Sox2 and βIII-Tubulin as Biomarkers of Drug Resistance in Poorly Differentiated Sinonasal Carcinomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Histological Classification of Sinonasal Tumors
2.3. Immunohistochemistry
2.4. HPV DNA and IDH2 Mutation Detection
2.5. Statistical Analysis
3. Results
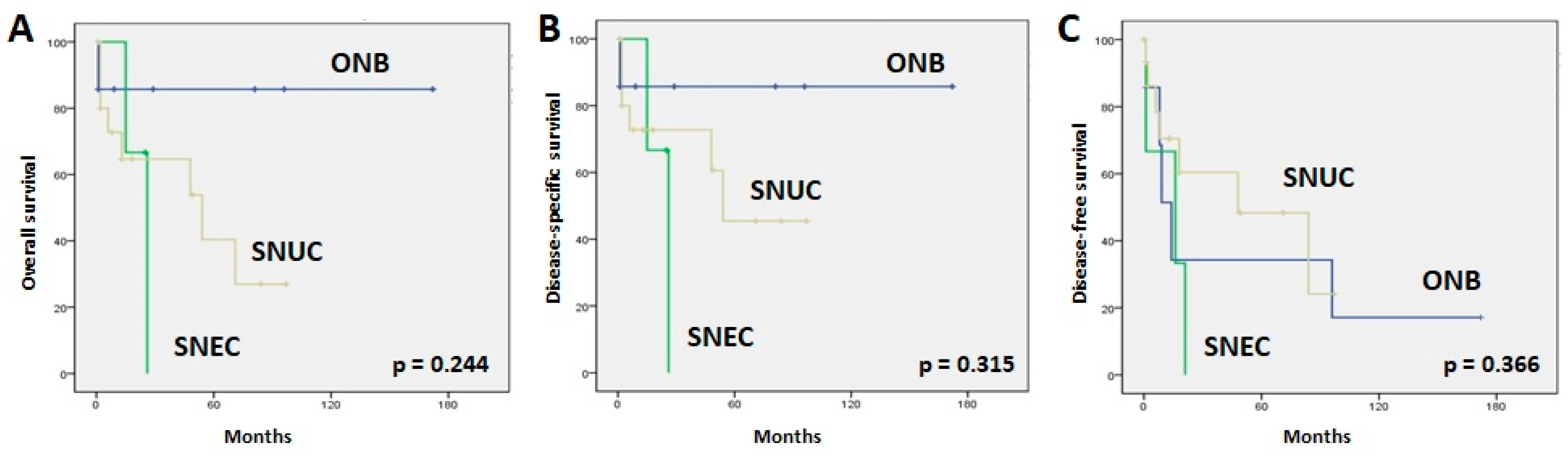
3.1. Clinicopathological Data and Follow-Up
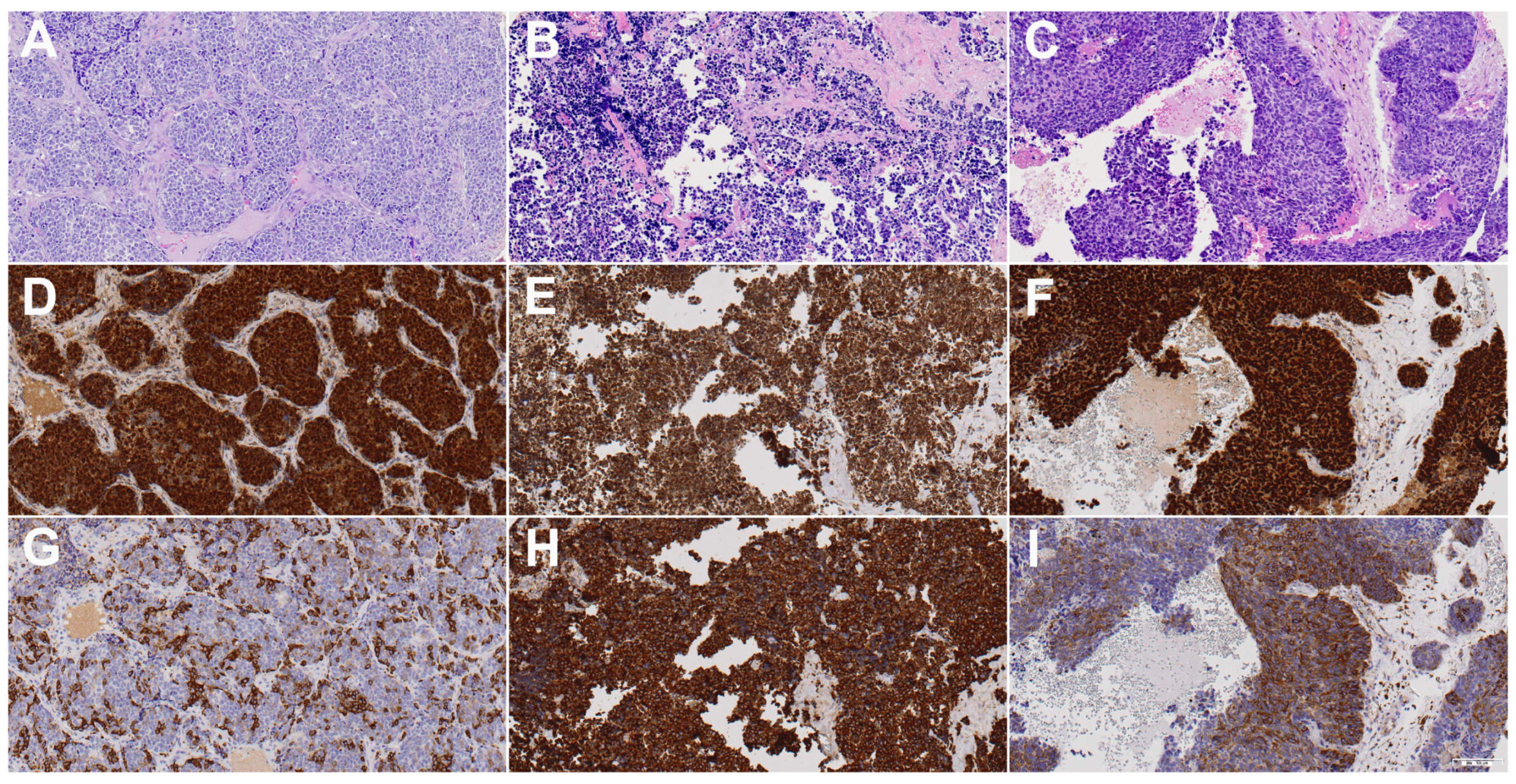
3.2. SOX2 Expression
3.3. βIII-Tubulin Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, S.Y.; Bell, D.; Hanna, E.Y. Esthesioneuroblastoma, Neuroendocrine Carcinoma, and Sinonasal Undifferentiated Carcinoma: Differentiation in Diagnosis and Treatment. Int. Arch. Otorhinolaryngol. 2014, 18, S149–S156. [Google Scholar] [CrossRef]
- López-Hernández, A.; Vivanco, B.; Franchi, A.; Bloemena, E.; Cabal, V.N.; Potes, S.; Riobello, C.; García-Inclán, C.; López, F.; Llorente, J.L.; et al. Genetic Profiling of Poorly Differentiated Sinonasal Tumours. Sci. Rep. 2018, 8, 3998. [Google Scholar] [CrossRef]
- Rooper, L.M. Proceedings of the 2023 North American Society of Head and Neck Pathology Companion Meeting, New Orleans, LA, March 12, 2023: Navigating New Developments in High Grade Sinonasal Neuroendocrine and Neuroectodermal Neoplasms. Head Neck Pathol. 2023, 17, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Zunitch, M.J.; Fisch, A.S.; Lin, B.; Barrios-Camacho, C.M.; Faquin, W.C.; Tachie-Baffour, Y.; Louie, J.D.; Jang, W.; Curry, W.T.; Gray, S.T.; et al. Molecular Evidence for Olfactory Neuroblastoma as a Tumor of Malignant Globose Basal Cells. Mod. Pathol. 2023, 36, 100122. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Taboni, S.; Contro, G.; Nicolai, P. Precision Medicine in the Treatment of Malignancies Involving the Ventral Skull Base: Present and Future. In Critical Issues in Head and Neck Oncology, Proceedings of the Eighth THNO Meeting, Amsterdam, The Netherlands, 11–13 November 2021; Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, J.-P., Nicolai, P., O’Sullivan, B., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 237–291. [Google Scholar]
- Lopez, D.C.; Wadley, A.E.; London, N.R. Emerging Concepts in Sinonasal Tumor Research. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 33–39. [Google Scholar] [CrossRef]
- Guilmette, J.; Sadow, P.M. High-Grade Sinonasal Carcinoma: Classification Through Molecular Profiling. Arch. Pathol. Lab. Med. 2019, 143, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, M.A.; Riobello, C.; García-Marín, R.; Cabal, V.N.; Suárez-Fernández, L.; López, F.; Llorente, J.L. Translational Genomics of Sinonasal Cancers. Semin. Cancer Biol. 2020, 61, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Romani, C.; Bignotti, E.; Mattavelli, D.; Bozzola, A.; Lorini, L.; Tomasoni, M.; Ardighieri, L.; Rampinelli, V.; Paderno, A.; Battocchio, S.; et al. Gene Expression Profiling of Olfactory Neuroblastoma Helps Identify Prognostic Pathways and Define Potentially Therapeutic Targets. Cancers 2021, 13, 2527. [Google Scholar] [CrossRef]
- Mody, M.D.; Saba, N.F. Multimodal Therapy for Sinonasal Malignancies: Updates and Review of Current Treatment. Curr. Treat. Options Oncol. 2020, 21, 4. [Google Scholar] [CrossRef]
- Chen, M.Y.; Wen, X.; Wei, Y.; Chen, L.; Huang, Z.X.; Lu, T.; Zheng, N.Z.; Li, J.; Wen, W.P.; Wen, Y.H. Oncologic outcome of multimodality treatment for sinonasal malignancies: An 18-year experience. Front. Oncol. 2022, 12, 958142. [Google Scholar] [CrossRef] [PubMed]
- Llorente, J.L.; López, F.; Suárez, C.; Hermsen, M.A. Sinonasal Carcinoma: Clinical, Pathological, Genetic and Therapeutic Advances. Nat. Rev. Clin. Oncol. 2014, 11, 460–472. [Google Scholar] [CrossRef]
- Thawani, R.; Kim, M.S.; Arastu, A.; Feng, Z.; West, M.T.; Taflin, N.F.; Thein, K.Z.; Li, R.; Geltzeiler, M.; Lee, N.; et al. The contemporary management of cancers of the sinonasal tract in adults. CA Cancer J. Clin. 2023, 73, 72–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Coleman, J.H.; Peterson, J.N.; Zunitch, M.J.; Jang, W.; Herrick, D.B.; Schwob, J.E. Injury Induces Endogenous Reprogramming and Dedifferentiation of Neuronal Progenitors to Multipotency. Cell Stem Cell 2017, 21, 761–774.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Reed, R.R.; Lane, A.P. Chronic Inflammation Directs an Olfactory Stem Cell Functional Switch from Neuroregeneration to Immune Defense. Cell Stem Cell 2019, 25, 501–513.e5. [Google Scholar] [CrossRef]
- Alqadah, A.; Hsieh, Y.-W.; Xiong, R.; Lesch, B.; Chang, C.; Chuang, C.-F. A Universal Transportin Protein Drives Stochastic Choice of Olfactory Neurons via Specific Nuclear Import of a Sox-2-Activating Factor. Proc. Natl. Acad. Sci. USA 2019, 116, 201908168. [Google Scholar] [CrossRef]
- Balachandran, S.; Narendran, A. The Developmental Origins of Cancer: A Review of the Genes Expressed in Embryonic Cells with Implications for Tumorigenesis. Genes 2023, 14, 604. [Google Scholar] [CrossRef] [PubMed]
- Holmes, Z.E.; Hamilton, D.J.; Hwang, T.; Parsonnet, N.V.; Rinn, J.L.; Wuttke, D.S.; Batey, R.T. The Sox2 Transcription Factor Binds RNA. Nat. Commun. 2020, 11, 1805. [Google Scholar] [CrossRef]
- Varzideh, F.; Gambardella, J.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Molecular mechanisms underlying pluripotency and self-renewal of embryonic stem cells. Int. J. Mol. Sci. 2023, 24, 8386. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, K.; Suzuki, A. The role of pioneer transcription factors in the induction of direct cellular reprogramming. Regen. Ther. 2023, 24, 112–116. [Google Scholar] [CrossRef]
- Menendez, S.T.; Rey, V.; Martinez-Cruzado, L.; Gonzalez, M.V.; Morales-Molina, A.; Santos, L.; Blanco, V.; Alvarez, C.; Estupiñan, O.; Allonca, E.; et al. SOX2 Expression and Transcriptional Activity Identifies a Subpopulation of Cancer Stem Cells in Sarcoma with Prognostic Implications. Cancers 2020, 12, 964. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, N.; Zhang, Y.; Jiang, W.; Fang, C.; Feng, Y.; Ma, H.; Jiang, F.; Dong, G. Self-restricted circular RNA circSOX2 suppressed the malignant progression in SOX2-amplified LUSC. Cell Death Dis. 2022, 13, 873. [Google Scholar] [CrossRef] [PubMed]
- Bensen, R.; Brognard, J. New therapeutic opportunities for the treatment of squamous cell carcinomas: A focus on novel driver kinases. Int. J. Mol. Sci. 2021, 22, 2831. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, Z.; Master, R.P.; Maharjan, C.K.; Carelock, M.E.; Reccoppa, T.B.A.; Kim, M.C.; Kolb, R.; Zhang, W. Breast cancer stem cells: Signaling pathways, cellular interactions, and therapeutic implications. Cancers 2022, 14, 3287. [Google Scholar] [CrossRef] [PubMed]
- Oria, V.O.; Zhang, H.; Zito, C.R.; Rane, C.K.; Ma, X.Y.; Provance, O.K.; Tran, T.T.; Adeniran, A.; Kluger, Y.; Sznol, M.; et al. Coupled fibromodulin and SOX2 signaling as a critical regulator of metastatic outgrowth in melanoma. Cell Mol. Life Sci. 2022, 23, 377. [Google Scholar] [CrossRef]
- Williams, A.; Gutgesell, L.; de Wet, L.; Selman, P.; Dey, A.; Avineni, M.; Kapoor, I.; Mendez, M.; Brown, R.; Lamperis, S.; et al. SOX2 expression in prostate cancer drives resistance to nuclear hormone receptor signaling inhibition through the WEE1/CDK1 signaling axis. Cancer Lett. 2023, 565, 216209. [Google Scholar] [CrossRef]
- Robinson, M.; Gilbert, S.F.; Waters, J.A.; Lujano-Olazaba, O.; Lara, J.; Alexander, L.J.; Green, S.E.; Burkeen, G.A.; Patrus, O.; Sarwar, Z.; et al. Characterization of SOX2, OCT4 and NANOG in ovarian cancer tumor-initiating cells. Cancers 2021, 12, 262. [Google Scholar] [CrossRef]
- Schaefer, T.; Lengerke, C. SOX2 protein biochemistry in stemness, reprogramming, and cancer: The PI3K/AKT/SOX2 axis and beyond. Oncogene 2020, 39, 278–292. [Google Scholar] [CrossRef]
- González-Márquez, R.; Llorente, J.L.; Rodrigo, J.P.; García-Pedrero, J.M.; Álvarez-Marcos, C.; Suárez, C.; Hermsen, M.A. SOX2 expression in hypopharyngeal, laryngeal, and sinonasal squamous cell carcinoma. Hum. Pathol. 2014, 45, 851–857. [Google Scholar] [CrossRef]
- Freier, K.; Knoepfle, K.; Flechtenmacher, C.; Pungs, S.; Devens, F.; Toedt, G.; Hofele, C.; Joos, S.; Lichter, P.; Radlwimmer, B. Recurrent Copy Number Gain of Transcription Factor SOX2 and Corresponding High Protein Expression in Oral Squamous Cell Carcinoma. Genes Chromosomes Cancer 2010, 49, 9–16. [Google Scholar] [CrossRef]
- Granda-Díaz, R.; Menéndez, S.T.; Pedregal Mallo, D.; Hermida-Prado, F.; Rodríguez, R.; Suárez-Fernández, L.; Vallina, A.; Sánchez-Canteli, M.; Rodríguez, A.; Fernández-García, M.S.; et al. The Novel Role of SOX2 as an Early Predictor of Cancer Risk in Patients with Laryngeal Precancerous Lesions. Cancers 2019, 11, 286. [Google Scholar] [CrossRef]
- Schröck, A.; Göke, F.; Wagner, P.; Bode, M.; Franzen, A.; Braun, M.; Huss, S.; Agaimy, A.; Ihrler, S.; Menon, R.; et al. Sex Determining Region Y-Box 2 (SOX2) Amplification Is an Independent Indicator of Disease Recurrence in Sinonasal Cancer. PLoS ONE 2013, 8, e59201. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Chen, Y.; Zhan, X.; Wu, T.; Chen, B.; Sun, G.; Yan, S.; Xu, L. The Role of SOX2 Overexpression in Prognosis of Patients with Solid Tumors: A Meta-Analysis and System Review. Medicine 2020, 99, e19604. [Google Scholar] [CrossRef] [PubMed]
- Dorna, D.; Paluszczak, J. Targeting Cancer Stem Cells as a Strategy for Reducing Chemotherapy Resistance in Head and Neck Cancers. J. Cancer Res. Clin. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kanakkanthara, A.; Miller, J.H. βIII-tubulin overexpression in cancer: Causes, consequences, and potential therapies. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188607. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Luo, X.-P.; Xian, L. The Prognostic Role of the Class III β-Tubulin in Non-Small Cell Lung Cancer (NSCLC) Patients Receiving the Taxane/Vinorebine-Based Chemotherapy: A Meta-Analysis. PLoS ONE 2014, 9, e93997. [Google Scholar] [CrossRef]
- Höflmayer, D.; Öztürk, E.; Schroeder, C.; Hube-Magg, C.; Blessin, N.C.; Simon, R.; Lang, D.S.; Neubauer, E.; Göbel, C.; Heinrich, M.-C.; et al. High Expression of Class III β-Tubulin in Upper Gastrointestinal Cancer Types. Oncol. Lett. 2018, 16, 7139–7145. [Google Scholar] [CrossRef]
- Lebok, P.; Öztürk, M.; Heilenkötter, U.; Jaenicke, F.; Müller, V.; Paluchowski, P.; Geist, S.; Wilke, C.; Burandt, E.; Lebeau, A.; et al. High Levels of Class III β-Tubulin Expression Are Associated with Aggressive Tumor Features in Breast Cancer. Oncol. Lett. 2016, 11, 1987–1994. [Google Scholar] [CrossRef]
- Öztop, S.; Işik, A.; Güner, G.; Gürdal, H.; Karabulut, E.; Yilmaz, E.; Akyol, A. Class III β-Tubulin Expression in Colorectal Neoplasms Is a Potential Predictive Biomarker for Paclitaxel Response. Anticancer Res. 2019, 39, 655–662. [Google Scholar] [CrossRef]
- Hinsch, A.; Chaker, A.; Burdelski, C.; Koop, C.; Tsourlakis, M.C.; Steurer, S.; Rink, M.; Eichenauer, T.S.; Wilczak, W.; Wittmer, C.; et al. βIII-Tubulin Overexpression Is Linked to Aggressive Tumor Features and Genetic Instability in Urinary Bladder Cancer. Hum. Pathol. 2017, 61, 210–220. [Google Scholar] [CrossRef]
- Nawara, H.M.; Afify, S.M.; Hassan, G.; Zahra, M.H.; Seno, A.; Seno, M. Paclitaxel-based chemotherapy targeting cancer stem cells from mono- to combination therapy. Biomedicines 2021, 9, 500. [Google Scholar] [CrossRef]
- Pernar Kovač, M.; Tadić, V.; Kralj, J.; Duran, G.E.; Stefanelli, A.; Stupin Polančec, D.; Dabelić, S.; Bačić, N.; Tomicic, M.T.; Heffeter, P.; et al. Carboplatin-induced upregulation of pan β-tubulin and class III β-tubulin is implicated in acquired resistance and cross-resistance of ovarian cancer. Cell Mol. Life Sci. 2023, 80, 294. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Ki, M.-S.; Kim, K.; Shim, H.-J.; Hwang, J.-E.; Bae, W.-K.; Chung, I.-J.; Lee, D.-H.; Lee, J.-K.; Yoon, T.-M.; et al. Different Protein Expression Associated with Chemotherapy Response in Oropharyngeal Cancer According to HPV Status. BMC Cancer 2014, 14, 824. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Kim, T.M.; Jeon, Y.K.; Kwon, T.-K.; Hah, J.H.; Lee, S.-H.; Kim, D.-W.; Wu, H.-G.; Rhee, C.-S.; Sung, M.-W.; et al. Class III Beta-Tubulin, but Not ERCC1, Is a Strong Predictive and Prognostic Marker in Locally Advanced Head and Neck Squamous Cell Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Nienstedt, J.C.; Gröbe, A.; Clauditz, T.; Simon, R.; Muenscher, A.; Knecht, R.; Sauter, G.; Moebius, C.; Blessmann, M.; Heiland, M.; et al. High-Level βIII-Tubulin Overexpression Occurs in Most Head and Neck Cancers but Is Unrelated to Clinical Outcome. J. Oral. Pathol. Med. 2017, 46, 986–990. [Google Scholar] [CrossRef]
- Parker, A.L.; Teo, W.S.; McCarroll, J.A.; Kavallaris, M. An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance. Int. J. Mol. Sci. 2017, 18, 1434. [Google Scholar] [CrossRef]
- Topcagic, J.; Feldman, R.; Ghazalpour, A.; Swensen, J.; Gatalica, Z.; Vranic, S. Comprehensive Molecular Profiling of Advanced/Metastatic Olfactory Neuroblastomas. PLoS ONE 2018, 13, e0191244. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA. Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Bishop, J.A. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2022, 16, 1–18. [Google Scholar] [CrossRef]
- Brcic, L.; Sherer, C.K.; Shuai, Y.; Hornick, J.L.; Chirieac, L.R.; Dacic, S. Morphologic and Clinicopathologic Features of Lung Squamous Cell Carcinomas Expressing Sox2. Am. J. Clin. Pathol. 2012, 138, 712–718. [Google Scholar] [CrossRef]
- Lu, Y.; Futtner, C.; Rock, J.R.; Xu, X.; Whitworth, W.; Hogan, B.L.M.; Onaitis, M.W. Evidence That SOX2 Overexpression Is Oncogenic in the Lung. PLoS ONE 2010, 5, e11022. [Google Scholar] [CrossRef]
- Sholl, L.M.; Barletta, J.A.; Yeap, B.Y.; Chirieac, L.R.; Hornick, J.L. Sox2 Protein Expression Is an Independent Poor Prognostic Indicator in Stage I Lung Adenocarcinoma. Am. J. Surg. Pathol. 2010, 34, 1193–1198. [Google Scholar] [CrossRef]
- Riobello, C.; López-Hernández, A.; Cabal, V.N.; García-Marín, R.; Suárez-Fernández, L.; Sánchez-Fernández, P.; Vivanco, B.; Blanco, V.; López, F.; Franchi, A.; et al. IDH2 Mutation Analysis in Undifferentiated and Poorly Differentiated Sinonasal Carcinomas for Diagnosis and Clinical Management. Am. J. Surg. Pathol. 2020, 44, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Gen, Y.; Yasui, K.; Zen, Y.; Zen, K.; Dohi, O.; Endo, M.; Tsuji, K.; Wakabayashi, N.; Itoh, Y.; Naito, Y.; et al. SOX2 Identified as a Target Gene for the Amplification at 3q26 That Is Frequently Detected in Esophageal Squamous Cell Carcinoma. Cancer Genet. Cytogenet. 2010, 202, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Hussenet, T.; Dali, S.; Exinger, J.; Monga, B.; Jost, B.; Dembelé, D.; Martinet, N.; Thibault, C.; Huelsken, J.; Brambilla, E.; et al. SOX2 Is an Oncogene Activated by Recurrent 3q26.3 Amplifications in Human Lung Squamous Cell Carcinomas. PLoS ONE 2010, 5, e8960. [Google Scholar] [CrossRef]
- Li, X.-L.; Eishi, Y.; Bai, Y.-Q.; Sakai, H.; Akiyama, Y.; Tani, M.; Takizawa, T.; Koike, M.; Yuasa, Y. Expression of the SRY-Related HMG Box Protein SOX2 in Human Gastric Carcinoma. Int. J. Oncol. 2004, 24, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive Genomic Analysis Identifies SOX2 as a Frequently Amplified Gene in Small-Cell Lung Cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.-M.; Su, Z.; Frye, C.; McClellan, S.; Allan, R.W.; Andrejewski, J.T.; Kelley, V.; Jorgensen, M.; Steindler, D.A.; Vieweg, J.; et al. Expression of Pluripotent Stem Cell Reprogramming Factors by Prostate Tumor Initiating Cells. J. Urol. 2010, 183, 2045–2053. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Chen, Q.; Xu, H.-M.; Ge, N.; Luo, R.-Z.; Shao, J.-Y.; He, Z.; Zeng, Y.-X.; Kang, T.; et al. Prognostic Significance of SOX2 Expression in Nasopharyngeal Carcinoma. Cancer Investig. 2012, 30, 79–85. [Google Scholar] [CrossRef]
- Lundberg, I.V.; Löfgren Burström, A.; Edin, S.; Eklöf, V.; Öberg, Å.; Stenling, R.; Palmqvist, R.; Wikberg, M.L. SOX2 Expression Is Regulated by BRAF and Contributes to Poor Patient Prognosis in Colorectal Cancer. PLoS ONE 2014, 9, e101957. [Google Scholar] [CrossRef]
- Bayo, P.; Jou, A.; Stenzinger, A.; Shao, C.; Gross, M.; Jensen, A.; Grabe, N.; Mende, C.H.; Rados, P.V.; Debus, J.; et al. Loss of SOX2 Expression Induces Cell Motility via Vimentin Up-Regulation and Is an Unfavorable Risk Factor for Survival of Head and Neck Squamous Cell Carcinoma. Mol. Oncol. 2015, 9, 1704–1719. [Google Scholar] [CrossRef]
- Person, F.; Wilczak, W.; Hube-Magg, C.; Burdelski, C.; Möller-Koop, C.; Simon, R.; Noriega, M.; Sauter, G.; Steurer, S.; Burdak-Rothkamm, S.; et al. Prevalence of βIII-Tubulin (TUBB3) Expression in Human Normal Tissues and Cancers. Tumour Biol. 2017, 39, 1010428317712166. [Google Scholar] [CrossRef]
- Reiman, T.; Lai, R.; Veillard, A.S.; Paris, E.; Soria, J.C.; Rosell, R.; Taron, M.; Graziano, S.; Kratzke, R.; Seymour, L.; et al. Cross-Validation Study of Class III Beta-Tubulin as a Predictive Marker for Benefit from Adjuvant Chemotherapy in Resected Non-Small-Cell Lung Cancer: Analysis of Four Randomized Trials. Ann. Oncol. 2012, 23, 86–93. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Ruan, L.; Zheng, L.-M.; Whyte, D.; Tzeng, C.-M.; Zhou, X.-W. Association between Class III β-Tubulin Expression and Response to Paclitaxel/Vinorebine-Based Chemotherapy for Non-Small Cell Lung Cancer: A Meta-Analysis. Lung Cancer Amst. Neth. 2012, 77, 9–15. [Google Scholar] [CrossRef]
- Lobert, S.; Graichen, M.E.; Hamilton, R.D.; Pitman, K.T.; Garrett, M.R.; Hicks, C.; Koganti, T. Prognostic Biomarkers for HNSCC Using Quantitative Real-Time PCR and Microarray Analysis: β-Tubulin Isotypes and the P53 Interactome. Cytoskeleton 2014, 71, 628–637. [Google Scholar] [CrossRef]
- Mariani, M.; Karki, R.; Spennato, M.; Pandya, D.; He, S.; Andreoli, M.; Fiedler, P.; Ferlini, C. Class III β-Tubulin in Normal and Cancer Tissues. Gene 2015, 563, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiong, X.; Sun, Y. Functional Characterization of SOX2 as an Anticancer Target. Signal Transduct. Target. Ther. 2020, 5, 135. [Google Scholar] [CrossRef]
- Karki, R.; Mariani, M.; Andreoli, M.; He, S.; Scambia, G.; Shahabi, S.; Ferlini, C. βIII-Tubulin: Biomarker of Taxane Resistance or Drug Target? Expert. Opin. Ther. Targets 2013, 17, 461–472. [Google Scholar] [CrossRef]
- Ferrandina, G.; Zannoni, G.F.; Martinelli, E.; Paglia, A.; Gallotta, V.; Mozzetti, S.; Scambia, G.; Ferlini, C. Class III Beta-Tubulin Overexpression Is a Marker of Poor Clinical Outcome in Advanced Ovarian Cancer Patients. Clin. Cancer Res. 2006, 12, 2774–2779. [Google Scholar] [CrossRef]
- Vasefifar, P.; Najafi, S.; Motafakkerazad, R.; Amini, M.; Safaei, S.; Najafzadeh, B.; Alemohammad, H.; Jafarlou, M.; Baradaran, B. Targeting Nanog Expression Increased Cisplatin Chemosensitivity and Inhibited Cell Migration in Gastric Cancer Cells. Exp. Cell Res. 2023, 429, 113681. [Google Scholar] [CrossRef] [PubMed]
- Classe, M.; Burgess, A.; El Zein, S.; Wassef, M.; Herman, P.; Mortuaire, G.; Leroy, X.; Malouf, G.G.; Verillaud, B. Evaluating the prognostic potential of the Ki67 proliferation index and tumor-infiltrating lymphocytes in olfactory neuroblastoma. Histopathology 2019, 75, 853–864. [Google Scholar] [CrossRef] [PubMed]
- London, N.R., Jr.; Rooper, L.M.; Bishop, J.A.; Xu, H.; Bernhardt, L.J.; Ishii, M.; Hann, C.L.; Taube, J.M.; Izumchenko, E.; Gaykalova, D.A.; et al. Expression of programmed cell death ligand 1 and associated lymphocyte infiltration in olfactory neuroblastoma. World Neurosurg. 2020, 135, e187–e193. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Fernández, E.; Hermsen, M.A.; Suárez-Fernández, L.; Vivanco, B.; Franchi, A.; García-Marín, R.; Cabal, V.N.; Codina-Martínez, H.; Lorenzo-Guerra, S.L.; Llorente, J.L.; et al. Biomarkers for Immunotherapy in poorly differentiated sinonasal tumors. Biomedicines 2022, 10, 2205. [Google Scholar] [CrossRef] [PubMed]
- Hieggelke, L.; Heydt, C.; Castiglione, R.; Rehker, J.; Merkelbach-Bruse, S.; Riobello, C.; Llorente, J.L.; Hermsen, M.A.; Buettner, R. Mismatch repair deficiency and somatic mutations in human sinonasal tumors. Cancers 2021, 13, 6081. [Google Scholar] [CrossRef] [PubMed]
- Abdelmeguid, A.S.; Bell, D.; Hanna, E.Y. Neuroendocrine Carcinoma and Sinonasal Undifferentiated Carcinoma. Adv. Otorhinolaryngol. 2020, 84, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Gadye, L.; Das, D.; Sanchez, M.A.; Street, K.; Baudhuin, A.; Wagner, A.; Cole, M.B.; Choi, Y.G.; Yosef, N.; Purdom, E.; et al. Injury Activates Transient Olfactory Stem Cell States with Diverse Lineage Capacities. Cell Stem Cell 2017, 21, 775–790.e9. [Google Scholar] [CrossRef]
- Ortiz-López, L.; González-Olvera, J.J.; Vega-Rivera, N.M.; García-Anaya, M.; Carapia-Hernández, A.K.; Velázquez-Escobar, J.C.; Ramírez-Rodríguez, G.B. Human Neural Stem/Progenitor Cells Derived from the Olfactory Epithelium Express the TrkB Receptor and Migrate in Response to BDNF. Neuroscience 2017, 355, 84–100. [Google Scholar] [CrossRef]
- Gay, L.M.; Kim, S.; Fedorchak, K.; Kundranda, M.; Odia, Y.; Nangia, C.; Battiste, J.; Colon-Otero, G.; Powell, S.; Russell, J.; et al. Comprehensive Genomic Profiling of Esthesioneuroblastoma Reveals Additional Treatment Options. Oncologist 2017, 22, 834–842. [Google Scholar] [CrossRef]
- Tompkins, D.H.; Besnard, V.; Lange, A.W.; Keiser, A.R.; Wert, S.E.; Bruno, M.D.; Whitsett, J.A. Sox2 Activates Cell Proliferation and Differentiation in the Respiratory Epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 101–110. [Google Scholar] [CrossRef]

| SNUC | SNEC | ONB | |
|---|---|---|---|
| Total Patients | 36 | 8 | 13 |
| Gender | |||
| Male | 19 | 3 | 4 |
| Female | 17 | 5 | 9 |
| Age | |||
| Average | 57 | 58 | 47 |
| Range | 31–85 | 40–77 | 20–69 |
| Location | |||
| Maxillary sinus | 6 | 0 | 0 |
| Ethmoid sinus | 30 | 8 | 13 |
| T classification | |||
| T2 | 14 | 4 | 6 |
| T3 | 2 | 1 | 2 |
| T4a | 19 | 2 | 5 |
| T4b | 1 | 1 | 0 |
| Disease stage | |||
| I-II | 14 | 4 | 6 |
| III-IV | 32 | 4 | 7 |
| Radiotherapy | |||
| No | 3 | 0 | 0 |
| Yes | 33 | 8 | 13 |
| Follow-up | |||
| Mean | 29 | 22 | 56 |
| Median | 13 | 25 | 29 |
| Range | 1–97 | 15–26 | 1–172 |
| Local Recurrence | |||
| No | 11 | 1 | 4 |
| Yes | 5 | 2 | 3 |
| Distant metastasis | |||
| No | 11 | 0 | 4 |
| Yes | 5 | 3 | 3 |
| Patient status | |||
| Alive | 8 | 1 | 6 |
| Died of disease | 6 | 2 | 1 |
| Died of other causes | 2 | 0 | 0 |
| Lost | 20 | 5 | 6 |
| SNUC | SNEC | ONB | |
|---|---|---|---|
| n = 36 | n = 8 | n = 13 | |
| Pancytokeratin | 97% | 100% | 38% |
| CK 5/6 | 0% | ND | ND |
| P40 | 0% | ND | ND |
| P16 | 27% | 0% | 0% |
| HPV | 0% | 0% | 0% |
| Synaptophysin/Chromogranin | 39% | 100% | 100% |
| NUT | 3% | 0% | 0% |
| SMARCB1 (INI-1) | 3% | 0% | 0% |
| SMARCA4 (BRG1) | 0% | 12.5% | 0% |
| IDH2 mut | 28% | 25% | 0% |
| SNUC | SNEC | ONB | |
|---|---|---|---|
| n = 36 | n = 8 | n = 13 | |
| Sox2 nuclear | 53% | 75% | 46% |
| βIII-tubulin strong | 64% | 75% | 85% |
| moderate | 8% | 12.5% | 8% |
| weak | 17% | 12.5% | 0% |
| negative | 11% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, L.; Fernández-Vañes, L.; Cabal, V.N.; García-Marín, R.; Suárez-Fernández, L.; Codina-Martínez, H.; Lorenzo-Guerra, S.L.; Vivanco, B.; Blanco-Lorenzo, V.; Llorente, J.L.; et al. Sox2 and βIII-Tubulin as Biomarkers of Drug Resistance in Poorly Differentiated Sinonasal Carcinomas. J. Pers. Med. 2023, 13, 1504. https://doi.org/10.3390/jpm13101504
López L, Fernández-Vañes L, Cabal VN, García-Marín R, Suárez-Fernández L, Codina-Martínez H, Lorenzo-Guerra SL, Vivanco B, Blanco-Lorenzo V, Llorente JL, et al. Sox2 and βIII-Tubulin as Biomarkers of Drug Resistance in Poorly Differentiated Sinonasal Carcinomas. Journal of Personalized Medicine. 2023; 13(10):1504. https://doi.org/10.3390/jpm13101504
Chicago/Turabian StyleLópez, Luis, Laura Fernández-Vañes, Virginia N. Cabal, Rocío García-Marín, Laura Suárez-Fernández, Helena Codina-Martínez, Sara L. Lorenzo-Guerra, Blanca Vivanco, Verónica Blanco-Lorenzo, José L. Llorente, and et al. 2023. "Sox2 and βIII-Tubulin as Biomarkers of Drug Resistance in Poorly Differentiated Sinonasal Carcinomas" Journal of Personalized Medicine 13, no. 10: 1504. https://doi.org/10.3390/jpm13101504
APA StyleLópez, L., Fernández-Vañes, L., Cabal, V. N., García-Marín, R., Suárez-Fernández, L., Codina-Martínez, H., Lorenzo-Guerra, S. L., Vivanco, B., Blanco-Lorenzo, V., Llorente, J. L., López, F., & Hermsen, M. A. (2023). Sox2 and βIII-Tubulin as Biomarkers of Drug Resistance in Poorly Differentiated Sinonasal Carcinomas. Journal of Personalized Medicine, 13(10), 1504. https://doi.org/10.3390/jpm13101504








