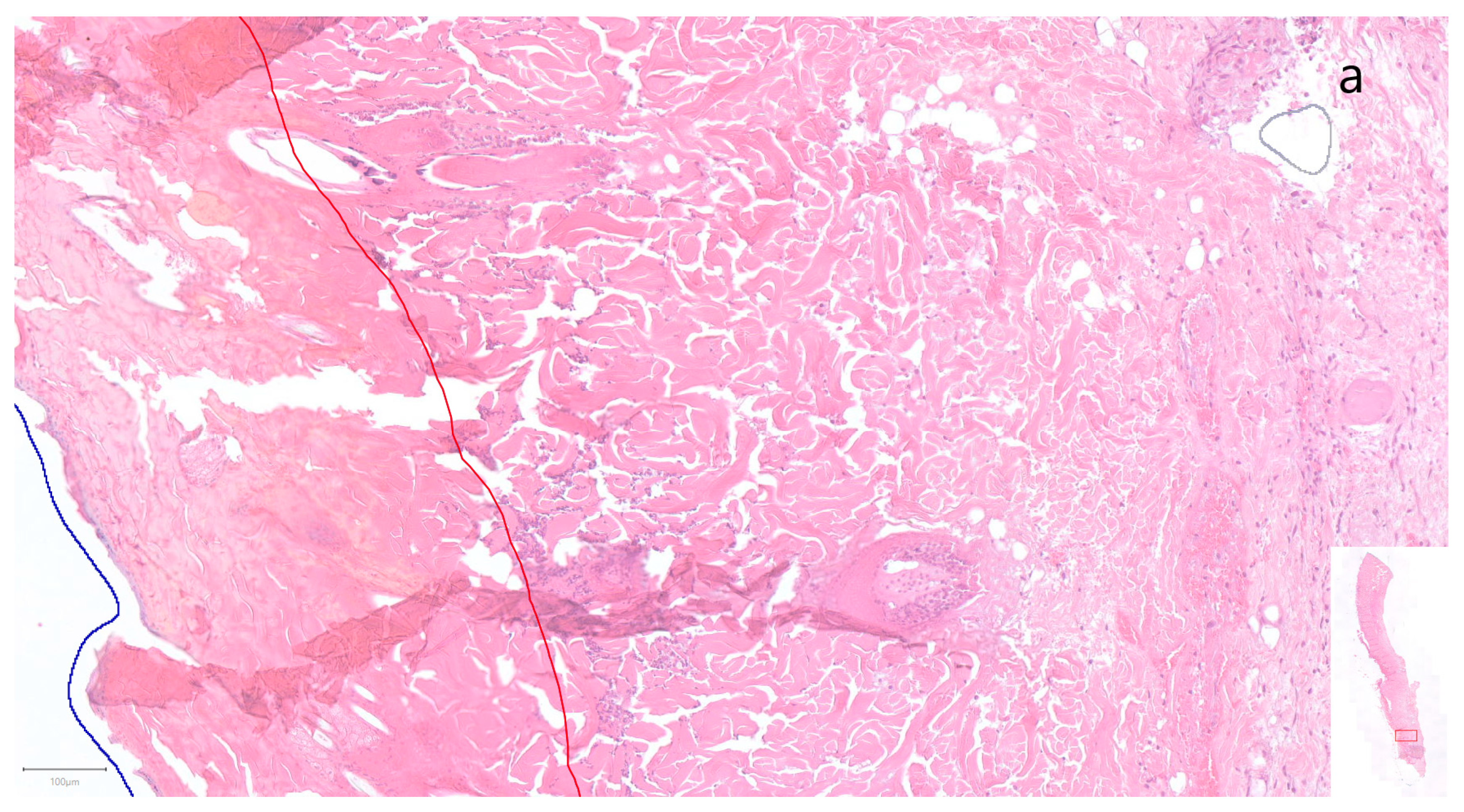
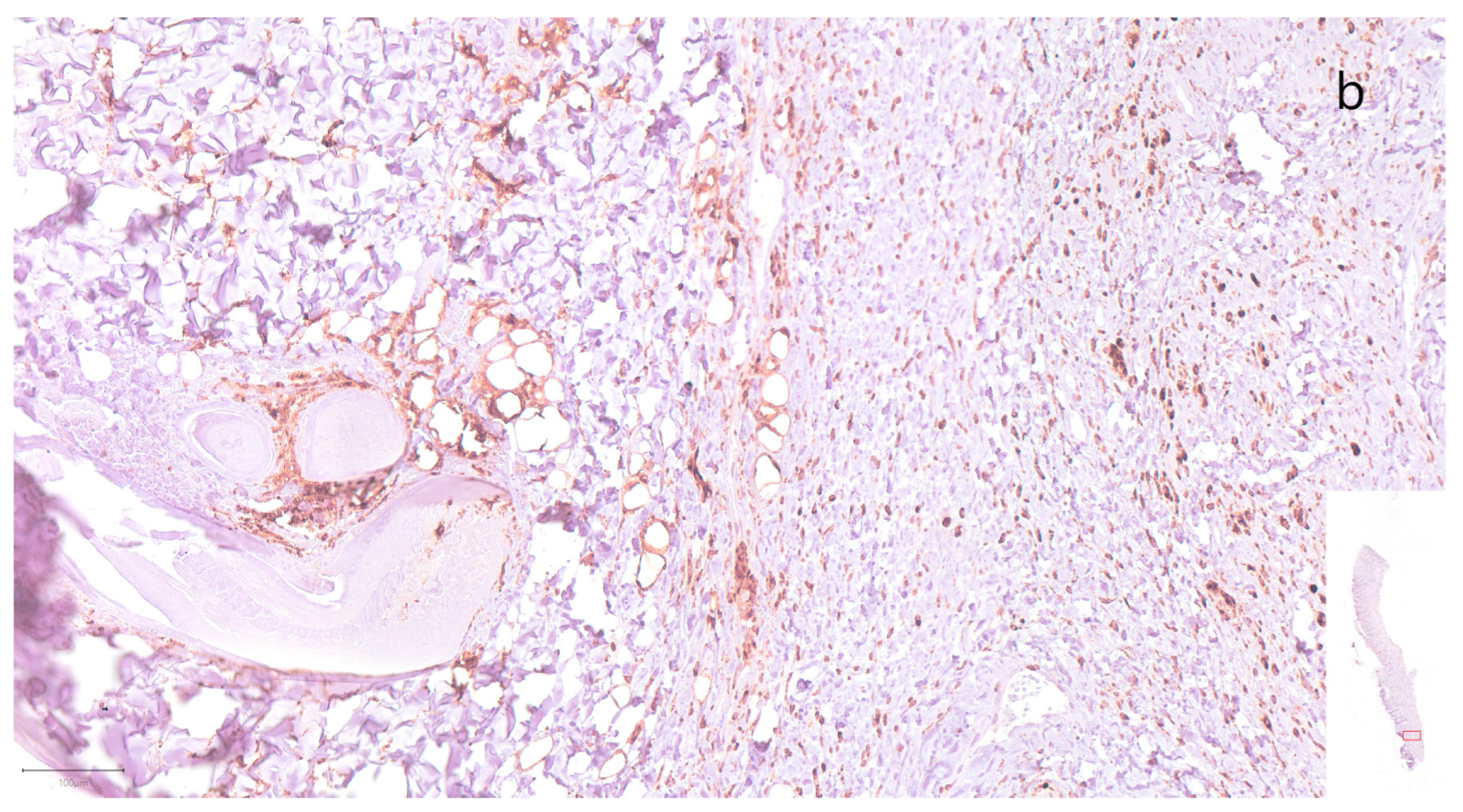
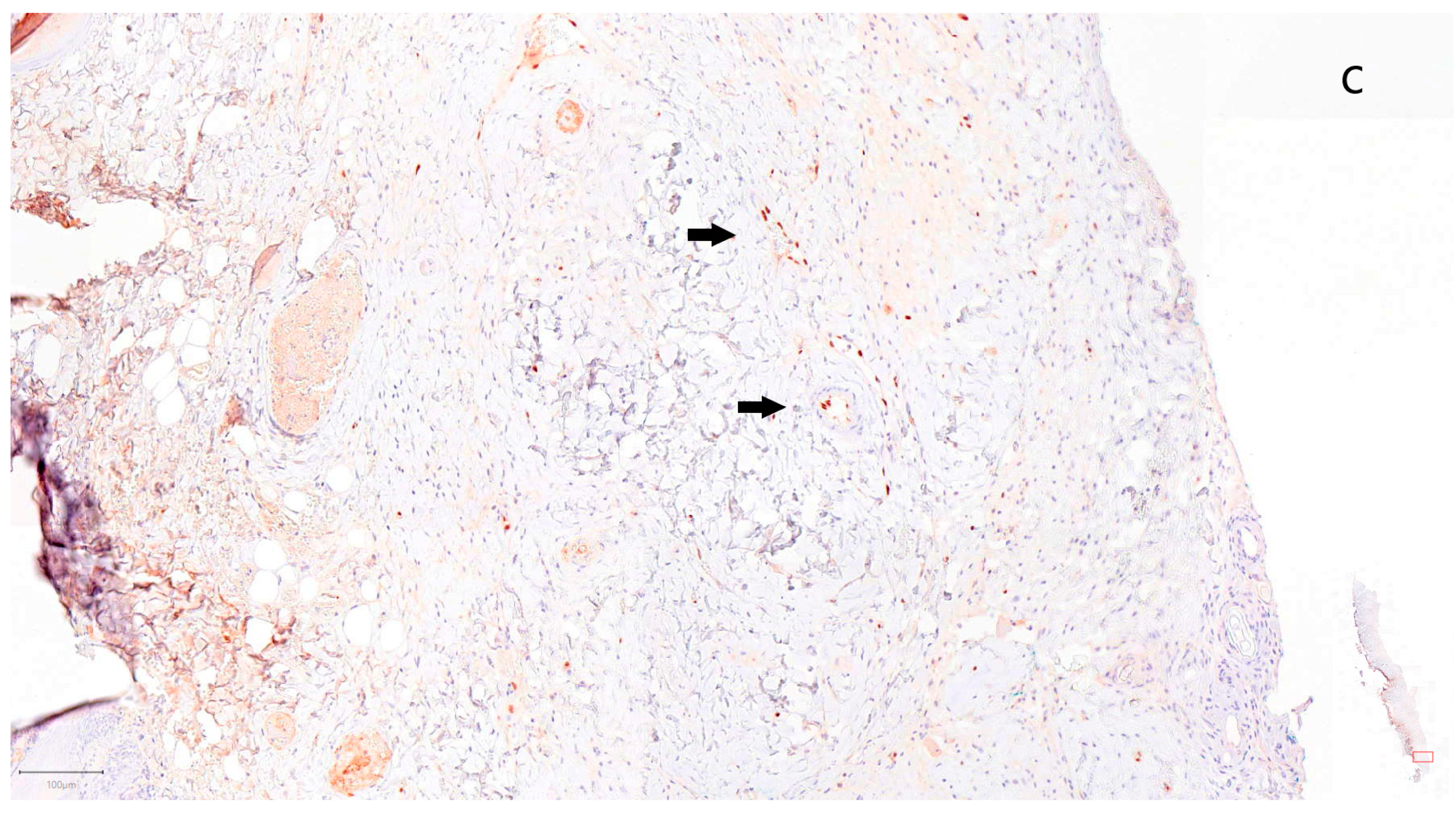
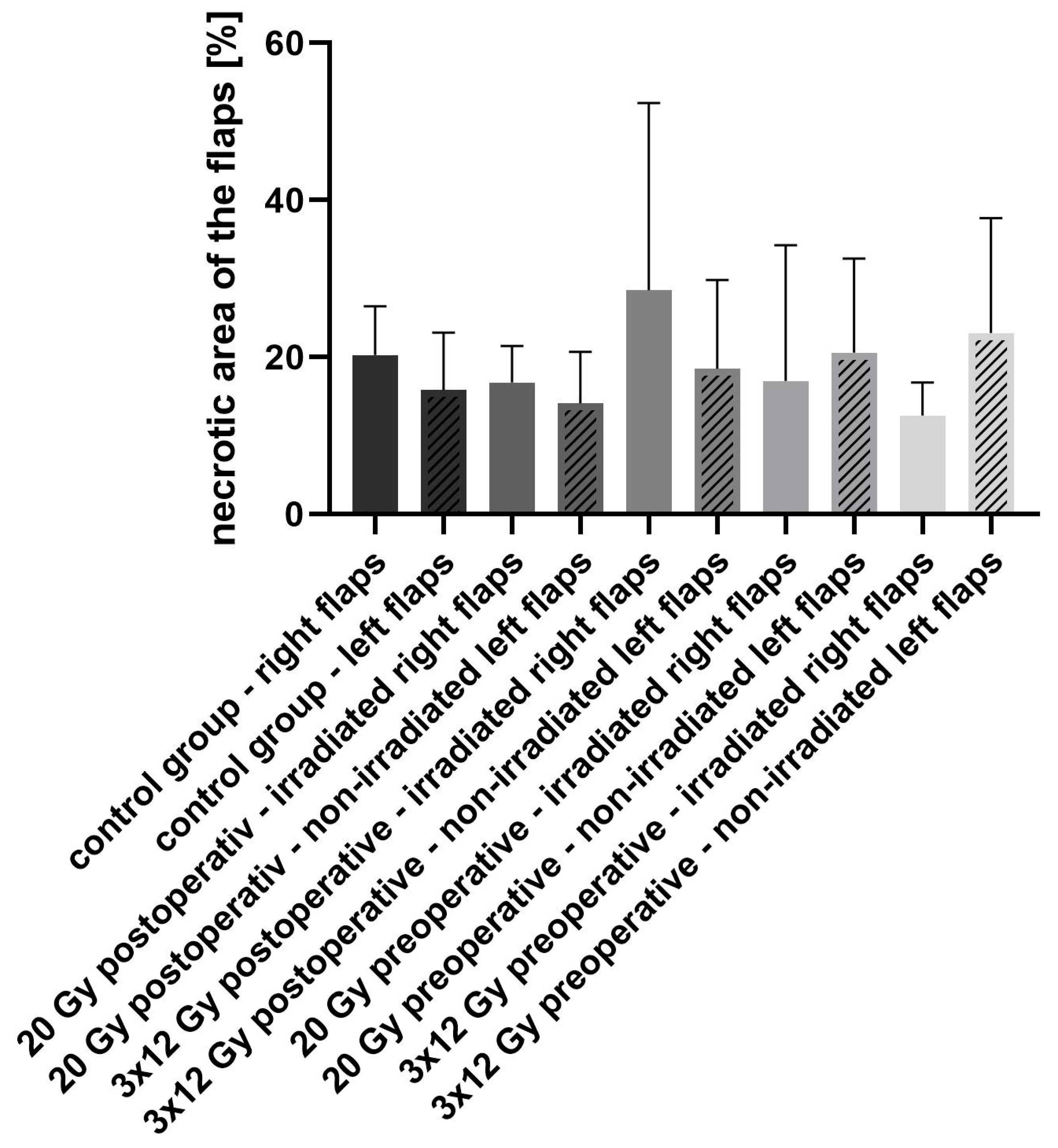
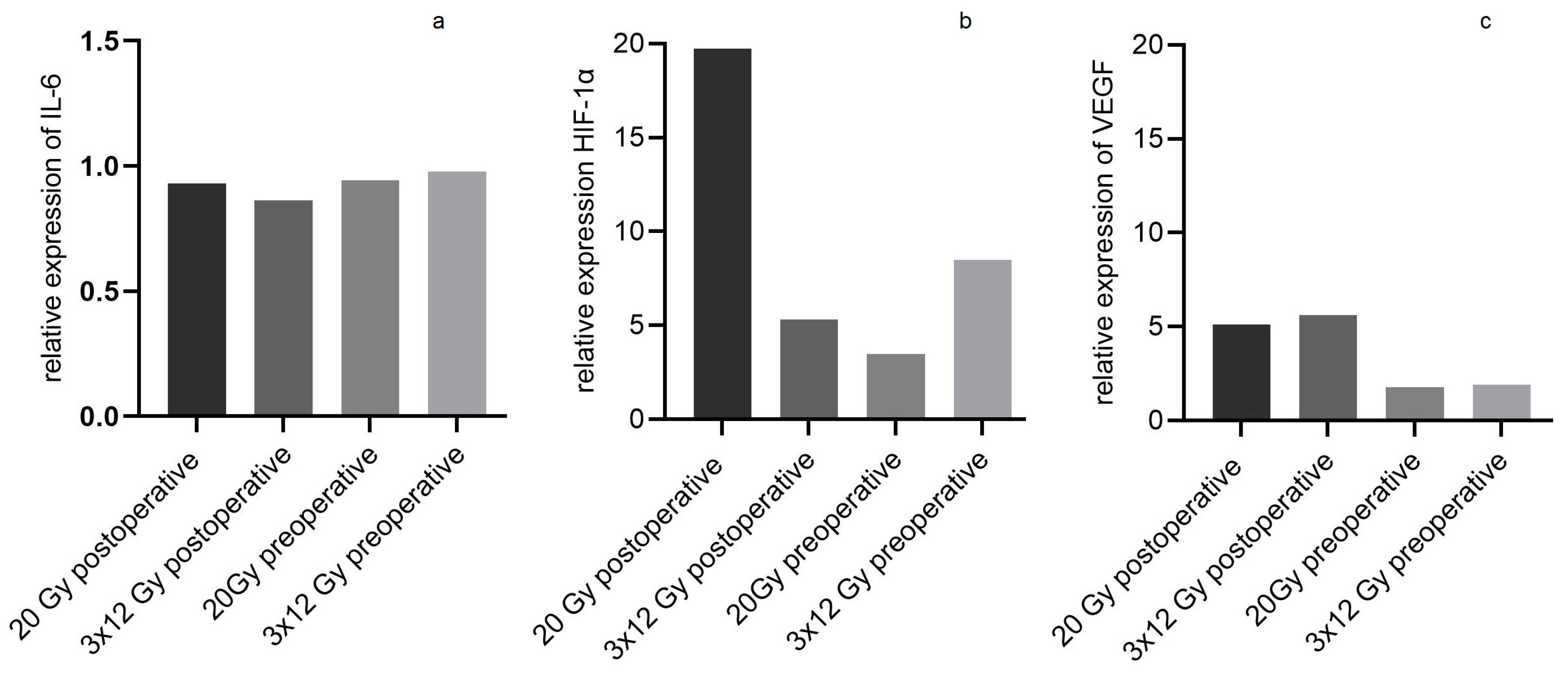
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, N.V.; Evans, G.R.; Kroll, S.S.; Baldwin, B.J.; Miller, M.J.; Reece, G.P.; Robb, G.L. Postoperative adjuvant irradiation: Effects on tranverse rectus abdominis muscle flap breast reconstruction. Plast. Reconstr. Surg. 2000, 106, 313–317. [Google Scholar] [CrossRef]
- Flacco, J.; Chung, N.; Blackshear, C.P.; Irizarry, D.; Momeni, A.; Lee, G.K.; Nguyen, D.; Gurtner, G.C.; Longaker, M.T.; Wan, D.C. Deferoxamine Preconditioning of Irradiated Tissue Improves Perfusion and Fat Graft Retention. Plast. Reconstr. Surg. 2018, 141, 655–665. [Google Scholar] [CrossRef]
- Chao, A.H.; Chang, D.W.; Shuaib, S.W.; Hanasono, M.M. The effect of neoadjuvant versus adjuvant irradiation on microvascular free flap reconstruction in sarcoma patients. Plast. Reconstr. Surg. 2012, 129, 675–682. [Google Scholar] [CrossRef]
- Kremer, T.; Cordts, T.; Hirche, C.; Hernekamp, F.; Radu, C.; Kneser, U. Reconstruction of Defects after Oncologic Resection and Radiation-Indications for Microsurgical Reconstruction. Handchir. Mikrochir. Plast. Chir. 2015, 47, 353–358. [Google Scholar]
- Olascoaga, A.; Vilar-Compte, D.; Poitevin-Chacón, A.; Contreras-Ruiz, J. Wound healing in radiated skin: Pathophysiology and treatment options. Int. Wound J. 2008, 5, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Myung, Y.; Son, Y.; Nam, T.H.; Kang, E.; Kim, E.K.; Kim, I.A.; Eom, K.Y.; Heo, C.Y.; Jeong, J.H. Objective assessment of flap volume changes and aesthetic results after adjuvant radiation therapy in patients undergoing immediate autologous breast reconstruction. PLoS ONE 2018, 13, e0197615. [Google Scholar] [CrossRef]
- Yarnold, J.; Brotons, M.C.V. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex ‘wound’. Radiother. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Mollà, M.; Panés, J. Radiation-induced intestinal inflammation. World J. Gastroenterol. 2007, 13, 3043–3046. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Breen, E.C. VEGF in biological control. J. Cell. Biochem. 2007, 102, 1358–1367. [Google Scholar] [CrossRef]
- Byrne, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J. Cell. Mol. Med. 2005, 9, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Hashimoto, I.; Abe, Y.; Ishida, S.; Kashiwagi, K.; Mineda, K.; Yamashita, Y.; Yamato, R.; Toda, A.; Fukunaga, Y.; Yoshimoto, S.; et al. Development of Skin Flaps for Reconstructive Surgery: Random Pattern Flap to Perforator Flap. J. Med. Investig. 2016, 63, 159–162. [Google Scholar] [CrossRef]
- McFarlane, R.M.; Deyoung, G.; Henry, R.A.; McFarlane, R.M. The Design of a Pedicle Flap in the Rat to Study Necrosis and Its Prevention. Plast. Reconstr. Surg. 1965, 35, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Schmauss, D.; Weinzierl, A.; Schmauss, V.; Harder, Y. Common Rodent Flap Models in Experimental Surgery. Eur. Surg. Res. 2018, 59, 255–264. [Google Scholar] [CrossRef]
- Adamson, J.E.; Horton, C.E.; Crawford, H.H.; Ayers, W.T., Jr. The effects of dimethyl sulfoxide on the experimental pedicle flap: A preliminary report. Plast. Reconstr. Surg. 1966, 37, 105–110. [Google Scholar] [CrossRef]
- Kelly, C.P.; Gupta, A.; Keskin, M.; Jackson, I.T. A new design of a dorsal flap in the rat to study skin necrosis and its prevention. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1553–1556. [Google Scholar] [CrossRef]
- Oh, M.; Chang, H.; Minn, K.W. The effects of tadalafil on axial-pattern skin flap survival in rats. Dermatol. Surg. 2008, 34, 626–630. [Google Scholar]
- Müller-Seubert, W.; Ostermaier, P.; Horch, R.E.; Distel, L.; Frey, B.; Cai, A.; Arkudas, A. Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography. J. Pers. Med. 2022, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Cury, V.; Bossini, P.S.; Fangel, R.; de Sousa Crusca, J.; Renno, A.C.; Parizotto, N.A. The effects of 660 nm and 780 nm laser irradiation on viability of random skin flap in rats. Photomed. Laser Surg. 2009, 27, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Luginbuhl, A.; Modest, M.; Yan, K.; Curry, J.; Heffelfinger, R. Novel irradiated axial rotational flap model in the rodent. JAMA Facial Plast. Surg. 2013, 15, 344–348. [Google Scholar] [CrossRef][Green Version]
- Angelos, P.C.; McCarn, K.E.; Winn, S.R.; Ghanem, T.; Kaurin, D.S.; Holland, J.; Wax, M.K. Development of an irradiated rodent model to study flap revascularization. Arch. Facial Plast. Surg. 2010, 12, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, N.; Farjah, G.H.; Ghadimi, B.; Zanjani, H.; Heshmatian, B. Acceleration of skin wound healing by low-dose indirect ionizing radiation in male rats. Kaohsiung J. Med. Sci. 2017, 33, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, Q.; Xu, W.; She, C.; Xie, Z.G.; Mao, Y.T.; Dong, Q.R.; Ling, M. Low-dose X-ray irradiation promotes osteoblast proliferation, differentiation and fracture healing. PLoS ONE 2014, 9, e104016. [Google Scholar] [CrossRef]
- Rutkowska, E.; Baker, C.; Nahum, A. Mechanistic simulation of normal-tissue damage in radiotherapy—Implications for dose-volume analyses. Phys. Med. Biol. 2010, 55, 2121–2136. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Nemiroff, P.M.; Merwin, G.E.; Brant, T.; Cassisi, N.J. Effects of hyperbaric oxygen and irradiation on experimental skin flaps in rats. Otolaryngol. Head Neck Surg. 1985, 93, 485–491. [Google Scholar] [CrossRef]
- Virolainen, P.; Aitasalo, K. Effect of postoperative irradiation on free skin flaps: An experimental study in rats. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2002, 36, 257–261. [Google Scholar] [CrossRef]
- Aitasalo, K.; Aro, H. Irradiation-induced hypoxia in bones and soft tissues: An experimental study. Plast. Reconstr. Surg. 1986, 77, 256–267. [Google Scholar] [CrossRef]
- Hammond, D.C.; Brooksher, R.D.; Mann, R.J.; Beernink, J.H. The dorsal skin-flap model in the rat: Factors influencing survival. Plast. Reconstr. Surg. 1993, 91, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Angel, M.F.; Kaufman, T.; Swartz, W.M.; Ramasastry, S.S.; Narayanan, K.; Futrell, J.W. Studies on the nature of the flap/bed interaction in rodents—Part I: Flap survival under varying conditions. Ann. Plast. Surg. 1986, 17, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, F.; Mathew, L.M.; Schwartz, R.A. Radiation dermatitis: An overview. Int. J. Dermatol. 2017, 56, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Barazzuol, L.; Coppes, R.P.; van Luijk, P. Prevention and treatment of radiotherapy-induced side effects. Mol. Oncol. 2020, 14, 1538–1554. [Google Scholar] [CrossRef]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Arias, J.I.; Aller, M.A.; Arias, J. Surgical inflammation: A pathophysiological rainbow. J. Transl. Med. 2009, 7, 19. [Google Scholar] [CrossRef]
- Karimipour, M.; Amanzade, V.; Jabbari, N.; Farjah, G.H. Effects of gamma-low dose irradiation on skin flap survival in rats. Phys. Med. 2017, 40, 104–109. [Google Scholar] [CrossRef]
- Yang, G.; Li, W.; Jiang, H.; Liang, X.; Zhao, Y.; Yu, D.; Zhou, L.; Wang, G.; Tian, H.; Han, F.; et al. Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int. J. Cancer 2016, 139, 2157–2168. [Google Scholar] [CrossRef]
- Rückert, M.; Deloch, L.; Frey, B.; Schlücker, E.; Fietkau, R.; Gaipl, U.S. Combinations of Radiotherapy with Vaccination and Immune Checkpoint Inhibition Differently Affect Primary and Abscopal Tumor Growth and the Tumor Microenvironment. Cancers 2021, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Rzeszowska-Wolny, J.; Przybyszewski, W.M.; Widel, M. Ionizing radiation-induced bystander effects, potential targets for modulation of radiotherapy. Eur. J. Pharmacol. 2009, 625, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects: Past history and future directions. Radiat. Res. 2001, 155, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Li, H.; Huang, H.; Xu, D.; Zhi, D.; Liu, D.; Zhang, Y. Increased expression of EMMPRIN and VEGF in the rat brain after gamma irradiation. J. Korean Med. Sci. 2012, 27, 291–299. [Google Scholar] [CrossRef][Green Version]
- Feng, C.J.; Guo, J.B.; Jiang, H.W.; Zhu, S.X.; Li, C.Y.; Cheng, B.; Chen, Y.; Wang, H.Y. Spatio-temporal localization of HIF-1alpha and COX-2 during irradiation-induced oral mucositis in a rat model system. Int. J. Radiat. Biol. 2008, 84, 35–45. [Google Scholar] [CrossRef]
- Corral, C.J.; Siddiqui, A.; Wu, L.; Farrell, C.L.; Lyons, D.; Mustoe, T.A. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch. Surg. 1999, 134, 200–205. [Google Scholar] [CrossRef]
- Suzuki, S.; Toyoma, S.; Kawasaki, Y.; Yamada, T. Irradiated fibroblasts increase interleukin-6 expression and induce migration of head and neck squamous cell carcinoma. PLoS ONE 2022, 17, e0262549. [Google Scholar] [CrossRef]
- Giglio, D.; Wasén, C.; Mölne, J.; Suchy, D.; Swanpalmer, J.; Jabonero Valbuena, J.; Tobin, G.; Ny, L. Downregulation of toll-like receptor 4 and IL-6 following irradiation of the rat urinary bladder. Clin. Exp. Pharmacol. Physiol. 2016, 43, 698–705. [Google Scholar] [CrossRef]
- Ohara, H.; Kishi, K.; Nakajima, T. Rat dorsal paired island skin flaps: A precise model for flap survival evaluation. Keio J. Med. 2008, 57, 211–216. [Google Scholar] [CrossRef][Green Version]
- Islam, M.M.; Khan, M.A.; Rahman, M.M. Preparation of gelatin based porous biocomposite for bone tissue engineering and evaluation of gamma irradiation effect on its properties. Mater. Sci. Eng. C 2015, 49, 648–655. [Google Scholar] [CrossRef]


| Gene | 5′-3′ Primer Sequence |
|---|---|
| GAPDH | For: GAAGGTCGGTGTGAACGGAT Rev: TGAACTTGCCGTGGGTAGAG |
| Interleukin 6 | For: GACTTCCAGCCAGTTGCCTT Rev: GCAGTGGCTGTCAACAACAT |
| HIF-1α | For: GCAACTGCCACCACTGATGA Rev: GCTGTCCGACTGTGAGTACC |
| VEGF | For: AATGATGAAGCCCTGGAGTG Rev: ATGCTGCAGGAAGCTCATCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Seubert, W.; Ostermaier, P.; Horch, R.E.; Distel, L.; Frey, B.; Erber, R.; Arkudas, A. The Influence of Different Irradiation Regimens on Inflammation and Vascularization in a Random-Pattern Flap Model. J. Pers. Med. 2023, 13, 1514. https://doi.org/10.3390/jpm13101514
Müller-Seubert W, Ostermaier P, Horch RE, Distel L, Frey B, Erber R, Arkudas A. The Influence of Different Irradiation Regimens on Inflammation and Vascularization in a Random-Pattern Flap Model. Journal of Personalized Medicine. 2023; 13(10):1514. https://doi.org/10.3390/jpm13101514
Chicago/Turabian StyleMüller-Seubert, Wibke, Patrick Ostermaier, Raymund E. Horch, Luitpold Distel, Benjamin Frey, Ramona Erber, and Andreas Arkudas. 2023. "The Influence of Different Irradiation Regimens on Inflammation and Vascularization in a Random-Pattern Flap Model" Journal of Personalized Medicine 13, no. 10: 1514. https://doi.org/10.3390/jpm13101514
APA StyleMüller-Seubert, W., Ostermaier, P., Horch, R. E., Distel, L., Frey, B., Erber, R., & Arkudas, A. (2023). The Influence of Different Irradiation Regimens on Inflammation and Vascularization in a Random-Pattern Flap Model. Journal of Personalized Medicine, 13(10), 1514. https://doi.org/10.3390/jpm13101514










