The Impact of Trophoblast Cell-Surface Antigen 2 Expression on the Survival of Patients with Gastrointestinal Tumors: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Databases and Search
2.3. Patients and Study Types
2.4. Study Outcomes
2.5. Quality Assessment
2.6. Protocol Registration
3. Results
3.1. Colorectal Cancer
3.2. Gastric Cancer
3.3. Pancreatic and Hepatobiliary Cancer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stepan, L.P.; Trueblood, E.S.; Hale, K.; Babcook, J.; Borges, L.; Sutherland, C.L. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: Potential implications as a cancer therapeutic target. J. Histochem. Cytochem. 2011, 59, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Cubas, R.; Zhang, S.; Li, M.; Chen, C.; Yao, Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol. Cancer 2010, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Groth, J.; Sossey-Alaoui, K.; Hawthorn, L.; Beall, S.; Geradts, J. Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin. Cancer Res. 2005, 11, 4357–4364. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Jia, L.; Bian, S.; Chang, X.; Zhang, Q.; Tang, Q.; Zhu, J.; Yang, Z.; Feng, Z. TROP2 Down-regulated DSG2 to Promote Gastric Cancer Cell Invasion and Migration by EGFR/AKT and DSG2/PG/β-Catenin Pathways. Curr. Cancer Drug Targets 2022, 22, 691–702. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, M.B.; Zhou, L.N.; Tang, M.; Liu, C.Y.; Lu, P.H. Impact of TROP2 expression on prognosis in solid tumors: A Systematic Review and Meta-analysis. Sci. Rep. 2016, 6, 33658. [Google Scholar] [CrossRef]
- Akarken, İ.; Dere, Y. Could trop-2 overexpression indicate tumor aggressiveness among prostatic adenocarcinomas? Ann. Diagn. Pathol 2021, 50, 151680. [Google Scholar] [CrossRef]
- Sin, S.T.K.; Li, Y.; Liu, M.; Ma, S.; Guan, X.Y. TROP-2 exhibits tumor suppressive functions in cervical cancer by dual inhibition of IGF-1R and ALK signaling. Gynecol. Oncol. 2019, 152, 185–193. [Google Scholar] [CrossRef]
- Zhang, K.; Jones, L.; Lim, S.; Maher, C.A.; Adkins, D.; Lewis, J.; Kimple, R.J.; Fertig, E.J.; Chung, C.H.; Van Tine, B.A.; et al. Loss of Trop2 causes ErbB3 activation through a neuregulin-1-dependent mechanism in the mesenchymal subtype of HNSCC. Oncotarget 2014, 5, 9281–9294. [Google Scholar] [CrossRef]
- Redlich, N.; Robinson, A.M.; Nickel, K.P.; Stein, A.P.; Wheeler, D.L.; Adkins, D.R.; Uppaluri, R.; Kimple, R.J.; Van Tine, B.A.; Michel, L.S. Anti-Trop2 blockade enhances the therapeutic efficacy of ErbB3 inhibition in head and neck squamous cell carcinoma. Cell Death Dis. 2018, 9, 5. [Google Scholar] [CrossRef]
- Omori, S.; Muramatsu, K.; Kawata, T.; Miyawaki, E.; Miyawaki, T.; Mamesaya, N.; Kawamura, T.; Kobayashi, H.; Nakashima, K.; Wakuda, K.; et al. Trophoblast cell-surface antigen 2 expression in lung cancer patients and the effects of anti-cancer treatments. J. Cancer Res. Clin. Oncol. 2022, 148, 2455–2463. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, 1. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Moser, P.; Krammel, C.; Gostner, J.M.; Margreiter, R.; Mitterer, M.; Gastl, G.; Spizzo, G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br. J. Cancer 2008, 99, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Ohmachi, T.; Tanaka, F.; Mimori, K.; Inoue, H.; Yanaga, K.; Mori, M. Clinical significance of TROP2 expression in colorectal cancer. Clin. Cancer Res. 2006, 12, 3057–3063. [Google Scholar] [CrossRef]
- Mühlmann, G.; Spizzo, G.; Gostner, J.; Zitt, M.; Maier, H.; Moser, P.; Gastl, G.; Zitt, M.; Müller, H.M.; Margreiter, R.; et al. TROP2 expression as prognostic marker for gastric carcinoma. J. Clin. Pathol. 2009, 62, 152–158. [Google Scholar] [CrossRef]
- Fang, Y.J.; Lu, Z.H.; Wang, G.Q.; Pan, Z.Z.; Zhou, Z.W.; Yun, J.P.; Zhang, M.F.; Wan, D.S. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int. J. Color. Dis. 2009, 24, 875–884. [Google Scholar] [CrossRef]
- Fang, Y.J.; Wang, G.Q.; Lu, Z.H.; Zhang, L.; Li, J.B.; Wu, X.J.; Ding, P.R.; Ou, Q.J.; Zhang, M.F.; Jiang, W.; et al. Different effects of ERβ and TROP2 expression in Chinese patients with early-stage colon cancer. Tumour Biol. 2012, 33, 2227–2235. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, H.Z.; Cai, J.H. Clinical investigation of TROP-2 as an independent biomarker and potential therapeutic target in colon cancer. Mol. Med. Rep. 2015, 12, 4364–4369. [Google Scholar] [CrossRef]
- Li, X.; Teng, S.; Zhang, Y.; Zhang, W.; Zhang, X.; Xu, K.; Yao, H.; Yao, J.; Wang, H.; Liang, X.; et al. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT. Oncotarget 2017, 8, 47052–47063. [Google Scholar] [CrossRef]
- Peng, J.; Ou, Q.; Deng, Y.; Xiao, B.; Zhang, L.; Li, J.; Li, Y.; Wan, D.; Lu, Z.; Fang, Y. TROP2 overexpression in colorectal liver oligometastases is associated with poor prognosis after liver resection. Ther. Adv. Med. Oncol. 2019, 11, 1758835919897543. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, S.; Yashiro, M.; Yamamoto, Y.; Sera, T.; Sugimoto, A.; Nishimura, S.; Togano, S.; Kuroda, K.; Yoshii, M.; Tamura, T.; et al. Clinicopathologic significance of TROP2 and phospho-TROP2 in gastric cancer. Mol. Clin. Oncol. 2021, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Trerotola, M.; Relli, V.; Lattanzio, R.; Tripaldi, R.; Vacca, G.; Ceci, M.; Boujnah, K.; Garbo, V.; Moschella, A.; et al. Trop-2 induces ADAM10-mediated cleavage of E-cadherin and drives EMT-less metastasis in colon cancer. Neoplasia 2021, 23, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Yokouchi, Y.; Kobayashi, M.; Ninomiya, H.; Sakakibara, R.; Subat, S.; Nagano, H.; Nomura, K.; Okumura, S.; Shibutani, T.; et al. Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget 2017, 8, 28725–28735. [Google Scholar] [CrossRef]
- Italiano, A.; Leroy, L.; Guegan, J.-P.; Cousin, S.; Peyraud, F.; Cabart, M.; Chomy, F.; Vanhersecke, L.; Bessede, A. TROP2 expression and response to immune checkpoint inhibition in patients with advanced non-small cell lung cancer. J. Clin. Oncol. 2023, 41, 9040-9040. [Google Scholar] [CrossRef]
- Izci, H.; Punie, K.; Waumans, L.; Laenen, A.; Wildiers, H.; Verdoodt, F.; Desmedt, C.; Ardui, J.; Smeets, A.; Han, S.N.; et al. Correlation of TROP-2 expression with clinical-pathological characteristics and outcome in triple-negative breast cancer. Sci. Rep. 2022, 12, 22498. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, F.; Fornili, M.; Boracchi, P.; Trerotola, M.; Relli, V.; Simeone, P.; La Sorda, R.; Lattanzio, R.; Querzoli, P.; Pedriali, M.; et al. Trop-2 is a determinant of breast cancer survival. PLoS ONE 2014, 9, e96993. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Z.; Zhu, J.; Xu, L.; Li, Y.; Duan, L.; Mao, Y.; Li, H. Overexpression of trophoblast cell surface antigen 2 as an independent marker for a poor prognosis and as a potential therapeutic target in epithelial ovarian carcinoma. Int. J. Exp. Pathol. 2016, 97, 150–158. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.; Filetti, M.; Falcone, R.; Altamura, V.; Paroni Sterbini, F.; Bria, E.; Fabi, A.; Giannarelli, D.; Scambia, G.; Daniele, G. Overview of Trop-2 in Cancer: From Pre-Clinical Studies to Future Directions in Clinical Settings. Cancers 2023, 15, 1744. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Jadid, H.; Denson, A.C.; Gray, J.E. Targeting Trop-2 in solid tumors: Future prospects. Onco Targets Ther. 2019, 12, 1781–1790. [Google Scholar] [CrossRef] [PubMed]

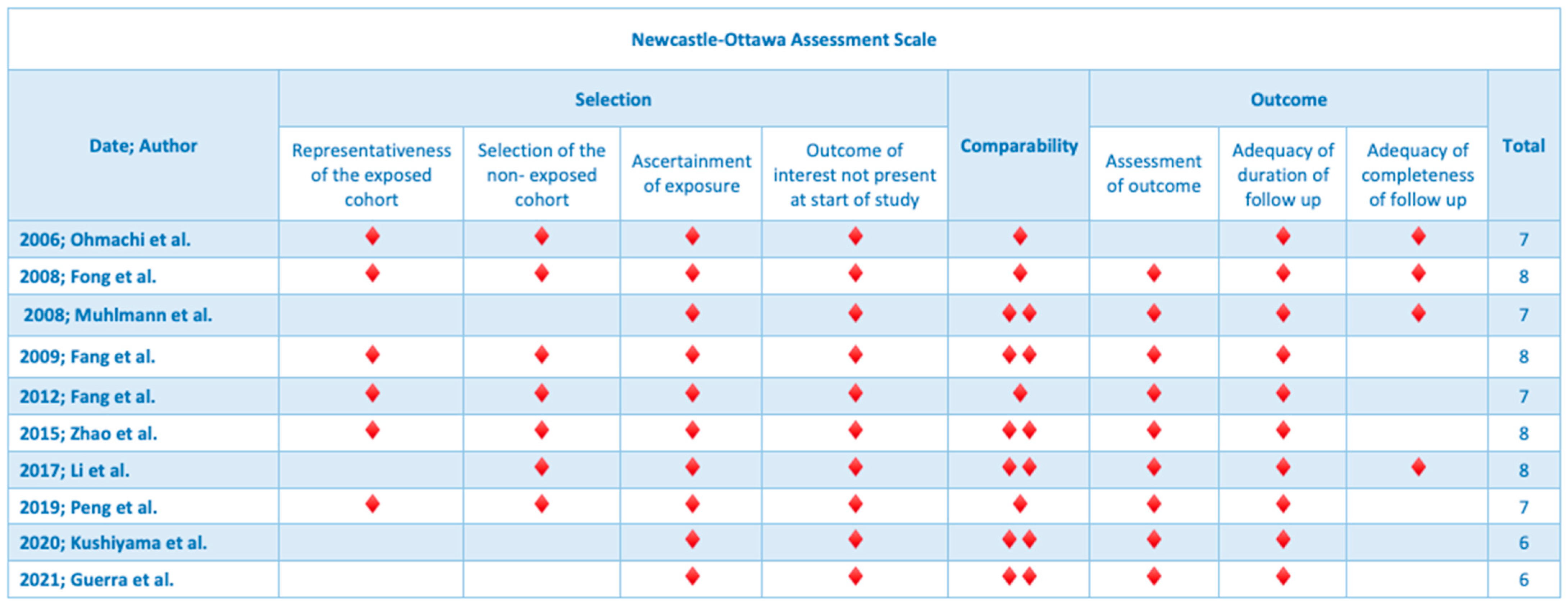
| Year; Author | Type of Study | Center/Country | Number of Patients | Age (Mean, Range) | Gender |
|---|---|---|---|---|---|
| 2006; Ohmachi et al. | Retrospective | Department of Surgery and Molecular Oncology, Medical Institute of Bioregulation, Kyushu University | N = 16 | 67 (33–84) | Males: 44 Female: 30 |
| 2008; Fong et al. | Retrospective | Department of Hematology and Oncology/Austria | N = 197 | 67 (37–91) | Males: 111 Female: 86 |
| 2008; Muhlmann et al. | Retrospective | Center of Operative Medicine, Department of Visceral, Transplant and Thoracic Surgery, Innsbruck Medical University | N = 104 | 70 (30–94) | Males: 70 Female: 34 |
| 2009; Fang et al. | Retrospective | Department of Colorectal Surgery, State Key Laboratory of Oncology in Southern China | N = 620 | 59 (15–86) | Males: 364 Female: 256 |
| 2012; Fang et al. | Retrospective | Department of Colorectal Surgery, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center | N = 220 | 59 (7–86) | Male: 130 Female: 90 |
| 2015; Zhao et al. | Retrospective | Department of Surgery, Hebei Medical University | N = 82 | 59.8 (35–90) | Male: 53 Female: 30 |
| 2017; Li et al. | Retrospective | Department of General Surgery, Changzheng Hospital, The Second Military Medical University | N = 105 | NA | Male:30 Female: 75 |
| 2019; Peng et al. | Retrospective | Department of Colorectal Surgery, Department of Experimental Research/China | N = 129 | 58 (25–78) | Males: 78 Female:51 |
| 2020; Kushiyama et al. | Retrospective | Departments of Gastroenterological Surgery, and Molecular Oncology and Therapeutics | N = 740 | ΝA | Males: 431 Female: 309 |
| 2021; Guerra et al. | Retrospective | Laboratory of Cancer Pathology, Center for Advanced Studies and Technology (CAST) | N = 80 | 72 (30–88) | Males: NA Female: NA |
| Year; Author | Type of Cancer | Stage | Surgery | Margins | Metastasis | Systematic Therapy |
|---|---|---|---|---|---|---|
| 2006; Ohmachi et al. | Colorectal cancer | NA | Yes: 34 | NA | Yes: 13 No: 61 | NA |
| 2008; Fong et al. | Pancreatic ductal adenocarcinoma | Ia: 6 Ib: 24 IIa: 34 IIb: 70 III: 20 IV: 26 | Yes: 143 No: 54 | R0: 86 R1: 42 | Yes: 25 No: 117 | NA |
| 2008; Muhlmann et al. | Gastric cancer | Ia: 15 Ib: 22 II: 30 III:19 IV: 18 | Yes: 104 Total gastrectomy: 47 Subtotal gastrectomy: 57 | R0: 97 R1: 7 | Yes:10 No: 94 | Neoadjuvant: 6 |
| 2009; Fang et al. | Colorectal cancer | I: 86 IIa: 146 IIb: 110 IIIa: 10 IIIb: 106 IIIc: 24 IV: 124 | Yes: 620 | NA | Yes: 225 No: 395 | Neoadjuvant: 311 Adjuvant: 533 |
| 2012; Fang et al. | Colorectal cancer | IIb: 220 | Yes: 220 | NA | NA | NA |
| 2015; Zhao et al. | Colorectal cancer | NA | Yes: 82 | NA | NA | 0 |
| 2017; Li et al. | Gallbladder cancer | I–II: 38 III–IV: 67 | Yes: 105 | NA | NA | NA |
| 2019; Peng et al. | Colorectal primary tumor and liver metastasis | NA | Yes: 129 | NA | Yes: 129 | Neoadjuvant: 31 Adjuvant: 87 Radiation: 5 |
| 2020; Kushiyama et al. | Gastric carcinoma | NA | Yes: 740 | NA | Yes: 30/No: 710 | 0 |
| 2021; Guerra et al. | Colorectal cancer metastasis | NA | Yes: 80 | NA | Yes: 43/No: 37 | NA |
| Year; Author | Type of Biopsy | Type of TROP-2 Antibody | TROP-2 Score | Follow-Up (Median) | Primary Outcome |
|---|---|---|---|---|---|
| 2006; Ohmachi et al. | Formalin-fixed, paraffin-embedded | Purified recombinant human TROP-2 extracellular domain (R&D Systems, Inc., Minneapolis, MN, USA) (5 Ag/mL) | NA | NA | Overall survival |
| 2008; Fong et al. | Formalin-fixed, paraffin-embedded | Purified goat polyclonal antibody detecting the recombinant human TROP-2 extracellular domain at a dilution of 1:50 | Proportion: 0: none, 1: <10%, 2: 10–50%, 3: 51–80%, 4: >80%) | NA | Overall survival |
| Intensity: 0, 1: no staining, 2: weak, 3: moderate, 4: strong) | |||||
| 2008; Muhlmann et al. | Formalin-fixed, paraffin-embedded | Recombinant human TROP-2 extracellular domain at a dilution of 1:50 (AF650, R&D Systems, Minneapolis, MN, USA) | Proportion: 0: none, 1: 10%; 2: 10–50%; 3: 51–80%; 4: 80% | NA | Overall survival |
| Intensity: 0: no staining, 1: weak, 2: moderate, 3: strong | |||||
| 2009; Fang et al. | Formalin-fixed, paraffin-embedded | Dilution of antibody for staining was 1:10 for TROP-2 (monoclonal goat, R&D Systems, Inc.) | Proportion: 0: none, 1: <10%, 2: 10–50%, 3: >50% | 52 months (range 1–130 months) | Overall survival |
| Intensity: 0: none, 1: weak, 2: moderate, 3: strong | |||||
| 2012; Fang et al. | Formalin-fixed, paraffin-embedded | Dilution of antibody for staining was 1:50 for TROP-2 (monoclonal goat, R&D systems, Inc.) | Proportion: 0: none, 1: <10%, 2: 10–50%, 3: >50% | 103 months (range, 1–167 months) | Overall survival |
| Intensity: 0: negative, 1: weak, 2: moderate, 3: positive | |||||
| 2015; Zhao et al. | Formalin-fixed, paraffin-embedded | Rabbit anti-human TROP-2 polyclonal antibody (1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) | NA | NA | Lymph node metastasis |
| 2017; Li et al. | Formalin-fixed, paraffin-embedded | NA | NA | NA | Overall survival |
| 2019; Peng et al. | Formalin-fixed, paraffin-embedded | A primary TROP-2 antibody (1:500 dilution, ab214488; Abcam, Cambridge, UK) | Proportion: 0: <5%, 1: 5–24%, 2: 25–49%, 3: 50–74%, 4: 75–100% | 35 months (2–143) | Overall survival |
| Intensity: 0: negative, 1: weak, 2: moderate, 3: strong | |||||
| 2020; Kushiyama et al. | Formalin-fixed, paraffin-embedded | Anti-mouse antibody for TROP-2 (1:250, sc-376746, Santa Cruz Biotechnology) | Proportion: 0: 0%, 1: 1–30%, 2: 31–70%, 3: 71–100% | NA | Overall survival |
| Intensity: 0, 0: 1, weak: 2: moderate, 3: strong | |||||
| 2021; Guerra et al. | Formalin-fixed, paraffin-embedded | Anti-goat (sc-2020; Santa Cruz Biotechnology) pAbs | NA | 400 months | Overall survival |
| Year; Author | TROP-2 Expression | Overall Survival (Median, Range) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| TROP-2 (−) | TROP-2 (+) | RR (95% C.I.) | RR (95% C.I.) | |||
| 2006; Ohmachi et al. | Yes: 34 Strong: 7 Moderate: 9 Weak: 18 | No: 0 | NA | NA | 2.02 (95% 1.22–3.50), p = 0.007 | 2.38 (95% 1.29–4.74), p = 0.005 |
| 2008; Fong et al. | Yes: 109 Strong: 57 Moderate: 52 Low: 76 0: 12 | No: 88 | 14 months | 8 months (p < 0.01) | NA | 1.8 (95% 1.1–3.1), p < 0.01 |
| 2008; Muhlmann et al. | Yes: 58 | No: 46 | 52 months (range 1–163): intestinal-type carcinoma 16 months (range 1–54): diffuse-type carcinoma | p = 0.97 | 6.3 (2.2 to 18.5) | |
| 2009; Fang et al. | Yes: 155 | No: 465 | NA | NA | 1.43 (95% 1.04–1.97), p = 0.03 | 1.14 (95% 0.55–1.38) |
| 2012; Fang et al. | NA | NA | NA | NA | 0.959 (95% 0.897–1.026) | NA |
| 2015; Zhao et al. | Yes: 75 | No: 7 | NA | NA | NA | NA |
| 2017; Li et al. | Yes: 65 | No: 40 | NA | NA | 0.259 (0.163–0.412), p = 0.000 | 0.463 (0.274–0.782), p = 0.004 |
| 2019; Peng et al. | Yes: metastasis 65/129 primary tumor 49/70 | No: metastasis 64/129 primary tumor 21/70 | NA | NA | 2.090 (95% 1.037–4.214) | 2.090 (95% 1.037–4.214), p < 0.039 |
| 2020; Kushiyama et al. | Yes: 330 Strong: 23 Moderate: 35 Moderate to strong: 58 | No: 410 | NA | NA | 1.562 (1.221–1.998), p = 0.0004 | 1.249 (0.959–1.628), p = 0.0994 |
| 2021; Guerra et al. | Yes: 65 High: 40 | No: 15 | NA | NA | 1.96, p = 0.00058 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liatsou, E.; Schizas, D.; Frountzas, M. The Impact of Trophoblast Cell-Surface Antigen 2 Expression on the Survival of Patients with Gastrointestinal Tumors: A Systematic Review. J. Pers. Med. 2023, 13, 1445. https://doi.org/10.3390/jpm13101445
Liatsou E, Schizas D, Frountzas M. The Impact of Trophoblast Cell-Surface Antigen 2 Expression on the Survival of Patients with Gastrointestinal Tumors: A Systematic Review. Journal of Personalized Medicine. 2023; 13(10):1445. https://doi.org/10.3390/jpm13101445
Chicago/Turabian StyleLiatsou, Efstathia, Dimitrios Schizas, and Maximos Frountzas. 2023. "The Impact of Trophoblast Cell-Surface Antigen 2 Expression on the Survival of Patients with Gastrointestinal Tumors: A Systematic Review" Journal of Personalized Medicine 13, no. 10: 1445. https://doi.org/10.3390/jpm13101445
APA StyleLiatsou, E., Schizas, D., & Frountzas, M. (2023). The Impact of Trophoblast Cell-Surface Antigen 2 Expression on the Survival of Patients with Gastrointestinal Tumors: A Systematic Review. Journal of Personalized Medicine, 13(10), 1445. https://doi.org/10.3390/jpm13101445








