Pain Assessment Using the Analgesia Nociception Index (ANI) in Patients Undergoing General Anesthesia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Inclusion and Exclusion Criteria
- Participants (P): patients receiving surgery under general anesthesia
- Intervention (I): ANI monitoring
- Comparison (C): not applicable (NA)
- Outcome measurements (O): diagnostic accuracy of intra- or post-operative pain
- Q1-1
- sensitivity, specificity, and diagnostic odds ratio (DOR) of ANI for intra-operative pain stimuli
- Q1-2
- area under curve (AUC) of accuracy for intra-operative pain
- Q1-3
- sensitivity, specificity, and DOR of ANI for post-operative pain
- Study design (SD): RCTs; cohort, cross-sectional, and case–control studies; and case series
- Participants (P): patients receiving surgery under general anesthesia
- Intervention (I): intra-operative ANI value
- Comparison (C): intra-operative hemodynamic variables, including systolic blood pressure (SBP), mean arterial pressure (MAP), diastolic blood pressure (DBP), and heart rate (HR)
- Outcome measurements (O): correlation
- Study design (SD): RCTs; cohort, cross-sectional, and case–control studies; and case series
- Participants (P): patients receiving surgery under general anesthesia
- Intervention (I): ANI monitoring
- Comparison (C): non-use of ANI monitoring
- Outcome measurements (O): amount of intra-operative opioid use
- Study design (SD): RCTs; cohort, cross-sectional, and case–control studies; and case series
- Review articles, case reports, letters to the editor, and conference abstracts, as well as animal, preclinical, and all other non-relevant studies
- Studies with missing outcome measurements of interest
2.3. Information Source and Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Study Quality Assessment
2.7. Statistical Analysis
2.8. Quality of the Evidence
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Quality Assessment
3.4. Quality of the Evidence
3.5. Prediction of Intra-operative or Post-operative Pain
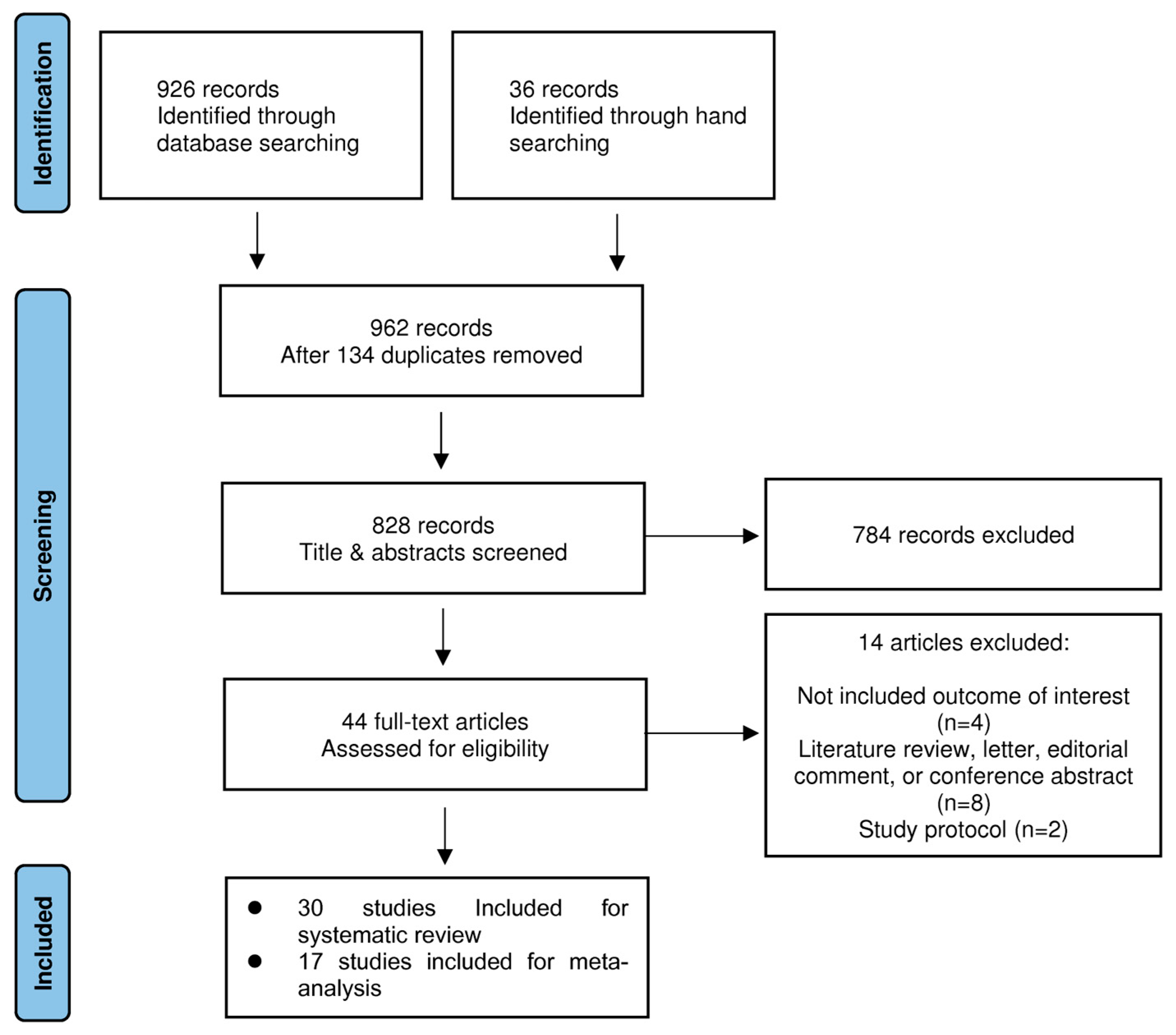
3.5.1. Prediction of Intra-operative Pain
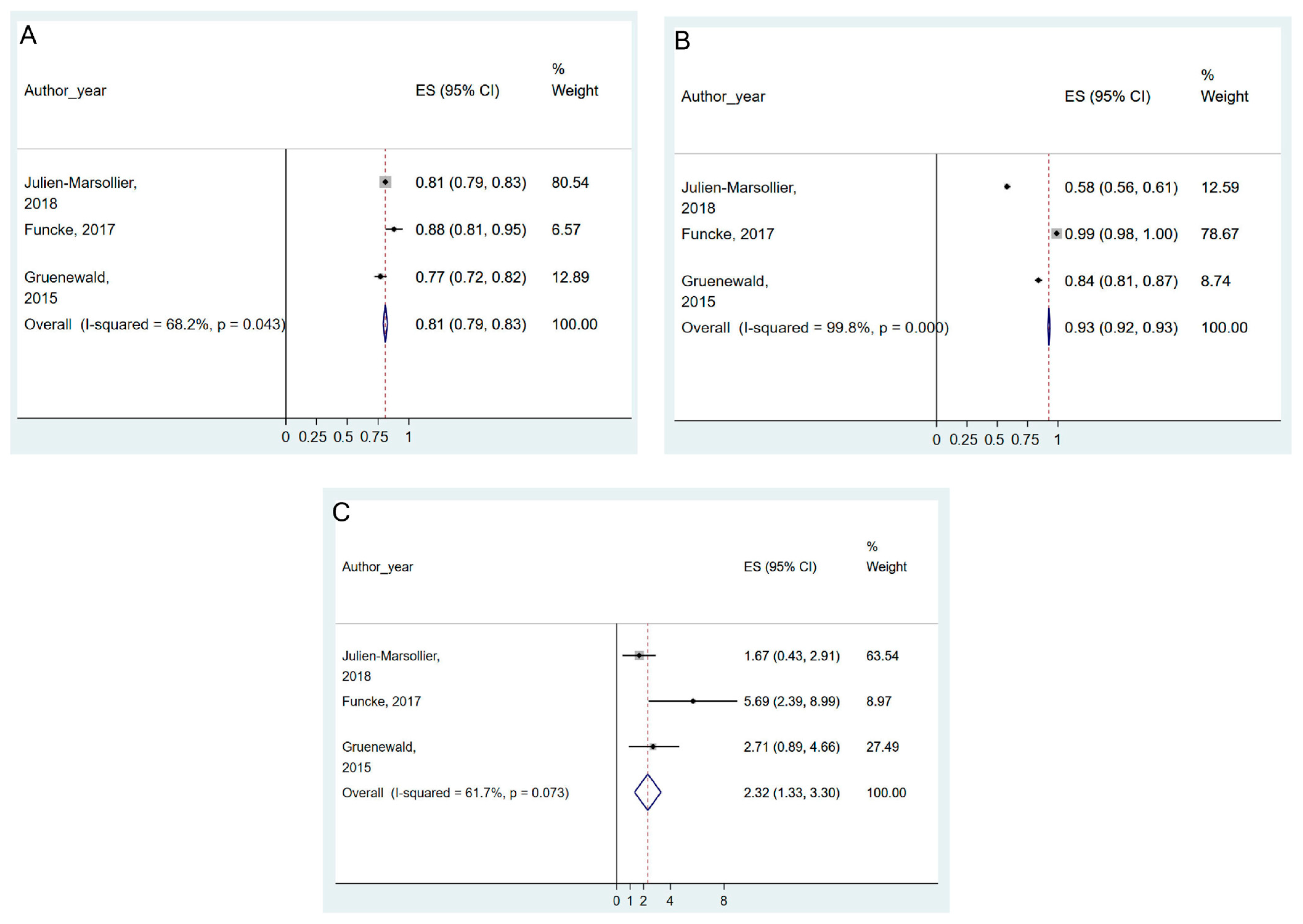
3.5.2. Prediction of Post-Operative Pain
3.6. Correlation between Hemodynamic Changes and ANI Monitoring
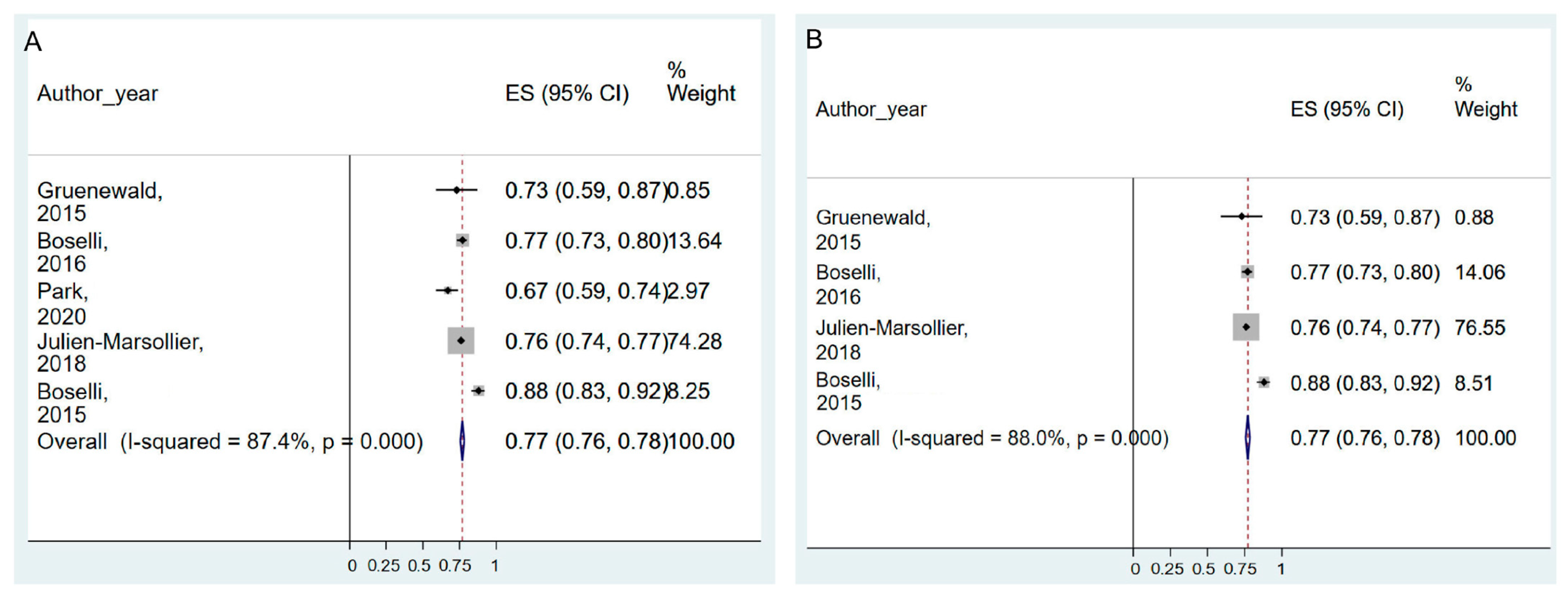
3.7. Intra-operative Opioid Consumption and ANI Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Additional Information: The Reasons of Excluded Articles Following Full-Text Review
- Abdullayev, R.; Uludag, O.; Celik, B. Analgesia Nociception Index: assessment of acute postoperative pain. Braz. J. Anesthesiol. 2019, 69, 396–402. https://dx.doi.org/10.1016/j.bjan.2019.01.003.
- 2.
- Boselli, E. Intérêt du monitorage du tonus parasympathique relatif par Analgesia/Nociception Index (ANI) chez les patients anesthésiés ou conscients. Douleurs Évaluation Diagn. Trait. 2018, 19, 205–210. https://doi.org/10.1016/j.douler.2018.07.008.
- 3.
- Boselli, E.; Jeanne, M. Analgesia/nociception index for the assessment of acute postoperative pain. Br. J. Anaesth. 2014, 112, 936–937. https://doi.org/10.1093/bja/aeu116.
- 4.
- Boselli, E.; Bouvet, L.; Allaouchiche, B. Analgesia monitoring using Analgesia/Nociception Index: Results of clinical studies in awake and anesthetized patients. Prat. En Anesth. Reanim. 2015, 19, 78–86. https://doi.org/10.1016/j.pratan.2015.03.006.
- 5.
- Daccache, G.; Caspersen, E.; Pegoix, M.; Monthe-Sagan, K.; Berger, L.; Fletcher, D.; Hanouz, J.L. A targeted remifentanil administration protocol based on the analgesia nociception index during vascular surgery. Anaesth. Crit. Care Pain Med. 2017, 36, 229–232. https://dx.doi.org/10.1016/j.accpm.2016.08.006.
- 6.
- Eisenried, A.; Akhbardeh, A.; Yeomans, D.C.; Tzabazis, A.Z. Objective measurement of pain perception in volunteers and anesthetized patients. Anesth. Analg. 2016, 122, S352. https://doi.org/10.1213/01.ane.0000499505.96779.a0.
- 7.
- Gazi, M.; Abitagaoglu, S.; Turan, G.; Koksal, C.; Akgun, F.N.; Ari, D.E. Evaluation of the effects of dexmedetomidine and remifentanil on pain with the analgesia nociception index in the perioperative period in hysteroscopies under general anesthesia. A randomized prospective study. Saudi Med. J. 2018, 39, 1017–1022. https://dx.doi.org/10.15537/smj.2018.10.23098.
- 8.
- Ledowski, T. Analgesia-nociception index. Br. J. Anaesth. 2014, 112, 937. https://dx.doi.org/10.1093/bja/aeu113.
- 9.
- Ledowski, T. Monitoring nociception—getting ‘there yet’ might be easier with a road map. Br. J. Anaesth. 2017, 119, 716–717. https://doi.org/10.1093/bja/aex277.
- 10.
- Ledowski, T.; Tiong, W.S.; Lee, C.; Wong, B.; Fiori, T.; Parker, N. Is acute postoperative pain reflected by a change in parasympathetic cardiac tone? Evaluation of the Analgesia Nociception Index. Anaesth. Intensive Care 2012, 40, 1064.
- 11.
- Lee, J.H.; Choi, B.M.; Jung, Y.R.; Lee, Y.H.; Bang, J.Y.; Noh, G.J. Evaluation of Surgical Pleth Index and Analgesia Nociception Index as surrogate pain measures in conscious postoperative patients: an observational study. J. Clin. Monit. Comput. 2020, 34, 1087–1093. https://doi.org/10.1007/s10877-019-00399-5.
- 12.
- Ramos-Luengo, A.; Gardeta Pallarés, A.; Asensio Merino, F. Usefulness of ANI (analgesia nociception index) monitoring for outpatient saphenectomy surgery outcomes: an observational study. J. Clin. Monit. Comput. 2021, 35, 491–497. https://doi.org/10.1007/s10877-020-00491-1.
- 13.
- Turan, G.; Ar, A.Y.; Kuplay, Y.Y.; Demiroluk, O.; Gazi, M.; Akgun, N.; Celikoglu, E. Analgesia Nociception Index for perioperative analgesia monitoring in spinal surgery. Braz. J. Anesthesiol. 2017, 67, 370–375. https://doi.org/10.1016/j.bjan.2017.03.004.
- 14.
- Logier, R.; Jeanne, M.; De Jonckheere, J.; Dassonneville, A.; Delecroix, M.; Tavernier, B. PhysioDoloris: a monitoring device for analgesia/nociception balance evaluation using heart rate variability analysis. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010, 2010, 1194–1197. https://dx.doi.org/10.1109/IEMBS.2010.5625971.
References
- Hochhausen, N.; Ritter, M.; KÖny, M.; Rossaint, R.; Czaplik, M. 33rd Congress of the Scandinavian Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol. Scand. 2015, 59 (Suppl. S121), 1–60. [Google Scholar]
- Logier, R.; Jeanne, M.; Tavernier, B.; De Jonckheere, J. Pain/analgesia evaluation using heart rate variability analysis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Pain-autonomic interactions. Neurol. Sci. 2006, 27 (Suppl. S2), S130–S133. [Google Scholar] [CrossRef]
- Gruenewald, M.; Herz, J.; Schoenherr, T.; Thee, C.; Steinfath, M.; Bein, B. Measurement of the nociceptive balance by analgesia nociception index and surgical pleth index during sevoflurane-remifentanil anesthesia. Minerva Anestesiol. 2015, 81, 480–489. [Google Scholar]
- Upton, H.D.; Ludbrook, G.L.; Wing, A.; Sleigh, J.W. Intraoperative “analgesia nociception index”-guided fentanyl administration during sevoflurane anesthesia in lumbar discectomy and laminectomy: A randomized clinical trial. Anesth. Analg. 2017, 125, 81–90. [Google Scholar] [CrossRef]
- Julien-Marsollier, F.; Rachdi, K.; Caballero, M.J.; Ayanmanesh, F.; Vacher, T.; Horlin, A.L.; Skhiri, A.; Brasher, C.; Michelet, D.; Dahmani, S. Evaluation of the analgesia nociception index for monitoring intraoperative analgesia in children. Br. J. Anaesth. 2018, 121, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Daniela-Ionescu, M.; Bégou, G.; Bouvet, L.; Dabouz, R.; Magnin, C.; Allaouchiche, B. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI). Br. J. Anaesth. 2013, 111, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Szental, J.A.; Webb, A.; Weeraratne, C.; Campbell, A.; Sivakumar, H.; Leong, S. Postoperative pain after laparoscopic cholecystectomy is not reduced by intraoperative analgesia guided by analgesia nociception index (ANI®) monitoring: A randomized clinical trial. Br. J. Anaesth. 2015, 114, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Chichester, UK, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Choi, G.J.; Kang, H. Introduction to Umbrella Reviews as a Useful Evidence-Based Practice. J. Lipid Atheroscler. 2023, 12, 3–11. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Rutjes, A.W.; Reitsma, J.B.; Bossuyt, P.M.; Kleijnen, J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef]
- Irwig, L.; Macaskill, P.; Glasziou, P.; Fahey, M. Meta-analytic methods for diagnostic test accuracy. J. Clin. Epidemiol. 1995, 48, 119–130, discussion 131–112. [Google Scholar] [CrossRef]
- Moses, L.E.; Shapiro, D.; Littenberg, B. Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat. Med. 1993, 12, 1293–1316. [Google Scholar] [CrossRef]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Daccache, G.; Caspersen, E.; Pegoix, M.; Monthé-Sagan, K.; Berger, L.; Fletcher, D.; Hanouz, J.L. A targeted remifentanil administration protocol based on the analgesia nociception index during vascular surgery. Anaesth. Crit. Care Pain Med. 2017, 36, 229–232. [Google Scholar] [CrossRef]
- Gazi, M.; Abitagaoglu, S.; Turan, G.; Koksal, C.; Akgun, F.N.; Ari, D.E. Evaluation of the effects of dexmedetomidine and remifentanil on pain with the analgesia nociception index in the perioperative period in hysteroscopies under general anesthesia. A randomized prospective study. Saudi Med. J. 2018, 39, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Luengo, A.; Gardeta Pallarés, A.; Asensio Merino, F. Usefulness of ANI (analgesia nociception index) monitoring for outpatient saphenectomy surgery outcomes: An observational study. J. Clin. Monit. Comput. 2021, 35, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Turan, G.; Ar, A.Y.; Kuplay, Y.Y.; Demiroluk, O.; Gazi, M.; Akgun, N.; Celikoglu, E. Analgesia Nociception Index for perioperative analgesia monitoring in spinal surgery. Braz. J. Anesthesiol. 2017, 67, 370–375. [Google Scholar] [CrossRef]
- Boselli, E. Intérêt du monitorage du tonus parasympathique relatif par Analgesia/Nociception Index (ANI) chez les patients anesthésiés ou conscients. Douleurs Évaluation-Diagn.-Trait. 2018, 19, 205–210. [Google Scholar] [CrossRef]
- Boselli, E.; Bouvet, L.; Allaouchiche, B. Analgesia monitoring using Analgesia/Nociception Index: Results of clinical studies in awake and anesthetized patients. Prat. Anesth. Reanim. 2015, 19, 78–86. [Google Scholar] [CrossRef]
- Boselli, E.; Jeanne, M. Analgesia/nociception index for the assessment of acute postoperative pain. Br. J. Anaesth. 2014, 112, 936–937. [Google Scholar] [CrossRef]
- Ledowski, T. Analgesia-nociception index. Br. J. Anaesth. 2014, 112, 937. [Google Scholar] [CrossRef][Green Version]
- Ledowski, T. Monitoring nociception—Getting ‘there yet’ might be easier with a road map. Br. J. Anaesth. 2017, 119, 716–717. [Google Scholar] [CrossRef]
- Eisenried, A.; Akhbardeh, A.; Yeomans, D.C.; Tzabazis, A.Z. Objective measurement of pain perception in volunteers and anesthetized patients. Anesth. Analg. 2016, 122, S352. [Google Scholar] [CrossRef]
- Ledowski, T.; Tiong, W.S.; Lee, C.; Wong, B.; Fiori, T.; Parker, N. Is acute postoperative pain reflected by a change in parasympathetic cardiac tone? Evaluation of the Analgesia Nociception Index. Anaesth. Intensive Care 2012, 40, 1064. [Google Scholar]
- Logier, R.; Jeanne, M.; De Jonckheere, J.; Dassonneville, A.; Delecroix, M.; Tavernier, B. PhysioDoloris: A monitoring device for analgesia/nociception balance evaluation using heart rate variability analysis. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 1194–1197. [Google Scholar] [CrossRef]
- Abdullayev, R.; Uludag, O.; Celik, B. Analgesia Nociception Index: Assessment of acute postoperative pain. Braz. J. Anesthesiol. 2019, 69, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, B.M.; Jung, Y.R.; Lee, Y.H.; Bang, J.Y.; Noh, G.J. Evaluation of Surgical Pleth Index and Analgesia Nociception Index as surrogate pain measures in conscious postoperative patients: An observational study. J. Clin. Monit. Comput. 2020, 34, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Bouvet, L.; Bégou, G.; Dabouz, R.; Davidson, J.; Deloste, J.Y.; Rahali, N.; Zadam, A.; Allaouchiche, B. Prediction of immediate postoperative pain using the analgesia/nociception index: A prospective observational study. Br. J. Anaesth. 2014, 112, 715–721. [Google Scholar] [CrossRef]
- Boselli, E.; Bouvet, L.; Bégou, G.; Torkmani, S.; Allaouchiche, B. Prediction of hemodynamic reactivity during total intravenous anesthesia for suspension laryngoscopy using Analgesia/Nociception Index (ANI): A prospective observational study. Minerva Anestesiol. 2015, 81, 288–297. [Google Scholar]
- Boselli, E.; Logier, R.; Bouvet, L.; Allaouchiche, B. Prediction of hemodynamic reactivity using dynamic variations of Analgesia/Nociception Index (∆ANI). J. Clin. Monit. Comput. 2016, 30, 977–984. [Google Scholar] [CrossRef]
- Charier, D.; Vogler, M.C.; Zantour, D.; Pichot, V.; Martins-Baltar, A.; Courbon, M.; Roche, F.; Vassal, F.; Molliex, S. Assessing pain in the postoperative period: Analgesia Nociception IndexTM versus pupillometry. Br. J. Anaesth. 2019, 123, e322–e327. [Google Scholar] [CrossRef]
- Coulombe, M.A.; Décary, E.; Maximos, S.; Brulotte, V.; Drolet, P.; Tanoubi, I.; Issa, R.; Zaphiratos, V.; Verdonck, O.; Fortier, L.P.; et al. Assessing the antinociceptive effect of nitrous oxide to tetanic stimulation in anaesthetised patients with new intra-operative nociception monitors: An observational study. Eur. J. Anaesthesiol. 2021, 38, 512–523. [Google Scholar] [CrossRef]
- Gruenewald, M.; Ilies, C.; Herz, J.; Schoenherr, T.; Fudickar, A.; Höcker, J.; Bein, B. Influence of nociceptive stimulation on analgesia nociception index (ANI) during propofol-remifentanil anaesthesia. Br. J. Anaesth. 2013, 110, 1024–1030. [Google Scholar] [CrossRef]
- Jeanne, M.; Clément, C.; De Jonckheere, J.; Logier, R.; Tavernier, B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J. Clin. Monit. Comput. 2012, 26, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Jeanne, M.; Delecroix, M.; De Jonckheere, J.; Keribedj, A.; Logier, R.; Tavernier, B. Variations of the analgesia nociception index during propofol anesthesia for total knee replacement. Clin. J. Pain 2014, 30, 1084–1088. [Google Scholar] [CrossRef]
- Kommula, L.K.; Bansal, S.; Umamaheswara Rao, G.S. Analgesia Nociception Index Monitoring during Supratentorial Craniotomy. J. Neurosurg. Anesthesiol. 2019, 31, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Köprülü, A.Ş.; Haspolat, A.; Gül, Y.G.; Tanrikulu, N. Can postoperative pain be predicted? New parameter: Analgesia nociception index. Turk. J. Med. Sci. 2020, 50, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Averhoff, L.; Tiong, W.S.; Lee, C. Analgesia Nociception Index (ANI) to predict intraoperative haemodynamic changes: Results of a pilot investigation. Acta Anaesthesiol. Scand. 2014, 58, 74–79. [Google Scholar] [CrossRef]
- Park, C.; Yang, M.H.; Choi, B.; Jeon, B.; Lee, Y.H.; Shin, H.; Lee, B.; Choi, B.M.; Noh, G.J. Performance of the nasal photoplethysmographic index as an analgesic index during surgery under general anaesthesia. Sci. Rep. 2020, 10, 7130. [Google Scholar] [CrossRef]
- Susano, M.J.; Vide, S.; Ferreira, A.D.; Amorim, P. Effects of varying remifentanil concentrations on Analgesia Nociception Index® under propofol: An observational study. J. Clin. Monit. Comput. 2021, 35, 199–205. [Google Scholar] [CrossRef]
- Gall, O.; Champigneulle, B.; Schweitzer, B.; Deram, T.; Maupain, O.; Montmayeur Verchere, J.; Orliaguet, G. Postoperative pain assessment in children: A pilot study of the usefulness of the analgesia nociception index. Br. J. Anaesth. 2015, 115, 890–895. [Google Scholar] [CrossRef]
- Sabourdin, N.; Arnaout, M.; Louvet, N.; Guye, M.L.; Piana, F.; Constant, I. Pain monitoring in anesthetized children: First assessment of skin conductance and analgesia-nociception index at different infusion rates of remifentanil. Paediatr. Anaesth. 2013, 23, 149–155. [Google Scholar] [CrossRef]
- Funcke, S.; Sauerlaender, S.; Pinnschmidt, H.O.; Saugel, B.; Bremer, K.; Reuter, D.A.; Nitzschke, R. Validation of innovative techniques for monitoring nociception during general anesthesia: A clinical study using tetanic and intracutaneous electrical stimulation. Anesthesiology 2017, 127, 272–283. [Google Scholar] [CrossRef]
- Le Gall, L.; David, A.; Carles, P.; Leuillet, S.; Chastel, B.; Fleureau, C.; Dewitte, A.; Ouattara, A. Benefits of intraoperative analgesia guided by the Analgesia Nociception Index (ANI) in bariatric surgery: An unmatched case-control study. Anaesth. Crit. Care Pain Med. 2019, 38, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, V.; Schreiberova, J.; Bartos, M.; Kukralova, L.; Dostal, P. Surgical pleth index and analgesia nociception index for intraoperative analgesia in patients undergoing neurosurgical spinal procedures: A comparative randomized study. Minerva Anestesiol. 2019, 85, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Dundar, N.; Kus, A.; Gurkan, Y.; Toker, K.; Solak, M. Analgesia nociception index (ani) monitoring in patients with thoracic paravertebral block: A randomized controlled study. J. Clin. Monit. Comput. 2018, 32, 481–486. [Google Scholar] [CrossRef]
- Theerth, K.; Sriganesh, K.; Chakrabarti, D.; Reddy, K.; Rao, G. Analgesia nociception index and hemodynamic changes during skull pin application for supratentorial craniotomies in patients receiving scalp block versus pin-site infiltration: A randomized controlled trial. Saudi J. Anaesth. 2019, 13, 306–311. [Google Scholar] [CrossRef]
- Theerth, K.A.; Sriganesh, K.; Reddy, K.M.; Chakrabarti, D.; Umamaheswara Rao, G.S. Analgesia Nociception Index-guided intraoperative fentanyl consumption and postoperative analgesia in patients receiving scalp block versus incision-site infiltration for craniotomy. Minerva Anestesiol. 2018, 84, 1361–1368. [Google Scholar] [CrossRef]
- Tribuddharat, S.; Sathitkarnmanee, T.; Sukhong, P.; Thananun, M.; Promkhote, P.; Nonlhaopol, D. Comparative study of analgesia nociception index (ANI) vs. standard pharmacokinetic pattern for guiding intraoperative fentanyl administration among mastectomy patients. BMC Anesthesiol. 2021, 21, 50. [Google Scholar] [CrossRef]
- Yi, D.; Wei, B.; Zhang, L.-P.; Guo, X.-Y. Analgesia nociception index guided remifentanil administration during general anesthesia in posterior lumbar spinal surgery. Basic Clin. Med. 2015, 35, 1341. [Google Scholar]
- Sabourdin, N.; Burey, J.; Tuffet, S.; Thomin, A.; Rousseau, A.; Al-Hawari, M.; Taconet, C.; Louvet, N.; Constant, I. Analgesia Nociception Index-Guided Remifentanil versus Standard Care during Propofol Anesthesia: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 333. [Google Scholar] [CrossRef]
- Sriganesh, K.; Theerth, K.A.; Reddy, M.; Chakrabarti, D.; Rao, G.S.U. Analgesia nociception index and systemic haemodynamics during anaesthetic induction and tracheal intubation: A secondary analysis of a randomised controlled trial. Indian J. Anaesth. 2019, 63, 100–105. [Google Scholar] [CrossRef]
- Logier, R.; De Jonckheere, J.; Delecroix, M.; Keribedj, A.; Jeanne, M.; Jounwaz, R.; Tavernier, B. Heart rate variability analysis for arterial hypertension etiological diagnosis during surgical procedures under tourniquet. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; Volume 2011, pp. 3776–3779. [Google Scholar] [CrossRef]
- Gruenewald, M.; Ilies, C. Monitoring the nociception-anti-nociception balance. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 235–247. [Google Scholar] [CrossRef]
- Graça, R.; Lobo, F.A. Analgesia Nociception Index (ANI) and ephedrine: A dangerous liasion. J. Clin. Monit. Comput. 2021, 35, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, M.; Willms, S.; Broch, O.; Kott, M.; Steinfath, M.; Bein, B. Sufentanil administration guided by surgical pleth index vs. standard practice during sevoflurane anaesthesia: A randomized controlled pilot study. Br. J. Anaesth. 2014, 112, 898–905. [Google Scholar] [CrossRef] [PubMed]
| Author (Year), Country | Study Design | Characteristics of Participants (Number) | Anesthetic Agents | Primary Outcome or Purpose |
|---|---|---|---|---|
| Julien-Marsollier (2018) France [6] | Observational study | ENT, abdominal, urological, or orthopedic surgery involving incision or laparoscopic trocar placement (n = 49) | Propofol Sevoflurane Remifentanil Atracurium | Assessing the diagnostic value of monitoring the ANI to detect surgical stimulation in children |
| Funcke (2017) Turkey [52] | Observational study | Open radical prostatectomy (n = 37) | Propofol Remifentanil | Correlation between ANI and remifentanil dose administered |
| Gruenewald (2015) Germany [4] | Observational study | Elective surgery (n = 27) | Sevoflurane Remifentanil Rocuronium | Comparing variations of ANI for noxious stimulations (laryngeal mask airway insertion, tetanic stimulation, intubation) according to remifentanil dose administered |
| Boselli (2016) France [39] | Observational study | ENT or lower limb orthopedic surgery (n = 128) | Propofol Ketamine Desflurane Remifentanil Cisatracurium | Comparing dynamic variations of ANI and static values to predict hemodynamic reactivity |
| Park (2020) Korea [48] | Observational study | Stomach surgery, colorectal surgery, hepatobiliary surgery (n = 81) | Propofol Remifentanil Rocuronium | Evaluating the performance of NPI in comparison with the SPI and the ANI in patients under general anesthesia with target-controlled infusion of propofol and remifentanil |
| Theerth (2018) India [57] | RCT single blind parallel assignment | Brain tumor operation (n = 57) Scalp block (n = 29) Incision infiltration (n = 28) | Thiopentone Sevoflurane Fentanyl Vecuronium | Evaluating intra-operative fentanyl consumption |
| Susano (2021) Portugal [49] | Observational study | Elective craniotomy (n = 16) | Propofol Remifentanil Rocuronium | Evaluating ability of the ANI monitor to detect standard noxious stimulus (tetanic stimulation) in patients under total intravenous anesthesia with propofol and remifentanil |
| Coulombe (2021) Canada [41] | Observational study | Elective abdominal surgery via laparotomy (n = 30) | Propofol Desflurane Remifentanil Rocuronium | Documenting ANI variations after standard nociceptive stimulation at 0, 20, and 50% of inhaled N2O |
| KÖPRÜLÜ (2020) Turkey [46] | Observational study | Laparoscopic cholecystectomy (n = 36) NRS ≤ 3 group (n = 17) NRS 4–6 group (n = 11) NRS ≥ 7 group (n = 8) | Midazolam Propofol Sevoflurane Fentanyl Remifentanil Rocuronium | Determining whether or not a correlation exists between the ANI values recorded at the completion of an operation and immediately before and after extubation and the NRS values recorded in the PACU in a group of patients who underwent laparoscopic cholecystectomy, with the goal of evaluating the potential use of ANI values for the prediction of post-operative pain levels |
| Sabourdin (2013) France [51] | Observational study Pilot study | Middle-ear surgery (n = 12) | Propofol Sevoflurane Desflurane Remifentanil | Describing the profiles of ANI after a standardized nociceptive stimulation, in anesthetized children, at different infusion rates of remifentanil |
| Ledowski (2014) Australia [47] | Observational study Pilot study | Elective surgery (n = 30) | Propofol Sevoflurane Fentanyl Rocuronium | Determining whether changes to the ANI would coincide with or precede observed haemodynamic changes |
| Gruenewald (2013) Germany [42] | Observational study | Elective surgery (n = 25) | Propofol Remifentanil | Challenging the ability of ANI to detect standardized noxious stimulation. |
| Boselli (2013) France [7] | Observation study | ENT, plastic surgery (n = 200) | Ketamine Propofol Sevoflurane Desflurane Remifentanil | Using ANI in the assessment of immediate post-operative pain in adult patients undergoing general anesthesia. |
| Boselli (2014) France [37] | Observation study | ENT, lower extremity surgery (n = 200) | Ketamine Propofol Sevoflurane Desflurane Remifentanil Cisatracurium | Performing ANI measurements at arousal from general anesthesia to predict immediate post-operative pain on arrival in PACU. |
| Charier (2019) France [40] | Observational study | Elective surgery (n = 345) | Intravenous anesthetic agents Opioid Volatile anesthetics | Comparing the respective values of ANI, PD, PLR, and VCPD with post-operative VAS scores |
| Gall (2015) France [50] | Observational study Pilot study | Elective surgery (n = 32) Imaging procedure (n = 30) | No restriction on the anesthetic technique | Investigating the relationship between the ANI and objective measurements of pain intensity during the recovery phase after procedures under general anesthesia in children |
| Sriganesh (2019) India [61] | RCT secondary analysis of Theerth (2018) | Brain tumor surgery (n = 57) Scalp block (n = 29) Incision infiltration (n = 28) | Thiopentone Sevoflurane Fentanyl Vecuronium | Observing ANI changes during direct laryngoscopy and tracheal intubation |
| Theerth (2019) India [56] | RCT single blind parallel assignment | Brain tumor operation (n = 57) Scalp block (n = 29) Pin-site infiltration (n = 28) | Thiopentone Sevoflurane Fentanyl Vecuronium | Comparing ANI and hemodynamic changes during skull pin insertion |
| Jeanne (2012) France [43] | Observational study | Laparoscopic appendectomy or cholecystectomy (n = 15) | Propofol Sevoflurane Remifentanil Cisatracurium | Comparing ANI with heart rate and systolic blood pressure during various noxious stimuli |
| Kommula (2019) India [45] | Observational study | Craniotomy (n = 21) | Propofol Sevoflurane Fentanyl Vecuronium | Monitoring analgesia during craniotomy using ANI monitor and comparing it with cardiovascular parameters |
| Boselli (2015) France [38] | Observation study | Suspension laryngoscopy (n = 50) | Propofol Remifentanil | Evaluating the performance of ANI to predict hemodynamic reactivity during suspension laryngoscopy. |
| Jeanne (2014) France [44] | Observational study | Total knee replacement (n = 27) | Propofol Sufentanil | Determining (1) whether ANI variations could detect early HemodR during propofol anesthesia for total knee replacement, (2) whether ANI measures are coherent with pain after surgery when patients are in PACU, and (3) the threshold predictive of HemodR to prepare for an interventional clinical trial that would measure the benefit of using the ANI monitor to adapt opioids during general anesthesia |
| Tribuddharat (2021) Thailand [58] | RCT double blind parallel assignment | Mastectomy ANI group (n = 30) Anesthesiologist’s judgment (n = 30) | Propofol Desflurane Fentanyl Cisatracurium | Comparing the efficacy of ANI with standard pharmacokinetic pattern to guide intra-operative fentanyl administration. |
| Upton (2017) Australia [5] | RCT single blind parallel assignment | Discectomy/laminectomy ANI (n = 24) Anesthesiologist’s judgment group (n = 26) | Propofol Sevoflurane Fentanyl Rocuronium | Documenting post-operative NRS pain score from 0 to 90 min of PACU stay Investigating the effect of intra-operative ANI-guided fentanyl administration on post-operative pain |
| Szental (2015) Australia [8] | RCT single blind parallel assignment | Laparoscopic cholecystectomy ANI group (n = 59) Anesthesiologist’s judgment group (n = 60) | Propofol Sevoflurane Desflurane Fentanyl Morphine Neuromuscular blocking agent (anesthetists’ choice) | Assessing post-operative moderate/severe pain (VAS ≥ 50 mm) at any of the four time points in the first post-operative hour |
| Dundar (2018) Turkey [55] | RCT single blind parallel assignment | Breast surgery ANI group (n = 22) Anesthesiologist’s judgment group (n = 22) | Propofol Sevoflurane Fentanyl Rocuronium | Measuring total intra-operative remifentanil consumption Evaluating the effectiveness of ANI monitoring during intra-operative period |
| Duan (2015) China [59] | RCT single blind parallel assignment | Posterior lumbar spine surgery ANI group (n = 28) Anesthesiologist’s judgment (n = 29) | Propofol Remifentanil Vecuronium | Investigating the effect of intra-operative ANI-guided remifentanil administration |
| Sabourdin (2022) France [60] | RCT single blind parallel assignment | Gynecologic surgery ANI group (n = 38) Anesthesiologist’s judgment group (n = 40) | Propofol Remifentanil Atracurium | Measuring ntra-operative remifentanil consumption |
| Dostalova (2019) Czech Republic [54] | RCT single blind parallel assignment | Spine surgery ANI group (n = 24) SPI group (n = 24) Anesthesiologist’s judgment group (n = 24) | Propofol Desflurane Sufentanil Atracurium | Measuring total intra-operative dose of sufentanil Comparing patterns of intra-operative use of opioids, post-operative cortisol levels and post-operative pain scores |
| Le Gall (2019) France [53] | Unmatched case control study | Bariatric surgery ANI group (n = 30) from prospective cohort Anesthesiologist’s judgment (n = 30) from retrospective cohort | Propofol Sevoflurane Sufentanil Succinylcholine | Measuring mean hourly intra-operative sufentanil requirement Comparing intra-operative opioid consumption |
| Quadas-2 | ||||||
|---|---|---|---|---|---|---|
| Study | Domain | |||||
| Patient Selection | Index Test | Reference Standard | Flow and Timing | |||
| Julien-Marsollier (2018) [6] | Low | Some concerns | Low | Low | ||
| Funcke (2017) [52] | Low | Some concerns | Low | Low | ||
| Gruenewald (2015) [4] | Low | Some concerns | Low | Low | ||
| Boselli (2013) [7] | Low | Some concerns | Low | Low | ||
| Boselli (2014) [37] | Low | Some concerns | Low | Low | ||
| Charier (2019) [40] | Low | Some concerns | Low | Low | ||
| Gall (2015) [50] | Low | Some concerns | Low | Low | ||
| RoBANS | ||||||
| Study | Domain | |||||
| Selection of Participants | Confounding Variables | Intervention Measurement | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | |
| Julien-Marsollier (2018) [6] | Unclear | No | No | Yes | No | No |
| Funcke (2017) [52] | Unclear | No | No | Yes | Yes | No |
| Gruenewald (2015) [4] | Unclear | No | No | Yes | Yes | No |
| Boselli (2016) [39] | Unclear | No | No | Yes | Yes | No |
| Park (2020) [48] | Unclear | No | No | Yes | Yes | No |
| Susano (2021) [49] | No | No | No | Yes | No | No |
| Coulombe (2021) [41] | Unclear | No | No | Yes | No | No |
| KÖPRÜLÜ (2020) [46] | Unclear | No | No | Yes | No | No |
| Sabourdin (2013) [51] | Unclear | No | No | Yes | No | No |
| Ledowski (2014) [47] | Unclear | No | No | Yes | No | No |
| Gruenewald (2013) [42] | Unclear | No | No | Yes | Yes | No |
| Boselli (2013) [7] | Unclear | No | No | Yes | No | No |
| Boselli (2014) [37] | Unclear | No | No | Yes | No | No |
| Charier (2019) [40] | Unclear | No | No | Yes | No | No |
| Gall (2015) [50] | Unclear | No | No | Yes | No | No |
| Jeanne (2012) [43] | Unclear | No | No | Yes | No | No |
| Kommula (2019) [45] | Unclear | No | No | Yes | No | No |
| Boselli (2015) [38] | Unclear | No | No | No | No | No |
| Jeanne (2014) [44] | Unclear | No | No | No | No | No |
| Le Gall (2019) [53] | Unclear | No | No | Yes | No | No |
| Sriganesh (2019) [61] | Unclear | No | No | No | No | No |
| RoB 2.0 | ||||||
| Study | Domain | |||||
| Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Bias | |
| Theerth (2018) [57] | Some concerns | Some concerns | Low risk | Low risk | Low risk | High risk |
| Theerth (2019) [61] | Some concerns | Some concerns | Low risk | Low risk | Low risk | High risk |
| Tribuddharat (2021) [58] | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Upton (2017) [5] | Some concerns | Some concerns | Low risk | Low risk | Low risk | High risk |
| Szental (2015) [8] | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Dundar (2018) [55] | Some concerns | Some concerns | Low risk | Low risk | Low risk | High risk |
| Duan (2015) [59] | Some concerns | Some concerns | Low risk | Low risk | Low risk | High risk |
| Sabourdin (2022) [60] | Some concerns | Some concerns | Low risk | Low risk | Low risk | High risk |
| Dostalova (2019) [54] | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Outcome | Number of Studies | Sensitivity | Specificity | Diagnostic Odd Ratio | Quality of Evidence | Certainty of Evidence | |||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | Hetero- Geneity | Specificity (95% CI) | Hetero- Geneity | Diagnostic Odd Ratio (95% CI) | Hetero- Geneity | ||||
| Prediction of intra-operative pain | 8 studies | 0.81 (0.79–0.83) | I2 = 68.2%; Pchi2 = 0.043 | 0.93 (0.92–0.93) | I2 = 99.8%; Pchi2 < 0.001 | 2.32 (1.33–3.30) | I2 = 61.7%; Pchi2 = 0.073 | Not serious | ⨁◯◯◯ Very low |
| Prediction of post-operative pain | 5 studies | 0.90 (0.87–0.93) | I2 = 58.7%; Pchi2 = 0.064 | 0.51 (0.49–0.52) | I2 = 99.9%; Pchi2 < 0.001 | 3.38 (2.87–3.88) | I2 = 81.2%; Pchi2 = 0.001 | Not serious | ⨁◯◯◯ Very low |
| Outcome | Number of Studies | Quality Assessment | Hetero-Geneity | SMD (95% CI) | Quality | ||||
| ROB | Inconsistency | Indirectness | Imprecision | Publication Bias | |||||
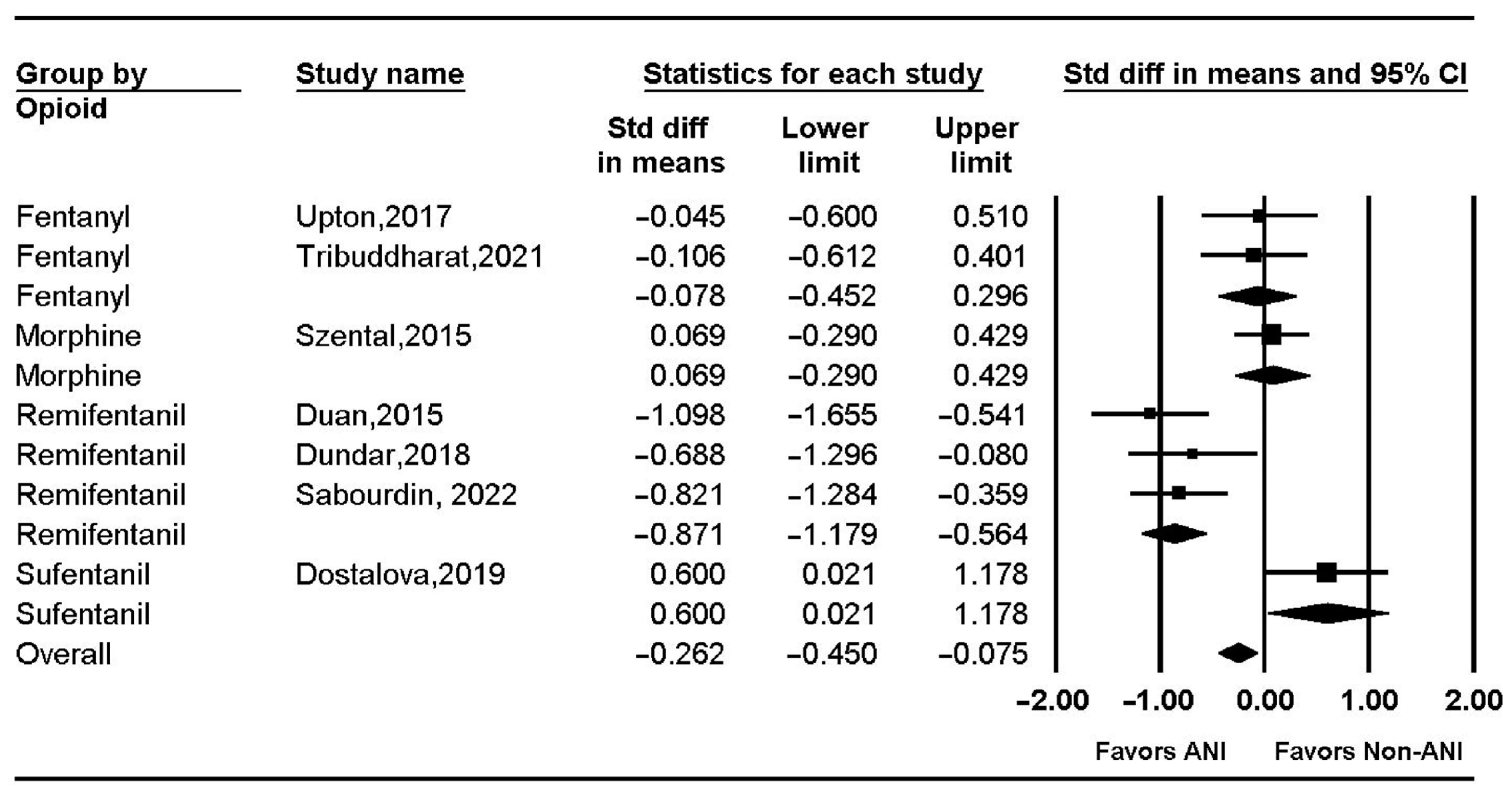
| Intra-operative opioid use | 8 studies | serious | serious | not serious | not serious | NA | I2 = 79.2%, Pchi2 < 0.001 | −0.262 (−0.450–−0.075) | ⨁⨁◯◯ Low |
| Author (Year) | Main Results | |||
|---|---|---|---|---|
| Theerth (2018) [57] | ANI variable [threshold] | AUC (95CI, SD) | ||
| ANIi-HR [≤50] ANIm-HR [≤50] ANIi-MBP [≤50] ANIm-MBP [≤50] | 0.687 0.672 0.599 0.588 | |||
| Jeanne (2014) [44] | ANI variable [threshold] | AUC (95CI, SD) | ||
| ANI [≤63] | 0.92 | |||
| sensitivity = 80%, specificity = 95%, PPV = 94%, and NPV = 79% | ||||
| No stimulation Medain [IQR] | Hemodynamic reactivity Medain [IQR] | p-Value | ||
| Patients with hemodynamic reactivity | 82.5 [30.3] | 47.5 [22.5] | <0.0001 | |
| No stimulation Medain [IQR] | Tibial cut Medain [IQR] | |||
| Patients without hemodynamic reactivity | 80.5 [45] | 62 [23] | 0.5 | |
| Susano (2021) [49] | Remifentanil effect site concentration | ANI before tetanic stimulation Mean ± SD | ANI after tetanic stimulation Mean ± SD | p-Value |
| 0.5 ng/mL | 56 ± 16 | 49 ± 15 | 0.002 | |
| 1.5 ng/mL | 68 ± 22 | 62 ± 22 | 0.012 | |
| 3.0 ng/mL | 66 ± 18 | 59 ± 16 | 0.009 | |
| 5.0 ng/mL | 72 ± 16 | 69 ± 14 | 0.253 | |
| 7.0 ng/mL | 71 ± 18 | 70 ± 16 | 0.655 | |
| Gruenewald (2013) [42] | Remifentanil effect site concentration | ANI before tetanic stimulation Medain [IQR] | ANI after tetanic stimulation Medain [IQR] | p-Value |
| 0 ng/mL 2 ng/mL 4 ng/mL | 61 [48–72] 71 [61–88] 88 [70–98] | 24 [12–35] 30 [20–40] 13 [5–27] | <0.05 <0.05 <0.05 | |
| Coulombe (2021) [41] | Type of stimulus | Prestimulation ANI Median [IQR] | Poststimulation ANI Median [IQR] | p-Value |
| Intubation Incision | 46.8 [39.1–59.2] 73.9 [52.7–87.7] | 35.2 [28.6–45.0] 55.7 [45.8–72.1] | <0.0001 0.001 | |
| Sabourdin (2013) [51] | ANI was significantly decreased compared with prestimulation values for all remifentanil infusion rates (p < 0.05) Stimulation type (tetanic stimulation) Remifentanil infusion rate (0, 2, 0.16, 0.12, 0.08, and 0.04 mcg/kg/min) | |||
| Ledowski (2014) [47] | Type of stimulus | Prestimulation ANI Mean ± SD | Poststimulation ANI Mean ± SD | p-Value |
| Airway manipulation Skin incision Fentanyl bolus | 52.4 ± 19.8 62.7 ± 18.7 53.3 ± 17.9 | 33.0 ± 11.9 37.9 ± 13.7 59.4 ± 18.7 | <0.001 <0.001 <0.05 | |
| The predictive probability value (Pk) for ANI to predict > 10% changes in HR was 0.61 (SE 0.09) and the Pk for >10% changes in SBP 0.59 (SE 0.06). | ||||
| Köprülü (2020) [46] | ANI is ineffective in the prediction of potential post-operative pain Pearson correlation: Group I: NRS ≤ 3, r = 0.016 (weak); Group II: NRS 4–6, r = −0.286 (weak); Group III: NRS ≥ 7, r = −0.293 (weak) | |||
| Le Gall (2019) [53] | There was significant reduction of sufentanil use in ANI group compared with non-ANI group (0.15 ± 0.05 vs. 0.17 ± 0.05 mcg/kg/h, p = 0.038). | |||
| Author (Year) | ANI Variable | Hemodynamic Variables | Correlation Estimate | p-Value |
|---|---|---|---|---|
| Sriganesh (2019) [61] | ANIi | HR MBP | −0.405 −0.415 | <0.001 <0.001 |
| Theerth (2019) [56] | ANIi ANIm (2 min average) | ANIi-HR ANIi-MBP ANIm-HR ANIm-MBP | −0.594 −0.534 −0.161 −0.091 | <0.001 <0.001 0.007 0.085 |
| Jeanne (2012) [43] | ANI (2 min average) | SBP | −0.348 | <0.01 |
| Kommula (2019) [45] | ANI average | HR MAP | −0.280 −0.258 | <0.0001 <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.K.; Choi, G.J.; Oh, K.S.; Lee, S.P.; Kang, H. Pain Assessment Using the Analgesia Nociception Index (ANI) in Patients Undergoing General Anesthesia: A Systematic Review and Meta-Analysis. J. Pers. Med. 2023, 13, 1461. https://doi.org/10.3390/jpm13101461
Kim MK, Choi GJ, Oh KS, Lee SP, Kang H. Pain Assessment Using the Analgesia Nociception Index (ANI) in Patients Undergoing General Anesthesia: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2023; 13(10):1461. https://doi.org/10.3390/jpm13101461
Chicago/Turabian StyleKim, Min Kyoung, Geun Joo Choi, Kyung Seo Oh, Sang Phil Lee, and Hyun Kang. 2023. "Pain Assessment Using the Analgesia Nociception Index (ANI) in Patients Undergoing General Anesthesia: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 13, no. 10: 1461. https://doi.org/10.3390/jpm13101461
APA StyleKim, M. K., Choi, G. J., Oh, K. S., Lee, S. P., & Kang, H. (2023). Pain Assessment Using the Analgesia Nociception Index (ANI) in Patients Undergoing General Anesthesia: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 13(10), 1461. https://doi.org/10.3390/jpm13101461









