Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions
Abstract
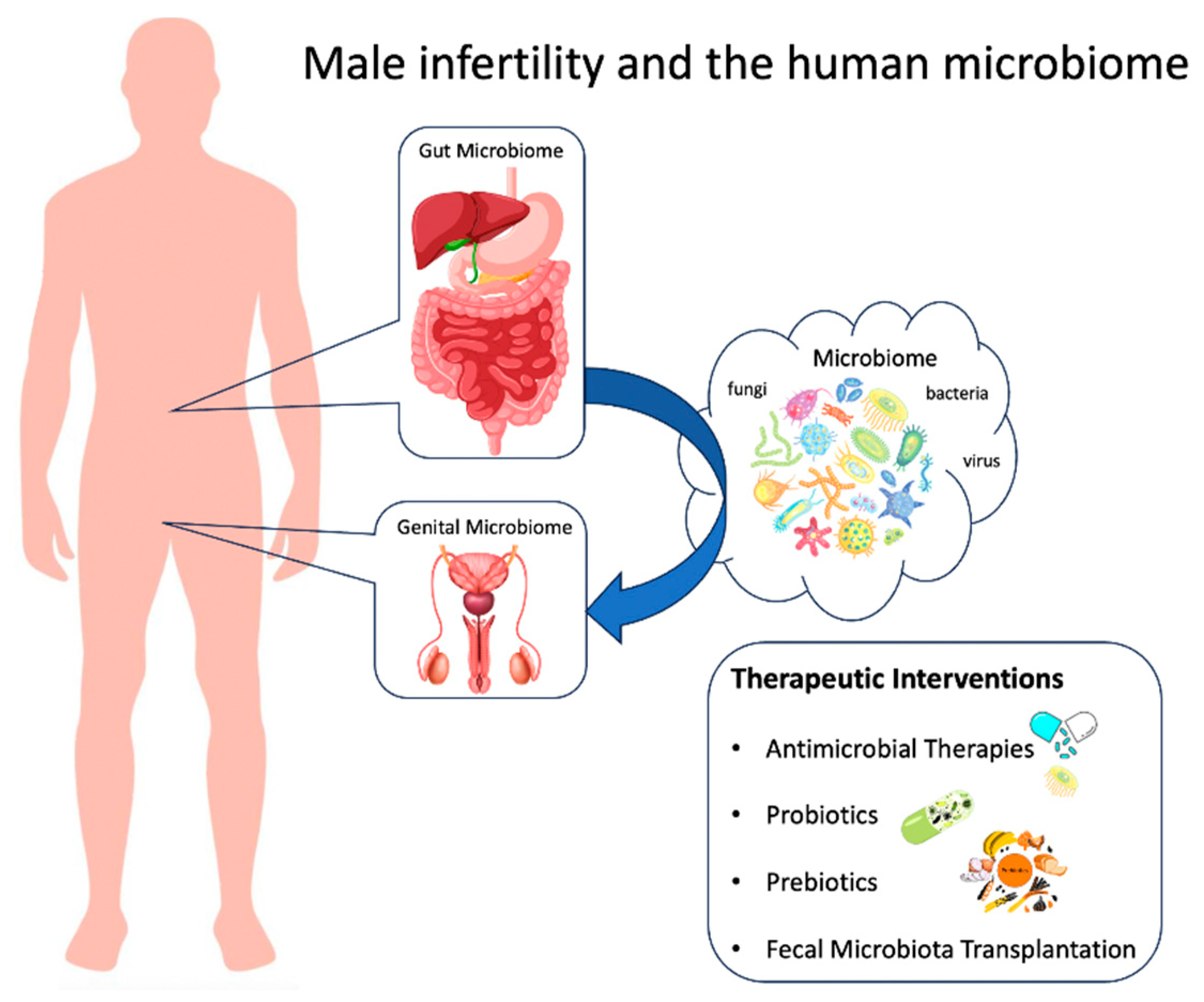
:1. Introduction
2. Microbiome and Male Reproductive Health
2.1. Introduction to the Human Microbiome and Its Significance
2.2. Mechanism of Action of the Microbiome Elements in Healthy Conditions
2.3. Microbiota in the Male Reproductive System
3. Microbial Dysbiosis and Male Infertility
3.1. Definition and Characterization of Microbial Dysbiosis
3.2. Evidence Linking Microbial Dysbiosis to Male Infertility
- 1.
- 2.
- Proliferation of pathogenic species: Dysbiosis can lead to an overgrowth of pathogenic species in the male genital tract. Bacteria like Escherichia coli and Ureaplasma urealyticum are associated with chronic prostatitis and inflammation [77]. These species can induce OS, inflammation, and damage to the male reproductive system, impacting sperm quality and fertility [69].
- 3.
- Genital microbiome disruptions: Changes in the composition and function of the male genital tract’s microbiota are linked to male infertility. Such disruptions can cause inflammation, oxidative stress, and sperm cell damage [69].
3.3. The Impact of Gut Microbial Dysbiosis on Male Infertility
3.4. Microbial Dysbiosis in the Reproductive System and Its Association with Male Infertility
3.5. Dysbiosis in Specific Regions of the Male Reproductive System
3.5.1. Rete Testis, Efferent Ducts, and Epididymis
3.5.2. Deferent Duct, Seminal Vesicles, and Prostate
3.5.3. Urethra and Coronal Sulcus
3.5.4. Semen
4. Mechanisms Linking Microbial Dysbiosis and Male Infertility
- Inflammation and immune response: A significant mechanism linking microbial dysbiosis to male infertility is the activation of inflammation and immune response. For instance, the oral microbiota, known for its dynamic and polymicrobial nature, can directly lead to diseases like dental caries and periodontitis [92]. These conditions manifest an inflammatory response triggered by the interaction between the microbiota and host factors, such as inflammation and dietary sugars [92]. In the context of male infertility, inflammation and immune response can negatively influence sperm function and overall fertility.
- 2.
- OS and its impact on sperm quality: OS, an imbalance between the ROS production and the body’s antioxidant defenses, is associated with male infertility [93]. While ROS are essential for reproduction, their overproduction can harm sperm DNA, impair sperm motility, and increase susceptibility to genetic anomalies [93]. OS can alter sperm morphology and reduce sperm concentration, affecting overall semen parameters [95]. Mechanisms through which OS affects sperm quality include lipid peroxidation, DNA damage, and compromised mitochondrial function [96].
- 3.
- Impaired sperm function and motility: Dysfunction in sperm movement and function are common in male infertility cases. The gut microbiota composition, influenced by factors like diet and the immune system, can affect sperm function and motility [97]. Dysbiosis in the gut microbiota, marked by reduced microbial diversity and the growth of specific bacterial taxa, has been linked to compromised sperm function and motility [97]. Factors such as oxidative stress, bacteriophage induction, and bacterial toxin release can initiate this dysbiosis [97].
5. Diagnostic Approaches for Assessing Microbial Dysbiosis in Male Infertility
5.1. Current Methods and Limitations
5.2. Advances in Molecular Techniques for Microbiota Analysis
5.2.1. Next-Generation Sequencing
5.2.2. Metatranscriptomics
5.2.3. Metaproteomics
5.2.4. Shotgun Metagenomics
5.2.5. Long-Read Sequencing
5.3. Biomarkers for Identifying Microbial Dysbiosis in Male Infertility
5.3.1. Seminal Oxidative Stress Markers
5.3.2. Seminal Inflammatory Markers
5.3.3. Microbial Biomarkers
5.3.4. Metabolomic Biomarkers
5.3.5. Epigenetic Biomarkers
6. Therapeutic Interventions for Modulating the Genital Microbiota
6.1. Targeted Antimicrobial Therapies
6.2. Probiotics and Their Potential Benefits
6.3. Prebiotics and Their Role in Restoring Microbial Balance
6.4. Fecal Microbiota Transplantation and Its Implications
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S.; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef]
- Dierickx, S.; De Proost, M.; Huang, A.Y.; Ceesay, S.; Clarke, E.; Balen, J. The Nairobi Summit and Reproductive Justice: Unmet Needs for People with Infertility. Am. J. Trop. Med. Hyg. 2021, 104, 812–813. [Google Scholar] [CrossRef]
- Mendiola, J.; Jørgensen, N.; Mínguez-Alarcón, L.; Sarabia-Cos, L.; López-Espín, J.J.; Vivero-Salmerón, G.; Ruiz-Ruiz, K.J.; Fernández, M.F.; Olea, N.; Swan, S.H.; et al. Sperm counts may have declined in young university students in Southern Spain. Andrology 2013, 1, 408–413. [Google Scholar] [CrossRef]
- Geoffroy-Siraudin, C.; Loundou, A.D.; Romain, F.; Achard, V.; Courbière, B.; Perrard, M.-H.; Durand, P.; Guichaoua, M.R. Decline of semen quality among 10 932 males consulting for couple infertility over a 20-year period in Marseille, France. Asian J. Androl. 2012, 14, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.I.; Martini, A.C.; Tissera, A.; Olmedo, J.; Senestrari, D.; De Cuneo, M.F.; Ruiz, R.D. Semen quality and aging: Analysis of 9.168 samples in Cordoba. Argentina. Arch. Esp. Urol. 2010, 63, 214–222. [Google Scholar]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Giwercman, A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum. Reprod. 2011, 26, 1012–1016. [Google Scholar] [CrossRef]
- Esteves, S.C.; Zini, A.; Aziz, N.; Alvarez, J.G.; Sabanegh, E.S., Jr.; Agarwal, A. Critical Appraisal of World Health Organization’s New Reference Values for Human Semen Characteristics and Effect on Diagnosis and Treatment of Subfertile Men. Urology 2012, 79, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Kayama, F.; Tatsuki, T.J.; Tsukamoto, T. Have sperm counts deteriorated over the past 20 years in healthy, young Japanese men? Results from the Sapporo area. J. Androl. 2001, 22, 40–44. [Google Scholar] [PubMed]
- Jensen, T.K.; Sobotka, T.; Hansen, M.A.; Pedersen, A.T.; Lutz, W.; Skakkebæk, N.E. Declining trends in conception rates in recent birth cohorts of native Danish women: A possible role of deteriorating male reproductive health. Int. J. Androl. 2008, 31, 81–92. [Google Scholar] [CrossRef]
- Carlsen, E.; Swan, S.H.; Petersen, J.H.; Skakkebæk, N.E. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum. Reprod. 2005, 20, 942–949. [Google Scholar] [CrossRef]
- Gyllenborg, J.; Skakkebæk, N.E.; Nielsen, N.C.; Keiding, N.; Giwercman, A. Secular and seasonal changes in semen quality among young Danish men: A statistical analysis of semen samples from 1927 donor candidates during 1977–1995. Int. J. Androl. 1999, 22, 28–36. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Mann, U.; Shiff, B.; Patel, P. Reasons for worldwide decline in male fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Ouabbou, S.; He, Y.; Butler, K.; Tsuang, M. Inflammation in Mental Disorders: Is the Microbiota the Missing Link? Neurosci. Bull. 2020, 36, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Wang, H.; Xu, A.; Gong, L.; Chen, Z.; Zhang, B.; Li, X. The Microbiome, an Important Factor That Is Easily Overlooked in Male Infertility. Front. Microbiol. 2022, 13, 831272. [Google Scholar] [CrossRef] [PubMed]
- Magill, R.G.; MacDonald, S.M. Male infertility and the human microbiome. Front. Reprod. Health 2023, 5, 1166201. [Google Scholar] [CrossRef]
- Potts, J.M.; Sharma, R.; Pasqualotto, F.; Nelson, D.; Hall, G.; Agarwal, A. Association of ureaplasma urealyticum with abnormal reactive oxygen species levels and absence of leukocytospermia. J. Urol. 2000, 163, 1775–1778. [Google Scholar] [CrossRef] [PubMed]
- Zeyad, A.; Hamad, M.; Amor, H.; Hammadeh, M.E. Relationships between bacteriospermia, DNA integrity, nuclear protamine alteration, sperm quality and ICSI outcome. Reprod. Biol. 2018, 18, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Merino, G.; Carranza-Lira, S.; Murrieta, S.; Rodriguez, L.; Cuevas, E.; Moran, C. Bacterial Infection and Semen Characteristics in Infertile Men. Arch. Androl. 1995, 35, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Sánchez, R.; Soto, L.; Risopatrón, J.; Villegas, J. Effect of Escherichia coli and its soluble factors on mitochondrial membrane potential, phosphatidylserine translocation, viability, and motility of human spermatozoa. Fertil. Steril. 2010, 94, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Berjis, K.; Ghiasi, M.; Sangy, S. Study of seminal infection among an infertile male population in Qom, Iran, and its effect on sperm quality. Iran. J. Microbiol. 2018, 10, 111–116. [Google Scholar]
- Ricci, S.; De Giorgi, S.; Lazzeri, E.; Luddi, A.; Rossi, S.; Piomboni, P.; De Leo, V.; Pozzi, G. Impact of asymptomatic genital tract infections on in vitro Fertilization (IVF) outcome. PLoS ONE 2018, 13, e0207684. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Khor, B.; Snow, M.; Herrman, E.; Ray, N.; Mansukhani, K.; Patel, K.A.; Said-Al-Naief, N.; Maier, T.R.; Machida, C.A. Interconnections between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance between Systemic Health and Disease. Microorganisms 2021, 9, 496. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z. Exploring the role of gut microbiome in male reproduction. Andrology 2022, 10, 441–450. [Google Scholar] [CrossRef]
- Velez, D.; Hwang, K. Personalized Medicine for the Infertile Male. Urol. Clin. N. Am. 2020, 47, 523–536. [Google Scholar] [CrossRef]
- Petrosino, J.F. The microbiome in precision medicine: The way forward. Genome Med. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Agharezaee, N.; Hashemi, M.; Shahani, M.; Gilany, K. Male Infertility, Precision Medicine and Systems Proteomics. J. Reprod. Infertil. 2018, 19, 185–192. [Google Scholar]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.J.; Sethi, S. COPD and the microbiome. Respirology 2016, 21, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2016, 595, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef]
- Khadka, V.D.; Key, F.M.; Romo-González, C.; Martínez-Gayosso, A.; Campos-Cabrera, B.L.; Gerónimo-Gallegos, A.; Lynn, T.C.; Durán-McKinster, C.; Coria-Jiménez, R.; Lieberman, T.D.; et al. The Skin Microbiome of Patients With Atopic Dermatitis Normalizes Gradually During Treatment. Front. Cell. Infect. Microbiol. 2021, 11, 720674. [Google Scholar] [CrossRef]
- Dickson, R.P.; Singer, B.H.; Newstead, M.W.; Falkowski, N.R.; Erb-Downward, J.R.; Standiford, T.J.; Huffnagle, G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016, 1, 16113. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Hurst, R.; Meader, E.; Gihawi, A.; Rallapalli, G.; Clark, J.; Kay, G.L.; Webb, M.; Manley, K.; Curley, H.; Walker, H.; et al. Microbiomes of Urine and the Prostate Are Linked to Human Prostate Cancer Risk Groups. Eur. Urol. Oncol. 2022, 5, 412–419. [Google Scholar] [CrossRef]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, So Is a Microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Hattori, M.; Taylor, T.D. The Human Intestinal Microbiome: A New Frontier of Human Biology. DNA Res. 2009, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Bishehsari, F. Microbes: Possible link between modern lifestyle transition and the rise of metabolic syndrome. Obes. Rev. 2018, 20, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Veneruso, I.; Cariati, F.; D’argenio, V. Microbiota and Human Reproduction: The Case of Female Infertility. BioTech 2020, 9, 12. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- García-Velasco, J.A.; Menabrito, M.; Catalán, I.B. What fertility specialists should know about the vaginal microbiome: A review. Reprod. Biomed. Online 2017, 35, 103–112. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, A.; Aguilera, M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients 2020, 12, 757. [Google Scholar] [CrossRef]
- Quaranta, G.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front. Immunol. 2019, 10, 2653. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Chia, N.; Nelson, H.; Segal, E.; Elinav, E. Microbiome at the Frontier of Personalized Medicine. Mayo Clin. Proc. 2017, 92, 1855–1864. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Broek, M.V.D.; Balzarini, J.; Vanderleyden, J.; Lebeer, S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol. Rev. 2013, 37, 762–792. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Veneruso, I.; Cariati, F.; D’argenio, V. Microbiota and Human Reproduction: The Case of Male Infertility. BioTech 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Zuber, A.; Peric, A.; Pluchino, N.; Baud, D.; Stojanov, M. Human Male Genital Tract Microbiota. Int. J. Mol. Sci. 2023, 24, 6939. [Google Scholar] [CrossRef]
- Tuddenham, S.; Ravel, J.; Marrazzo, J.M. Protection and Risk: Male and Female Genital Microbiota and Sexually Transmitted Infections. J. Infect. Dis. 2021, 223, S222–S235. [Google Scholar] [CrossRef]
- Motrich, R.D.; Salazar, F.C.; Breser, M.L.; Mackern-Oberti, J.P.; Godoy, G.J.; Olivera, C.; Paira, D.A.; Rivero, V.E. Implications of prostate inflammation on male fertility. Andrologia 2018, 50, e13093. [Google Scholar] [CrossRef]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; A Lozupone, C. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Bhatt, A.S. Microbes and microbiomes in 2020 and beyond. Nat. Commun. 2020, 11, 4988. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rana, M.; Qiu, E.; Albunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Suárez, J.P.; Maya, W.D.C. Microbiota, Prostatitis, and Fertility: Bacterial Diversity as a Possible Health Ally. Adv. Urol. 2021, 2021, 1007366. [Google Scholar] [CrossRef]
- Mändar, R.; Punab, M.; Korrovits, P.; Türk, S.; Ausmees, K.; Lapp, E.; Preem, J.-K.; Oopkaup, K.; Salumets, A.; Truu, J. Seminal microbiome in men with and without prostatitis. Int. J. Urol. 2017, 24, 211–216. [Google Scholar] [CrossRef]
- Fraczek, M.; Kurpisz, M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J. Androl. 2006, 28, 325–333. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. The human gut microbiota is neither an organ nor a commensal. FEBS Lett. 2020, 594, 3262–3271. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019, 13, 423–434. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: From fiction to function. Am. J. Physiol. Liver Physiol. 2019, 317, G602–G608. [Google Scholar] [CrossRef]
- Farahani, L.; Tharakan, T.; Yap, T.; Ramsay, J.W.; Jayasena, C.N.; Minhas, S. The semen microbiome and its impact on sperm function and male fertility: A systematic review and meta-analysis. Andrology 2021, 9, 115–144. [Google Scholar] [CrossRef]
- Huang, C.-P.; Zhu, H.; Xu, K.; Wang, S.; Fan, L.T.; Zhu, W. Mycoplasma and ureaplasma infection and male infertility: A systematic review and meta-analysis. Andrology 2015, 3, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, X.D.; Zhang, X.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, P.; Ge, W.; Feng, Y.; Li, L.; Sun, Z.; Zhang, H.; Shen, W. Alginate oligosaccharides improve germ cell development and testicular microenvironment to rescue busulfan disrupted spermatogenesis. Theranostics 2020, 10, 3308–3324. [Google Scholar] [CrossRef]
- Lundy, S.D.; Sangwan, N.; Parekh, N.V.; Selvam, M.K.P.; Gupta, S.; McCaffrey, P.; Bessoff, K.; Vala, A.; Agarwal, A.; Sabanegh, E.S.; et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur. Urol. 2021, 79, 826–836. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, Y.; Li, L.; Ge, W.; Yu, S.; Hao, Y.; Shen, W.; Han, X.; Ma, D.; Yin, S.; et al. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut 2021, 70, 222–225. [Google Scholar] [CrossRef]
- Verspoor, R.L.; Price, T.A.R.; Wedell, N. Selfish genetic elements and male fertility. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20200067. [Google Scholar] [CrossRef] [PubMed]
- D’argenio, V.; Dittfeld, L.; Lazzeri, P.; Tomaiuolo, R.; Tasciotti, E. Unraveling the Balance between Genes, Microbes, Lifestyle and the Environment to Improve Healthy Reproduction. Genes 2021, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Matthewman, C.; Narin, A.; Huston, H.; Hopkins, C.E. Systems to model the personalized aspects of microbiome health and gut dysbiosis. Mol. Asp. Med. 2023, 91, 101115. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Phukan, N.; Parsamand, T.; Brooks, A.E.S.; Nguyen, T.N.M.; Simoes-Barbosa, A. The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex. Transm. Infect. 2013, 89, 455–459. [Google Scholar] [CrossRef]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef]
- Allen-Vercoe, E. Bringing the gut microbiota into focus through microbial culture: Recent progress and future perspective. Curr. Opin. Microbiol. 2013, 16, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Ferrarese, R.; Locatelli, I.; Ventimiglia, E.; Ippolito, S.; Gallina, P.; Cesana, D.; Canducci, F.; Pagliardini, L.; Viganò, P.; et al. Testicular microbiome in azoospermic men—First evidence of the impact of an altered microenvironment. Hum. Reprod. 2018, 33, 1212–1217. [Google Scholar] [CrossRef]
- Weng, S.-L.; Chiu, C.-M.; Lin, F.-M.; Huang, W.-C.; Liang, C.; Yang, T.; Yang, T.-L.; Liu, C.-Y.; Wu, W.-Y.; Chang, Y.-A.; et al. Bacterial Communities in Semen from Men of Infertile Couples: Metagenomic Sequencing Reveals Relationships of Seminal Microbiota to Semen Quality. PLoS ONE 2014, 9, e110152. [Google Scholar] [CrossRef]
- Baud, D.; Pattaroni, C.; Vulliemoz, N.; Castella, V.; Marsland, B.J.; Stojanov, M. Sperm Microbiota and Its Impact on Semen Parameters. Front. Microbiol. 2019, 10, 234. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, M.; Baud, D.; Greub, G.; Vulliemoz, N. Male infertility: The intracellular bacterial hypothesis. New Microbes New Infect. 2018, 26, 37–41. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, H.; Jin, Z.; Hang, J.; Jiang, H.; Wu, H.; Zhang, Z. Characterization of Non-Obstructive Azoospermia in Men Using Gut Microbial Profiling. J. Clin. Med. 2023, 12, 701. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Schulte, R.T.; Ohl, D.A.; Sigman, M.; Smith, G.A. Sperm DNA damage in male infertility: Etiologies, assays, and outcomes. J. Assist. Reprod. Genet. 2009, 27, 3–12. [Google Scholar] [CrossRef]
- Ruiz, L.; García-Carral, C.; Rodriguez, J.M. Unfolding the Human Milk Microbiome Landscape in the Omics Era. Front. Microbiol. 2019, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Bui, A.D. Oxidation-reduction potential as a new marker for oxidative stress: Correlation to male infertility. Investig. Clin. Urol. 2017, 58, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Bajinka, O.; Tan, Y.; Abdelhalim, K.A.; Özdemir, G.; Qiu, X. Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express 2020, 10, 130. [Google Scholar] [CrossRef]
- Douglas, C.J.; Parekh, N.; Kahn, L.S.; Henkel, R.; Agarwal, A. A Novel Approach to Improving the Reliability of Manual Semen Analysis: A Paradigm Shift in the Workup of Infertile Men. World J. Men’s Health 2021, 39, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Selvam, M.K.P.; Baskaran, S. Proteomic Analyses of Human Sperm Cells: Understanding the Role of Proteins and Molecular Pathways Affecting Male Reproductive Health. Int. J. Mol. Sci. 2020, 21, 1621. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.L. Oxidation-reduction potential of semen: What is its role in the treatment of male infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Selvam, M.K.P.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Lanzillotti, C.; Mazziotta, C.; Tognon, M.; Martini, F. Epigenetics of Male Infertility: The Role of DNA Methylation. Front. Cell Dev. Biol. 2021, 9, 689624. [Google Scholar] [CrossRef]
- Behrouzi, A.; Nafari, A.H.; Siadat, S.D. The significance of microbiome in personalized medicine. Clin. Transl. Med. 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef] [PubMed]
- Farsimadan, M.; Motamedifar, M. Bacterial infection of the male reproductive system causing infertility. J. Reprod. Immunol. 2020, 142, 103183. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirsalehian, A.; Gilani, M.A.S.; Bahador, A.; Afraz, K. Association of asymptomatic Chlamydia trachomatis infection with male infertility and the effect of antibiotic therapy in improvement of semen quality in infected infertile men. Andrologia 2018, 50, e12944. [Google Scholar] [CrossRef] [PubMed]
- Bondaryk, M.; Kurzątkowski, W.; Staniszewska, M. Antifungal agents commonly used in the superficial and mucosal candidiasis treatment: Mode of action and resistance development. Adv. Dermatol. Allergol. 2013, 5, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, R.E.; Dutta, S.; Hamed, M.A.; Ajayi, A.F.; Sengupta, P.; Ahmad, G. Viral Infections and Male Infertility: A Comprehensive Review of the Role of Oxidative Stress. Front. Reprod. Health 2022, 4, 782915. [Google Scholar] [CrossRef] [PubMed]
- Drobnis, E.Z.; Nangia, A.K. Antivirals and Male Reproduction. Adv. Exp. Med. Biol. 2017, 1034, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Condorelli, R.A.; Russo, G.I.; La Vignera, S. Conservative Nonhormonal Options for the Treatment of Male Infertility: Antibiotics, Anti-Inflammatory Drugs, and Antioxidants. BioMed Res. Int. 2017, 2017, 4650182. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Palmisano, M.; Chironna, M.; Vacca, M.; Masciandaro, P.; Bassi, E.; Luigi, L.S.; Depalo, R. Impact of chronic viral diseases on semen parameters. Andrologia 2010, 42, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.; Donnerer, J.; Sator, K.; Staufer, K.; Scherzer, T.-M.; Dejaco, C.; Sator, M.; Kessler, H.; Ferenci, P. Seminal fluid ribavirin level and functional semen parameters in patients with chronic hepatitis C on antiviral combination therapy. J. Hepatol. 2010, 52, 812–816. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Ahmed, M.M.; Anjum, F.; Anwar, H.; Naqvi, S.A.R.; Zahra, T.; Batool, U. Post interferon therapy decreases male fertility through gonadotoxic effect. Pak. J. Pharm. Sci. 2018, 31, 1565–1570. [Google Scholar] [PubMed]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.; Mahasneh, A. Probiotics and the envisaged role in treating human infertility. Middle East Fertil. Soc. J. 2020, 25, 33. [Google Scholar] [CrossRef]
- Unno, T.; Choi, J.-H.; Hur, H.-G.; Sadowsky, M.J.; Ahn, Y.-T.; Huh, C.-S.; Kim, G.-B.; Cha, C.-J. Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J. Dairy Sci. 2015, 98, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444. [Google Scholar] [CrossRef]
- Mazanko, M.S.; Gorlov, I.F.; Prazdnova, E.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Chistyakov, V.A.; Tutelyan, A.V.; Komarova, Z.B.; Mosolova, N.I.; et al. Bacillus Probiotic Supplementations Improve Laying Performance, Egg Quality, Hatching of Laying Hens, and Sperm Quality of Roosters. Probiotics Antimicrob. Proteins 2018, 10, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Riesco, M.F.; Martínez-Vázquez, J.M.; Robles, V. Diet Supplemented with Antioxidant and Anti-Inflammatory Probiotics Improves Sperm Quality after Only One Spermatogenic Cycle in Zebrafish Model. Nutrients 2019, 11, 843. [Google Scholar] [CrossRef]
- Keshtmand, Z.; Akbaribazm, M.; Bagheri, Y.; Oliaei, R. The ameliorative effects of Lactobacillus coagulans and Lactobacillus casei probiotics on CCl4-induced testicular toxicity based on biochemical, histological and molecular analyses in rat. Andrologia 2021, 53, e13908. [Google Scholar] [CrossRef]
- Helli, B.; Kavianpour, M.; Ghaedi, E.; Dadfar, M.; Haghighian, H.K. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Hum. Fertil. 2022, 25, 499–507. [Google Scholar] [CrossRef]
- Barbonetti, A.; Cinque, B.; Vassallo, M.R.C.; Mineo, S.; Francavilla, S.; Cifone, M.G.; Francavilla, F. Effect of vaginal probiotic lactobacilli on in vitro–induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil. Steril. 2011, 95, 2485–2488. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Fic, M.; van de Wetering, T.; Folwarski, M.; Makarewicz, W. Therapeutic methods of gut microbiota modification in colorectal cancer management–fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 2020, 11, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Molina, N.M.; Sola-Leyva, A.; Saez-Lara, M.J.; Plaza-Diaz, J.; Tubić-Pavlović, A.; Romero, B.; Clavero, A.; Mozas-Moreno, J.; Fontes, J.; Altmäe, S. New Opportunities for Endometrial Health by Modifying Uterine Microbial Composition: Present or Future? Biomolecules 2020, 10, 593. [Google Scholar] [CrossRef]
- Fooks, L.J.; Gibson, G.R. Probiotics as modulators of the gut flora. Br. J. Nutr. 2002, 88, s39–s49. [Google Scholar] [CrossRef] [PubMed]
- van Nood, E.; Speelman, P.; Nieuwdorp, M.; Keller, J.J. Fecal microbiota transplantation. Curr. Opin. Gastroenterol. 2014, 30, 34–39. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Sklar, J.; Jiang, L.; Natarajan, L.; Knight, R.; Belkaid, Y. Host variables confound gut microbiota studies of human disease. Nature 2020, 587, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Bordalo Tonucci, L.; Dos Santos, K.M.; De Luces Fortes Ferreira, C.L.; Ribeiro, S.M.; De Oliveira, L.L.; Martino, H.S. Gut microbiota and probiotics: Focus on diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2017, 57, 2296–2309. [Google Scholar] [CrossRef]
- Xavier, M.J.; Salas-Huetos, A.; Oud, M.S.; Aston, K.I.; Veltman, J.A. Disease gene discovery in male infertility: Past, present and future. Hum. Genet. 2020, 140, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Bertolotto, M.; Maggi, M. Historical trends for the standards in scrotal ultrasonography: What was, what is and what will be normal. Andrology 2021, 9, 1331–1355. [Google Scholar] [CrossRef]
- Egle, U.T.; Egloff, N.; von Känel, R. Stressinduzierte Hyperalgesie (SIH) als Folge von emotionaler Deprivation und psychischer Traumatisierung in der Kindheit. Schmerz 2016, 30, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Tijani, H.A.; Bhattacharya, S. The role of intrauterine insemination in male infertility. Hum. Fertil. 2010, 13, 226–232. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y. Apoplastic Proteases: Powerful Weapons against Pathogen Infection in Plants. Plant Commun. 2020, 1, 100085. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Sun, X.; Yan, Y.; Yuan, Q.; Wang, J.; Shen, X. Metabolic Engineering of Microorganisms for the Production of Flavonoids. Front. Bioeng. Biotechnol. 2020, 8, 589069. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Zachariou, A.; Markou, E.; Dimitriadis, F.; Sofikitis, N.; Pournaras, S. Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions. J. Pers. Med. 2023, 13, 1491. https://doi.org/10.3390/jpm13101491
Kaltsas A, Zachariou A, Markou E, Dimitriadis F, Sofikitis N, Pournaras S. Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions. Journal of Personalized Medicine. 2023; 13(10):1491. https://doi.org/10.3390/jpm13101491
Chicago/Turabian StyleKaltsas, Aris, Athanasios Zachariou, Eleftheria Markou, Fotios Dimitriadis, Nikolaos Sofikitis, and Spyridon Pournaras. 2023. "Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions" Journal of Personalized Medicine 13, no. 10: 1491. https://doi.org/10.3390/jpm13101491
APA StyleKaltsas, A., Zachariou, A., Markou, E., Dimitriadis, F., Sofikitis, N., & Pournaras, S. (2023). Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions. Journal of Personalized Medicine, 13(10), 1491. https://doi.org/10.3390/jpm13101491






