The Impact of Metabolic Factors and Lipid-Lowering Drugs on Common Bile Duct Stone Recurrence after Endoscopic Sphincterotomy with Following Cholecystectomy
Abstract
1. Introduction
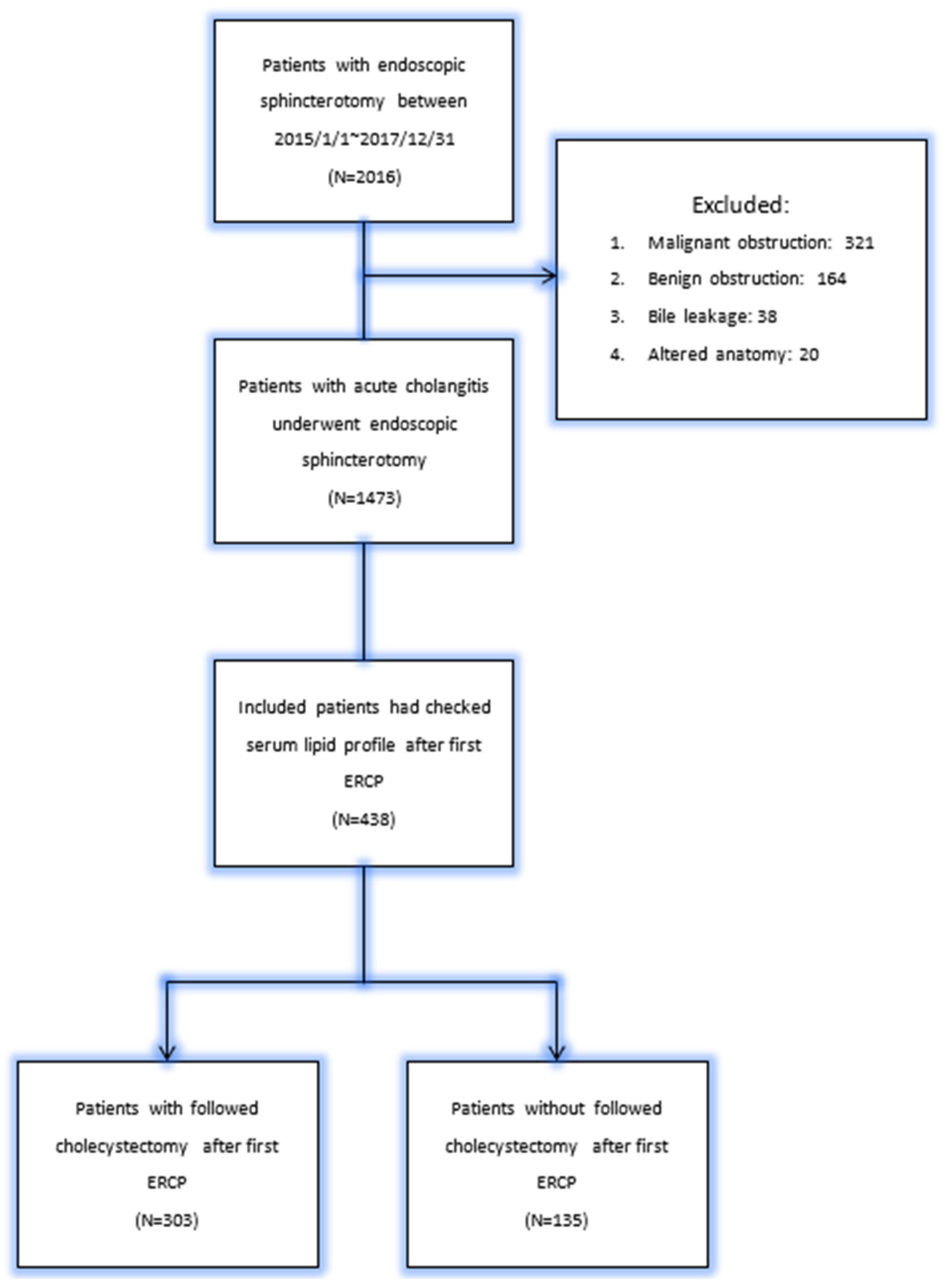
2. Material and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sultan, S.; Baillie, J. Recurrent bile duct stones after endoscopic sphincterotomy. Gut 2004, 53, 1725–1727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, M.E.; Chung, M.J.; Lee, D.-J.; Oh, T.G.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y.; Chung, J.B. Cholecystectomy for prevention of recurrence after endoscopic clearance of bile duct stones in Korea. Yonsei Med. J. 2016, 57, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Prat, F.; Malak, N.A.; Pelletier, G.; Buffet, C.; Fritsch, J.; Choury, A.D.; Altman, C.; Liguory, C.; Etienne, J.P. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology 1996, 110, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.S.; Yoo, B.M.; Kim, J.H.; Hwang, J.C.; Yang, M.J.; Lee, K.M.; Kim, S.S.; Noh, C.K. Evaluation of risk factors for recurrent primary common bile duct stone in patients with cholecystectomy. Scand. J. Gastroenterol. 2018, 53, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Kawabe, T.; Komatsu, Y.; Yoshida, H.; Isayama, H.; Sasaki, T.; Kogure, H.; Togawa, O.; Arizumi, T.; Matsubara, S.; et al. Endoscopic papillary balloon dilation for bile duct stone: Immediate and long-term outcomes in 1000 patients. Clin. Gastroenterol. Hepatol. 2007, 5, 130–137. [Google Scholar] [CrossRef]
- Sbeit, W.; Kadah, A.; Simaan, M.; Shahin, A.; Khoury, T. Predictors of recurrent bile duct stone after clearance by endoscopic retrograde cholangiopancreatography: A case-control study. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhou, M.; Liu, P.-P.; Hong, J.-B.; Li, G.-H.; Zhou, X.-J.; Chen, Y.-X. Causes associated with recurrent choledocholithiasis following therapeutic endoscopic retrograde cholangiopancreatography: A large sample sized retrospective study. World J. Clin. Cases 2019, 7, 1028. [Google Scholar] [CrossRef]
- Mazer, N.A.; Carey, M.C. Quasi-elastic light-scattering studies of aqueous biliary lipid systems. Cholesterol solubilization and precipitation in model bile solutions. Biochemistry 1983, 22, 426–442. [Google Scholar] [CrossRef]
- Crawford, J.M.; Möckel, G.; Crawford, A.; Hagen, S.; Hatch, V.; Barnes, S.; Godleski, J.; Carey, M. Imaging biliary lipid secretion in the rat: Ultrastructural evidence for vesiculation of the hepatocyte canalicular membrane. J. Lipid Res. 1996, 36, 2147–2163. [Google Scholar] [CrossRef]
- Angelico, F.; Del Ben, M.; Barbato, A.; Conti, R.; Urbinati, G. Ten-year incidence and natural history of gallstone disease in a rural population of women in central Italy. The Rome Group for the Epidemiology and Prevention of Cholelithiasis (GREPCO). Ital. J. Gastroenterol. Hepatol. 1997, 29, 249–254. [Google Scholar]
- Ostrow, J.D. The etiology of pigment gallstones. Hepatology 1984, 4, 215S–222S. [Google Scholar] [CrossRef] [PubMed]
- Maki, T. Pathogenesis of calcium bilirubinate gallstone: Role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann. Surg. 1966, 164, 90. [Google Scholar] [CrossRef] [PubMed]
- Cetta, F.M. Bile infection documented as initial event in the pathogenesis of brown pigment biliary stones. Hepatology 1986, 6, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Portincasa, P. An update on the pathogenesis of cholesterol gallstone disease. Curr. Opin. Gastroenterol. 2018, 34, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.A.; Wittenburg, H. Cholesterol gallstone susceptibility loci: A mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology 2006, 131, 1943–1970. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Cohen, D.E.; Carey, M.C. Biliary lipids and cholesterol gallstone disease. J. Lipid Res. 2009, 50, S406–S411. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Carey, M.C. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: Influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J. Lipid Res. 1996, 37, 606–630. [Google Scholar] [PubMed]
- Shabanzadeh, D.M.; Skaaby, T.; Sørensen, L.T.; Eugen-Olsen, J.; Jørgensen, T. Metabolic biomarkers and gallstone disease–a population-based study. Scand. J. Gastroenterol. 2017, 52, 1270–1277. [Google Scholar] [CrossRef]
- Soloway, R.D.; Trotman, B.W.; Ostrow, J.D. Pigment gallstones. Gastroenterology 1977, 72, 167–182. [Google Scholar] [CrossRef]
- Bouchier, I.A. The formation of gallstones. Keio J. Med. 1992, 41, 1–5. [Google Scholar] [CrossRef]
- Hayat, S.; Hassan, Z.; Changazi, S.H.; Zahra, A.; Noman, M.; Zain Ul Abdin, M.; Javed, H.; Ans, A.H. Comparative analysis of serum lipid profiles in patients with and without gallstones: A prospective cross-sectional study. Ann. Med. Surg. 2019, 42, 11–13. [Google Scholar] [CrossRef]
- Batajoo, H.; Hazra, N.K. Analysis of serum lipid profile in cholelithiasis patients. J. Nepal Health Res. Counc. 2013, 11, 53–55. [Google Scholar] [PubMed]
- Saraya, A.; Irshad, M.; Gandhi, B.M.; Tandon, R.K. Plasma lipid profile in gallstone patients from North India. Trop. Gastroenterol. 1995, 16, 16–21. [Google Scholar] [PubMed]
- Wang, J.; Shen, S.; Wang, B.; Ni, X.; Liu, H.; Ni, X.; Yu, R.; Suo, T.; Liu, H. Serum lipid levels are the risk factors of gallbladder stones: A population-based study in China. Lipids Health Dis. 2020, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Beckingham, I.; El Sayed, G.; Gurusamy, K.; Sturgess, R.; Webster, G.; Young, T. Updated guideline on the management of common bile duct stones (CBDS). Gut 2017, 66, 765–782. [Google Scholar] [CrossRef]
- Zhao, H.C.; He, L.; Zhou, D.C.; Geng, X.P.; Pan, F.M. Meta-analysis comparison of endoscopic papillary balloon dilatation and endoscopic sphincteropapillotomy. World J. Gastroenterol. 2013, 19, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, I.; Fujita, N.; Maguchi, H.; Hasebe, O.; Igarashi, Y.; Murakami, A.; Mukai, H.; Fujii, T.; Yamao, K.; Maeshiro, K.; et al. Long-term outcomes after endoscopic sphincterotomy versus endoscopic papillary balloon dilation for bile duct stones. Gastrointest. Endosc. 2010, 72, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Wu, H.; Huang, Q.; Zeng, A.; Yu, Z.; Zhong, Z. High Levels of Serum Triglyceride, Low-density Lipoprotein Cholesterol, Total Bile Acid, and Total Bilirubin are Risk Factors for Gallstones. Clin. Lab. 2021, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Banim, P.J.; Luben, R.N.; Bulluck, H.; Sharp, S.J.; Wareham, N.J.; Khaw, K.T.; Hart, A.R. The aetiology of symptomatic gallstones quantification of the effects of obesity, alcohol and serum lipids on risk. Epidemiological and biomarker data from a UK prospective cohort study (EPIC-Norfolk). Eur. J. Gastroenterol. Hepatol. 2011, 23, 733–740. [Google Scholar] [CrossRef]
- Janowitz, P.; Wechsler, J.; Kuhn, K.; Kratzer, W.; Tudyka, J.; Swobodnik, W.; Ditschuneit, H. The relationship between serum lipids, nucleation time, and biliary lipids in patients with gallstones. Clin. Investig. 1992, 70, 430–436. [Google Scholar] [CrossRef]
- Thornton, J.; Heaton, K.; Macfarlane, D. A relation between high-density-lipoprotein cholesterol and bile cholesterol saturation. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.J.; Fasulo, J.M. High density lipoproteins, but not other lipoproteins, provide a vehicle for sterol transport to bile. J. Clin. Investig. 1997, 99, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.C.; Halloran, L.G.; Vlahcevic, Z.R.; Gregory, D.H.; Swell, L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science 1978, 200, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.J.; Fasulo, J.M. Delineation of a novel hepatic route for the selective transfer of unesterified sterols from high-density lipoproteins to bile: Studies using the perfused rat liver. Hepatology 1999, 29, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mao, M.; Zhang, C.; Hu, F.; Cui, P.; Li, G.; Shi, J.; Wang, X.; Shan, X. Blood lipid metabolism and the risk of gallstone disease: A multi-center study and meta-analysis. Lipids Health Dis. 2022, 21, 26. [Google Scholar] [CrossRef]
- Holzbach, R.T.; Marsh, M.; Olszewski, M.; Holan, K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J. Clin. Investig. 1973, 52, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Chiu, T.H.T.; Chang, C.C.; Lin, M.N.; Lin, C.L. Plant-Based Diet, Cholesterol, and Risk of Gallstone Disease: A Prospective Study. Nutrients 2019, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Gong, K.; Shao, X. The relationship between serum lipids, apolipoproteins level and bile lipids level, chemical type of stone. Chin. Med. J. 1995, 75, 656–659, 708. [Google Scholar]
- Atamanalp, S.S.; Keles, M.S.; Atamanalp, R.S.; Acemoglu, H.; Laloglu, E. The effects of serum cholesterol, LDL, and HDL levels on gallstone cholesterol concentration. Pak. J. Med. Sci. 2013, 29, 187. [Google Scholar] [CrossRef]
- Rao, P.J.; Jarari, A.; El-Awami, H.; Patil, T.; El-Saiety, S. Lipid profile in bile and serum of cholelithiasis patients-A comparative study. J. Basic Med. Allied Sci. 2012, 1, 27–39. [Google Scholar]
- Krawczyk, M.; Gruenhage, F.; Mahler, M.; Tirziu, S.; Acalovschi, M.; Lammert, F. The common adiponutrin variant p.I148M does not confer gallstone risk but affects fasting glucose and triglyceride levels. J. Physiol. Pharmacol. 2011, 62, 369–375. [Google Scholar] [PubMed]
- Weerakoon, H.T.; Ranasinghe, S.; Navaratne, A.; Sivakanesan, R.; Galketiya, K.B.; Rosairo, S. Serum lipid concentrations in patients with cholesterol and pigment gallstones. BMC Res. Notes 2014, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, R.; Mohan, H.; Attri, A.K.; Kaur, J.; Punia, R.P. A comparative study of serum lipid profile and gallstone disease. Indian J. Pathol. Microbiol. 2007, 50, 308–312. [Google Scholar] [PubMed]
- Moazeni-Bistgani, M.; Kheiri, S.; Ghorbanpour, K. The effects of cholecystectomy on serum lipids during one year follow-up. Research 2014, 1, 1094. [Google Scholar] [CrossRef]
- Jonkers, I.J.; Smelt, A.H.; Ledeboer, M.; Hollum, M.E.; Biemond, I.; Kuipers, F.; Stellaard, F.; Boverhof, R.; Meinders, A.E.; Lamers, C.H.; et al. Gall bladder dysmotility: A risk factor for gall stone formation in hypertriglyceridaemia and reversal on triglyceride lowering therapy by bezafibrate and fish oil. Gut 2003, 52, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.S.; Magnuson, T.H.; Lillemoe, K.D.; Frasca, P.; Pitt, H.A. The role of bacteria in gallbladder and common duct stone formation. Ann. Surg. 1989, 209, 584. [Google Scholar] [CrossRef] [PubMed]
- Tazuma, S. Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract. Res. Clin. Gastroenterol. 2006, 20, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Lombardi, R.; Milone, M.R.; Pucci, B.; De Rienzo, S.; Budillon, A.; Bruzzese, F. Targeting mevalonate pathway in cancer treatment: Repurposing of statins. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 184–200. [Google Scholar] [CrossRef]
- Swarne, E.; Srikanth, M.; Shreyas, A.; Desai, S.; Mehdi, S.; Gangadharappa, H.; Krishna, K. Recent advances, novel targets and treatments for cholelithiasis; a narrative review. Eur. J. Pharmacol. 2021, 908, 174376. [Google Scholar]
- Kan, H.P.; Guo, W.B.; Tan, Y.F.; Zhou, J.; Liu, C.D.; Huang, Y.Q. Statin use and risk of gallstone disease: A meta-analysis. Hepatol. Res. 2015, 45, 942–948. [Google Scholar] [CrossRef]
- Kwon, M.J.; Lee, J.W.; Kang, H.S.; Lim, H.; Kim, E.S.; Kim, N.Y.; Choi, H.G.; Kim, M.J. Association between Gallstone Disease and Statin Use: A Nested Case-Control Study in Korea. Pharmaceuticals 2023, 16, 536. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Brauchli, Y.B.; KrńhenbŘhl, S.; Jick, S.S.; Meier, C.R. Statin use and risk of gallstone disease followed by cholecystectomy. JAMA 2009, 302, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Haal, S.; Guman, M.S.; Bruin, S.; Schouten, R.; van Veen, R.N.; Fockens, P.; Dijkgraaf, M.G.; Hutten, B.A.; Gerdes, V.E.; Voermans, R.P. Risk factors for symptomatic gallstone disease and gallstone formation after bariatric surgery. Obes. Surg. 2022, 32, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Caroli-Bosc, F.; Le Gall, P.; Pugliese, P.; Delabre, B.; Caroli-Bosc, C.; Demarquay, J.; Delmont, J.; Rampal, P.; Montet, J. General Practitioners’ Group of Vidauban. Role of fibrates and HMG-CoA reductase inhibitors in gallstone formation: Epidemiological study in an unselected population. Dig. Dis. Sci. 2001, 46, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; de Bari, O.; Liu, K.J.; Garruti, G.; Neuschwander-Tetri, B.A.; Wang, D.Q.H. Prevention of cholesterol gallstones by inhibiting hepatic biosynthesis and intestinal absorption of cholesterol. Eur. J. Clin. Investig. 2013, 43, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Mo, P.; Chen, H.; Jiang, X.; Hu, F.; Zhang, F.; Shan, G.; Chen, W.; Li, S.; Xu, G. Effect of hepatic NPC1L1 on cholesterol gallstone disease and its mechanism. Heliyon 2023, 9, e15757. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; Mendez-Sanchez, N.; Uribe, M.; Wang, D.Q. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 2008, 134, 2101–2110. [Google Scholar] [CrossRef]
- de Bari, O.; Neuschwander-Tetri, B.A.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Ezetimibe: Its novel effects on the prevention and the treatment of cholesterol gallstones and nonalcoholic fatty liver disease. J. Lipids 2012, 2012, 302847. [Google Scholar] [CrossRef]
- Ahmed, O.; Littmann, K.; Gustafsson, U.; Pramfalk, C.; Öörni, K.; Larsson, L.; Minniti, M.E.; Sahlin, S.; Camejo, G.; Parini, P.; et al. Ezetimibe in Combination With Simvastatin Reduces Remnant Cholesterol Without Affecting Biliary Lipid Concentrations in Gallstone Patients. J. Am. Heart Assoc. 2018, 7, e009876. [Google Scholar] [CrossRef]
| Overall (N = 303) | with Recurrence (N = 61) | without Recurrence (N = 242) | p Value | |
|---|---|---|---|---|
| Age (years) | 65 (55–74) | 68 (60–78) | 63 (54–73) | 0.031 * |
| Gender | 0.607 | |||
| Male | 180 (59.4%) | 38 (62.3%) | 142 (58.7%) | |
| Female | 123 (40.6%) | 23 (37.7%) | 100 (41.3%) | |
| BW (kg) | 67 (58–75) | 64 (59–70) | 68 (57–75) | 0.138 |
| BMI (kg/m2) | 25.0 (23.0–28.0) | 25.0 (23.1–27.3) | 25.0 (23.0–28.0) | 0.894 |
| Underlying disease | ||||
| HTN | 168 (55.4%) | 38 (62.3%) | 130 (53.7%) | 0.228 |
| DM | 104 (34.4%) | 23 (37.7%) | 81 (33.5%) | 0.534 |
| CAD | 27 (8.9%) | 4 (6.6%) | 23 (9.5%) | 0.470 |
| Stroke | 14 (4.6%) | 4 (6.6%) | 10 (4.1%) | 0.420 |
| Fatty liver | 0.692 | |||
| No | 124 (40.9%) | 23 (37.7%) | 101 (41.7%) | |
| Mild | 93 (30.7%) | 24 (39.3%) | 69 (28.5%) | |
| Moderate | 79 (26.1%) | 14 (23.0%) | 65 (26.9%) | |
| Severe | 7 (2.3%) | 0 (0.0%) | 7 (2.9%) | |
| HbA1C (%) | 6.1 (5.7–6.5) | 6.4 (5.9–6.7) | 6.0 (5.7–6.5) | 0.013 * |
| Liver function test upon diagnosis of cholangitis | ||||
| AST (U/L) | 101 (41–249) | 62 (31–189) | 107.5 (46.0–274.0) | 0.002 * |
| ALT (U/L) | 139 (46–264) | 85 (38–188) | 155 (67–292) | 0.005 * |
| Total bilirubin (mg/dL) | 2.0 (0.9–3.6) | 1.6 (0.7–2.6) | 2.0 (0.9–4.0) | 0.021 * |
| r-GT (U/L) | 212 (107–304) | 181 (105–228) | 255 (108–308) | 0.011 |
| ALK-p (U/L) | 144 (98–220) | 128 (90–195) | 146 (101–229) | 0.103 |
| Liver function test upon follow-up | ||||
| AST (U/L) | 25 (18–30) | 25 (20–32) | 24 (18–30) | 0.095 |
| ALT (U/L) | 23 (15–31) | 24 (17–34) | 23 (15–31) | 0.298 |
| Follow-up time (months) | 66.0 (51.1–82.0) | 79.9 (58.6–91.6) | 65.0 (50.1–81.3) | 0.007 * |
| Overall (N = 303) | with Recurrence (N = 61) | without Recurrence (N = 242) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||||
| BW (kg) | 67 (58–75) | 64 (59–70) | 68 (57–75) | 0.166 | |||
| BMI (kg/m2) | 25.0 (23.0–28.0) | 25.0 (23.1–27.3) | 25.0 (23.0–28.0) | 0.087 | |||
| Fatty liver | 0.692 | ||||||
| No | 124 (40.9%) | 23 (37.7%) | 101 (41.7%) | ||||
| Mild | 93 (30.7%) | 24 (39.3%) | 69 (28.5%) | ||||
| Moderate | 79 (26.1%) | 14 (23.0%) | 65 (26.9%) | ||||
| Severe | 7 (2.3%) | 0 (0.0%) | 7 (2.9%) | ||||
| Lipid profile | |||||||
| TG (mg/dL) | 117 (81–165) | 161 (114–222) | 108 (79–149) | 1.005 (1.002–1.008) | 0.004 * | 0.574 | |
| TG ≥ 150 | 49 (16.2%) | 20 (32.8%) | 29 (12.0%) | 3.583 (1.851–6.936) | 0.000 * | 0.084 | |
| TG < 150 | 254 (83.8%) | 41 (67.2%) | 213 (88.0%) | ||||
| LDL (mg/dL) | 97 (76–125) | 107 (81–139) | 92 (74–120) | 1.011 (1.003–1.019) | 0.004 * | 0.756 | |
| LDL ≥ 130 | 70 (23.1%) | 22 (36.1%) | 48 (19.8%) | 2.28 (1.238–4.199) | 0.008 * | 0.874 | |
| LDL < 130 | 233 (76.9%) | 39 (63.9%) | 194 (80.2%) | ||||
| HDL (mg/dL) | 45 (39–53) | 39 (32–49) | 46 (40–54) | 0.95 (0.926–0.975) | 0.000 * | 0.936 (0.908–0.966) | 0.000 * |
| HDL ≥ 40 | 226 (74.6%) | 29 (47.5%) | 197 (81.4%) | 0.207 (0.114–0.376) | 0.000 * | 0.199 (0.102–0.388) | 0.000 * |
| HDL < 40 | 77 (25.4%) | 32 (52.5%) | 45 (18.6%) | ||||
| Cholesterol (mg/dL) | 173.0 ± 42.1 | 186.9 ± 58.4 | 169.5 ± 36.2 | 1.010 (1.003–1.017) | 0.005 * | 0.146 | |
| Cholesterol ≥ 200 | 68 (22.4%) | 27 (44.3%) | 41 (16.9%) | 3.893 (2.122–7.141) | 0.000 * | 4.558 (1.625–12.787) | 0.004 * |
| Cholesterol < 200 | 235 (77.6%) | 34 (55.7%) | 201 (83.1%) | ||||
| HbA1C (%) | 6.1 (5.7–6.5) | 6.4 (5.9–6.7) | 6.0 (5.7–6.5) | 0.120 | 0.454 | ||
| HbA1C ≥ 6.5 | 91 (30%) | 25 (41.0%) | 66 (27.3%) | 1.852 (1.033–3.319) | 0.038 * | 0.695 | |
| HbA1C < 6.5 | 212 (70%) | 36 (59.0%) | 176 (72.7%) |
| Overall (N = 303) | with Recurrence (N = 61) | without Recurrence (N = 242) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||||
| Statin | 104 (34.3%) | 12 (19.7%) | 92 (38.0%) | 0.399 (0.202–0.790) | 0.008 * | 0.297 (0.132–0.665) | 0.003 * |
| Fenofibrate | 9 (3.0%) | 2 (3.3%) | 7 (2.9%) | 0.874 | 0.810 | ||
| Ezetimibe | 16 (5.3%) | 6 (9.8%) | 10 (4.1%) | 0.084 | 5.618 (1.659–19.025) | 0.006 * | |
| Ursodeoxycholic acid | 5 (1.7%) | 3 (4.9%) | 2 (0.8%) | 6.207 (1.014–38.004) | 0.048 * | 8.050 (1.188–54.573) | 0.033 * |
| Aspirin | 53 (17.5%) | 7 (11.5%) | 46 (19.0%) | 0.171 | 0.603 |
| Overall (N = 61) | HDL ≥ 40 (N = 29) | HDL < 40 (N = 32) | p-Value | |
|---|---|---|---|---|
| From first ERCP to recurrence (months) | 38.9 ± 36.0 | 39.3 ± 36.0 | 38.5 ± 36.6 | 0.935 |
| From cholecystectomy to recurrence (months) | 36.5 ± 47.9 | 37.7 ± 55.9 | 35.4 ± 40.2 | 0.856 |
| Overall (N = 61) | Cholesterol ≥ 200 (N = 27) | Cholesterol < 200 (N = 34) | p-Value | |
|---|---|---|---|---|
| From first ERCP to recurrence (months) | 38.9 ± 36.0 | 36.3 ± 36.2 | 40.9 ± 36.3 | 0.622 |
| From cholecystectomy to recurrence (months) | 36.5 ± 47.9 | 34.8 ± 51.6 | 37.9 ± 45.5 | 0.803 |
| Overall (N = 61) | with Statin (N = 12) | without Statin (N = 49) | p-Value | |
|---|---|---|---|---|
| From first ERCP to recurrence (months) | 38.9 ± 36.0 | 38.6 ± 32.9 | 39.0 ± 37.0 | 0.978 |
| From cholecystectomy to recurrence (months) | 36.5 ± 47.9 | 25.8 ± 39.4 | 39.1 ± 49.8 | 0.390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-F.; Wu, C.-H.; Sung, K.-F.; Tsou, Y.-K.; Lin, C.-H.; Lee, C.-W.; Lee, M.-H.; Liu, N.-J. The Impact of Metabolic Factors and Lipid-Lowering Drugs on Common Bile Duct Stone Recurrence after Endoscopic Sphincterotomy with Following Cholecystectomy. J. Pers. Med. 2023, 13, 1490. https://doi.org/10.3390/jpm13101490
Wang S-F, Wu C-H, Sung K-F, Tsou Y-K, Lin C-H, Lee C-W, Lee M-H, Liu N-J. The Impact of Metabolic Factors and Lipid-Lowering Drugs on Common Bile Duct Stone Recurrence after Endoscopic Sphincterotomy with Following Cholecystectomy. Journal of Personalized Medicine. 2023; 13(10):1490. https://doi.org/10.3390/jpm13101490
Chicago/Turabian StyleWang, Sheng-Fu, Chi-Huan Wu, Kai-Feng Sung, Yung-Kuan Tsou, Cheng-Hui Lin, Chao-Wei Lee, Mu-Hsien Lee, and Nai-Jen Liu. 2023. "The Impact of Metabolic Factors and Lipid-Lowering Drugs on Common Bile Duct Stone Recurrence after Endoscopic Sphincterotomy with Following Cholecystectomy" Journal of Personalized Medicine 13, no. 10: 1490. https://doi.org/10.3390/jpm13101490
APA StyleWang, S.-F., Wu, C.-H., Sung, K.-F., Tsou, Y.-K., Lin, C.-H., Lee, C.-W., Lee, M.-H., & Liu, N.-J. (2023). The Impact of Metabolic Factors and Lipid-Lowering Drugs on Common Bile Duct Stone Recurrence after Endoscopic Sphincterotomy with Following Cholecystectomy. Journal of Personalized Medicine, 13(10), 1490. https://doi.org/10.3390/jpm13101490







