Neutrophil-to-Lymphocyte Ratio as an Early Predictor of Symptomatic Anastomotic Leakage in Patients after Rectal Cancer Surgery: A Propensity Score-Matched Analysis
Abstract
1. Introduction
2. Methods
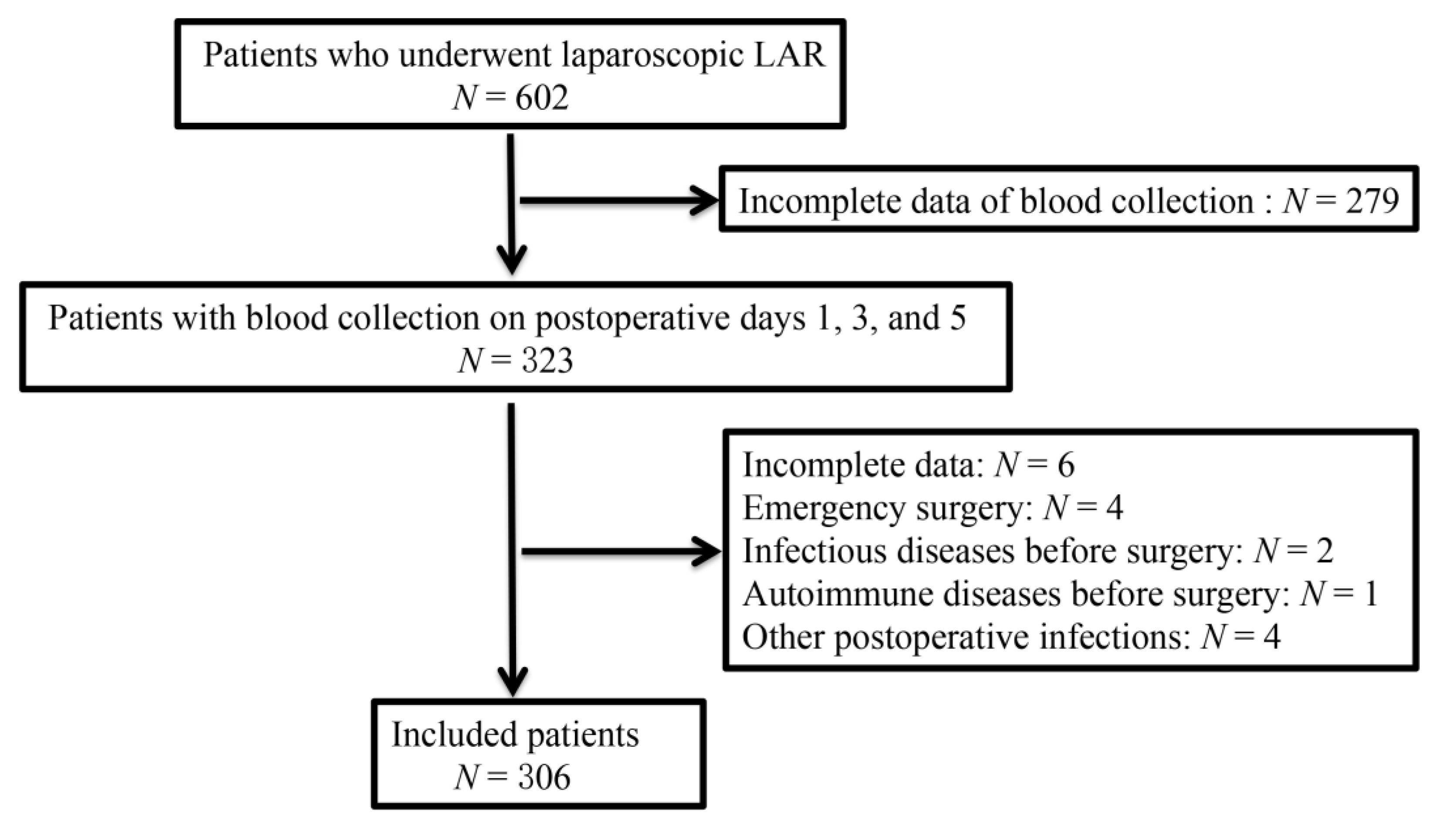
2.1. Patients
2.2. Inclusion and Exclusion Criteria
2.3. Surgical Procedure and Laboratory Testing
2.4. Definition
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Index in the Original Data
3.2. Matching of Covariates Using PSM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sciuto, A.; Merola, G.; De Palma, G.D.; Sodo, M.; Pirozzi, F.; Bracale, U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J. Gastroenterol. 2018, 24, 2247–2260. [Google Scholar] [CrossRef]
- Kang, C.Y.; Halabi, W.J.; Chaudhry, O.O.; Nguyen, V.; Pigazzi, A.; Carmichael, J.C.; Mills, S.; Stamos, M.J. Risk Factors for Anastomotic Leakage After Anterior Resection for Rectal Cancer. JAMA Surg. 2013, 148, 65–71. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Q.; Jindou, L.; Cheng, Y. The influence of anastomotic leakage for rectal cancer oncologic outcome: A systematic review and meta-analysis. J. Surg. Oncol. 2020, 121, 1283–1297. [Google Scholar] [CrossRef]
- Kehlet, H. Fast-Track Colonic Surgery: Status and Perspectives. Rectal Cancer Treat. 2005, 165, 8–13. [Google Scholar] [CrossRef]
- Gessler, B.; Eriksson, O.; Angenete, E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int. J. Color. Dis. 2017, 32, 549–556. [Google Scholar] [CrossRef]
- Sparreboom, C.L.; Van Groningen, J.T.; Lingsma, H.F.; Wouters, M.; Menon, A.G.; Kleinrensink, G.-J.; Jeekel, J.; Lange, J.F. Different Risk Factors for Early and Late Colorectal Anastomotic Leakage in a Nationwide Audit. Dis. Colon Rectum 2018, 61, 1258–1266. [Google Scholar] [CrossRef]
- den Dulk, M.; Noter, S.L.; Hendriks, E.R.; Brouwers, M.A.; van der Vlies, C.H.; Oostenbroek, R.J.; Menon, A.G.; Steup, W.H.; van de Velde, C.J. Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur. J. Surg. Oncol. 2009, 35, 420–426. [Google Scholar] [CrossRef]
- Nora, I.; Shridhar, R.; Huston, J.; Meredith, K. The accuracy of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as a marker for gastrointestinal malignancies. J. Gastrointest. Oncol. 2018, 9, 972–978. [Google Scholar] [CrossRef]
- Li, M.-X.; Liu, X.-M.; Zhang, X.-F.; Zhang, J.-F.; Wang, W.-L.; Zhu, Y.; Dong, J.; Cheng, J.-W.; Liu, Z.-W.; Ma, L.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int. J. Cancer 2013, 134, 2403–2413. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; He, G.; Li, X.; Zhan, F. Dynamics of neutrophil-to-lymphocyte ratio predict outcomes of metastatic colorectal carcinoma patients treated by FOLFOX. J. Gastrointest. Oncol. 2021, 12, 2846–2853. [Google Scholar] [CrossRef]
- Josse, J.M.; Cleghorn, M.C.; Ramji, K.M.; Jiang, H.; Elnahas, A.; Jackson, T.D.; Okrainec, A.; Quereshy, F.A. The neutrophil-to-lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Color. Dis. 2016, 18, O236–O242. [Google Scholar] [CrossRef]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; Beamishaj; Bashashati, M.; Millham, F.H.; Orgill, D.P.; et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, H.; Zheng, Z.; Liang, J.; Zhou, Z.; Wang, X. Predictive risk factors for anastomotic leakage after anterior resection of rectal cancer in elderly patients over 80 years old: An analysis of 288 consecutive patients. World, J. Surg. Oncol. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. International Union Against Cancer. In TNM Classification of Malignant Tumors, 8th ed.; Wiley: Chichester, UK, 2017. [Google Scholar]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Caputo, D.; Caricato, M.; Coppola, A.; La Vaccara, V.; Fiore, M.; Coppola, R. Neutrophil to Lymphocyte Ratio (NLR) and Derived Neutrophil to Lymphocyte Ratio (d-NLR) Predict Non-Responders and Postoperative Complications in Patients Undergoing Radical Surgery After Neo-Adjuvant Radio-Chemotherapy for Rectal Adenocarcinoma. Cancer Investig. 2016, 34, 440–451. [Google Scholar] [CrossRef]
- Andersson, B.; Ansari, D.; Nordén, M.; Nilsson, J.; Andersson, R. Surgical Stress Response After Colorectal Resection. Int. Surg. 2013, 98, 292–299. [Google Scholar] [CrossRef]
- Pantoja Pachajoa, D.A.; Gielis, M.; Palacios Huatuco, R.M.; Benitez, M.N.; Avila, M.N.; Doniquian, A.M.; Alvarez, F.A.; Parodi, M. Neutrophil-to-lymphocyte ratio vs C-reactive protein as early predictors of anastomotic leakage after colorectal surgery: A retrospective cohort study. Ann. Med. Surg. 2021, 64, 102201. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Deidda, S.; Maslyankov, S.; Paycheva, T.; Farag, A.; Mashhour, A.; Misiakos, E.; Papakonstantinou, D.; Mik, M.; Losinska, J.; et al. Blood cell count indexes as predictors of anastomotic leakage in elective colorectal surgery: A multicenter study on 1432 patients. World J. Surg. Oncol. 2020, 18, 1–8. [Google Scholar] [CrossRef]
- Mik, M.; Dziki, L.; Berut, M.; Trzcinski, R.; Dziki, A. Neutrophil to Lymphocyte Ratio and C-Reactive Protein as Two Predictive Tools of Anastomotic Leak in Colorectal Cancer Open Surgery. Dig. Surg. 2017, 35, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Qasem, E.; Dilaver, N.; Egan, R.; Bodger, O.; Kokelaar, R.; Evans, M.D.; Davies, M.; Beynon, J.; Harris, D. Inflammatory cell ratios predict major septic complications following rectal cancer surgery. Int. J. Color. Dis. 2018, 33, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.A.; Kunjuraman, B.; Bartolo, D.C.C. Neutrophil-to-lymphocyte ratio predicts anastomotic dehiscence. ANZ J. Surg. 2018, 88, E573–E577. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Lampe, P.; Mrowiec, S. The influence of nutritional status on the incidence of postoperative complications in patients following distal pancreatectomy. Gastroenterol. Rev. 2020, 15, 65–75. [Google Scholar] [CrossRef]
| Variable | All Patients | Anastomotic Leakage (N = 17) | Non-Anastomotic Leakage (N = 289) | p Value |
|---|---|---|---|---|
| Gender (n) | 0.008 * | |||
| Male | 176 | 15 (88.2) | 161 (55.7) | |
| Female | 130 | 2 (11.8) | 128 (44.3) | |
| Age (median with 95% CI), years | 49 (44.73–58.33) | 61 (59.32–61.89) | 0.002 * | |
| Distance from the anal verge (median with 95% CI), cm | 7 (5.91–7.98) | 10 (8.77–9.42) | 0.001 * | |
| Body mass index (median with 95% CI) kg/m2 | 22.64 (21.85–25.69) | 24.22 (23.73–24.54) | 0.675 | |
| Neoadjuvant therapy (n) | 4 (23.5) | 47 (16.3) | 0.655 | |
| American Society of Anesthesiologists score category (n) | 0.746 | |||
| I | 43 | 3 (17.7) | 40 (13.8) | |
| II | 234 | 12 (70.6) | 222 (76.8) | |
| III | 29 | 2 (11.8) | 27 (9.4) | |
| Smoking (n) | 6 (35.3) | 89 (30.8) | 0.697 | |
| Alcohol consumption (n) | 5 (29.4) | 48 (16.6) | 0.305 | |
| Previous history of abdominal surgery (n) | 2 (11.8) | 52 (18.0) | 0.743 | |
| Preoperative intestinal obstruction (n) | 0 (0) | 29 (10.0) | 0.344 | |
| Hypertension (n) | 2 (11.8) | 79 (27.3) | 0.258 | |
| Diabetes (n) | 0 (0) | 34 (11.8) | 0.270 | |
| Pulmonary insufficiency (n) | 5 (29.4) | 67 (23.2) | 0.769 | |
| Preoperative hemoglobin (median with 95% CI), g/L | 146 (127.61–150.74) | 134 (130.3–135) | 0.101 | |
| Preoperative white blood cell (median with 95% CI), 109/L | 6.49 (5.71–7.18) | 5.98 (6.03–7.25) | 0.622 | |
| Preoperative serum albumin (median with 95% CI), g/L | 46.7 (40.59–47.81) | 44.10 (42.63–43.91) | 0.077 | |
| Preoperative CEA (median with 95% CI), ng/ml | 3.13 (−3.30–25.95) | 3.58 (9.60–18.65) | 0.582 | |
| Duration of operation (median with 95% CI) (min) | 182 (185.56–232.91) | 190 (187.32–202.51) | 0.204 | |
| Intraoperative blood loss (median with 95% CI) (mL) | 50 (44.63–100.07) | 50 (51.09–66.11) | 0.075 | |
| Defunctioning stoma (n) | 7 (41.2) | 135 (46.7) | 0.582 | |
| Pathology type of tumor (n%) | 0.174 | |||
| Adenocarcinoma | 292 | 16 (94.1) | 276 (95.5) | |
| Mucinous carcinoma | 11 | 0 (0) | 11 (3.8) | |
| Signet-ring cell | 3 | 1 (5.9) | 2 (0.7) | |
| Size of tumor (median with 95% CI), cm | 3 (3.12–5.47) | 4 (3.61–3.96) | 0.570 | |
| Tumor differentiation (n) | 0.452 | |||
| Well | 21 | 1 (5.9) | 20 (6.9) | |
| Moderate | 235 | 12 (70.5) | 223 (77.2) | |
| Poor | 10 | 1 (5.9) | 9 (3.1) | |
| Well-moderate | 7 | 1 (5.9) | 6 (2.1) | |
| Moderate-poor | 33 | 2 (11.8) | 31 (10.7) | |
| Tumor type (n%) | 0.841 | |||
| Ulcer | 196 | 10 (58.8) | 186 (64.4) | |
| Uplift | 89 | 6 (35.3) | 83 (28.7) | |
| Infiltrating | 21 | 1 (5.9) | 20 (6.9) | |
| Pathological tumor (T) category (n) | 0.294 | |||
| T0 | 7 | 0 (0) | 7 (2.4) | |
| T1 | 21 | 1 (5.9) | 20 (6.9) | |
| T2 | 61 | 6 (35.3) | 55 (19) | |
| T3 | 169 | 6 (35.3) | 163 (56.4) | |
| T4 | 48 | 4 (23.5) | 44 (15.3) | |
| Pathological node (N) category (n) | 0.511 | |||
| N0 | 172 | 11 (64.7) | 161 (55.7) | |
| N1 | 80 | 5 (29.4) | 75 (26.00) | |
| N2 | 54 | 1 (5.9) | 53 (18.3) | |
| Pathological metastasis (M) category (n) | 0.138 | |||
| M0 | 294 | 15 (88.2) | 279 (96.5) | |
| M1 | 12 | 2 (11.8) | 10 (3.5) |
| Variable | Patients | Postoperative 1 Day | Postoperative 3 Day | Postoperative 5 Day |
|---|---|---|---|---|
| WBC (median with 95% CI) 109/L | Non-AL | 8.77 (8.77–9.61) | 7.53 (7.44–8.19) | 6.82 (6.99–7.72) |
| AL | 10.06 (9.15–10.02) | 8.66 (7.58–11.07) | 10.12 (7.85–12.23) | |
| p value | 0.054 | 0.042 * | 0.001 * | |
| Neutrophils (median with 95% CI) 109/L | Non-AL | 7.34 (7.36–8.09) | 5.32 (5.40–6.19) | 4.81 (5.02–5.71) |
| AL | 8.46 (6.80–10.53) | 6.71 (5.70–9.52) | 7.25 (6.16–10.41) | |
| p value | 0.134 | 0.042 * | <0.001 * | |
| Lymphocytes (median with 95% CI) 109/L | Non-AL | 0.84 (0.83–1.04) | 1.08 (1.03–1.30) | 1.22 (1.14–1.29) |
| AL | 1.01 (0.72–1.28) | 0.88 (0.71–1.43) | 0.84 (0.71–1.06) | |
| p value | 0.505 | 0.314 | 0.004 * | |
| Monocytes (median with 95% CI) 109/L | Non-AL | 0.46 (0.47–0.53) | 0.51 (0.51–0.60) | 0.55 (0.53–0.67) |
| AL | 0.58 (0.49–0.69) | 0.50 (0.42–0.69) | 0.53 (0.47–0.75) | |
| p value | 0.025 * | 0.705 | 0.823 | |
| Platelets (median with 95% CI) 109/L | Non-AL | 171 (169.67–188.06) | 180 (173.21–189.71) | 198 (198.98–216.22) |
| AL | 173 (156.04–200.20) | 170 (148.77–212.28) | 178 (145.19–208.70) | |
| p value | 0.745 | 0.931 | 0.062 | |
| NLR (median with 95% CI) 109/L | Non-AL | 8.76 (9.50–11.49) | 4.81 (5.58–7.13) | 4.16 (4.87–6.34) |
| AL | 10.62 (7.76–13.67) | 8.25 (6.44–16.49) | 9.98 (7.62–14.36) | |
| p value | 0.497 | 0.056 | <0.001 * | |
| LMR (median with 95% CI) 109/L | Non-AL | 1.78 (1.87–2.50) | 2.06 (2.16–2.67) | 2.13 (2.16–2.53) |
| AL | 1.77 (1.22–3.00) | 1.60 (1.46–2.58) | 1.77 (1.29–2.04) | |
| p value | 0.429 | 0.155 | 0.014 * | |
| PLR (median with 95% CI) 109/L | Non-AL | 200 (216.66–255.79) | 159.43 (175.17–206.47) | 170.68 (185.68–220.65) |
| AL | 187.60 (141.07–343.70) | 193.18 (162.02–311.79) | 163.70 (163.54–330.59) | |
| p value | 0.472 | 0.458 | 0.382 |
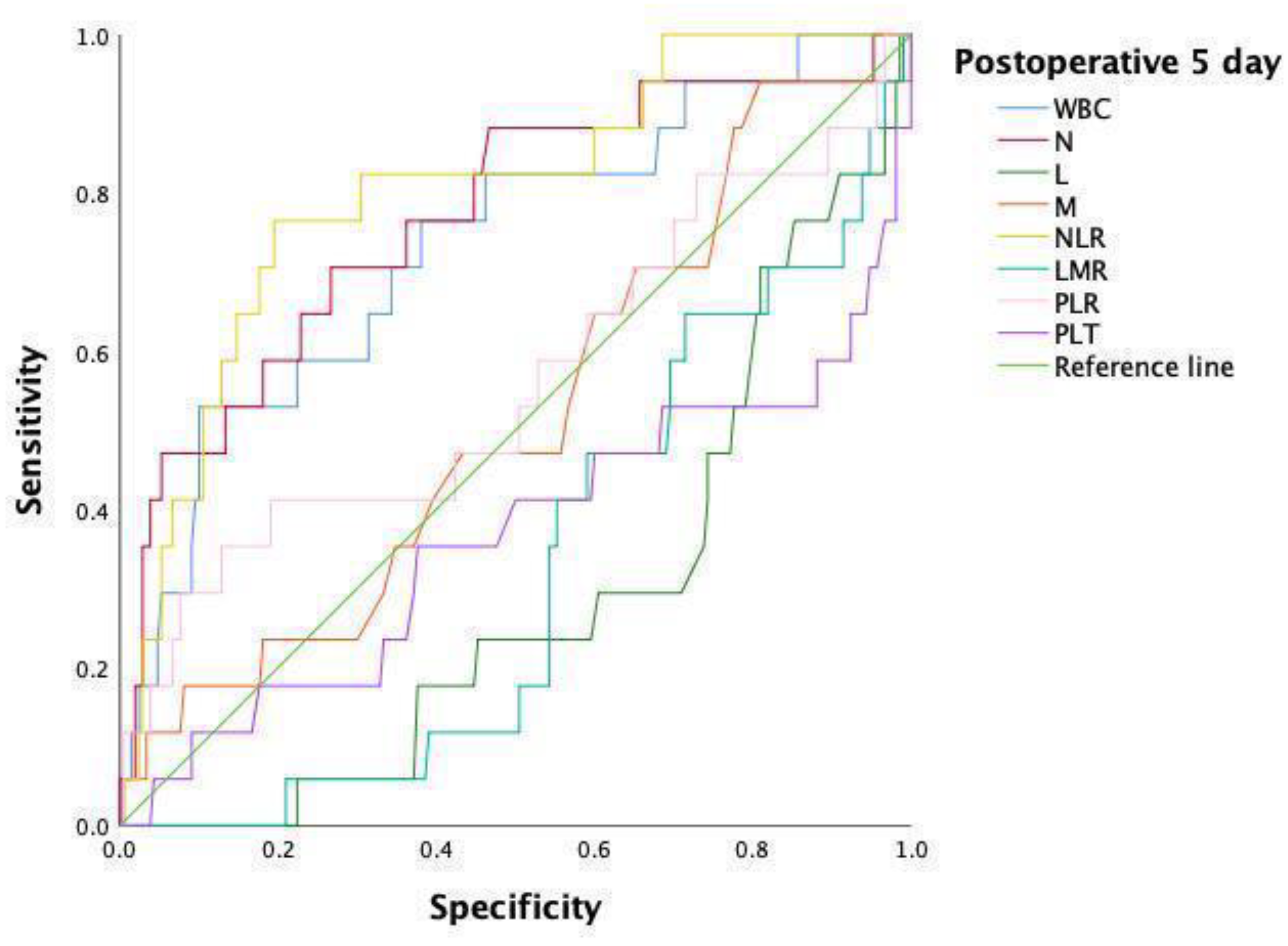
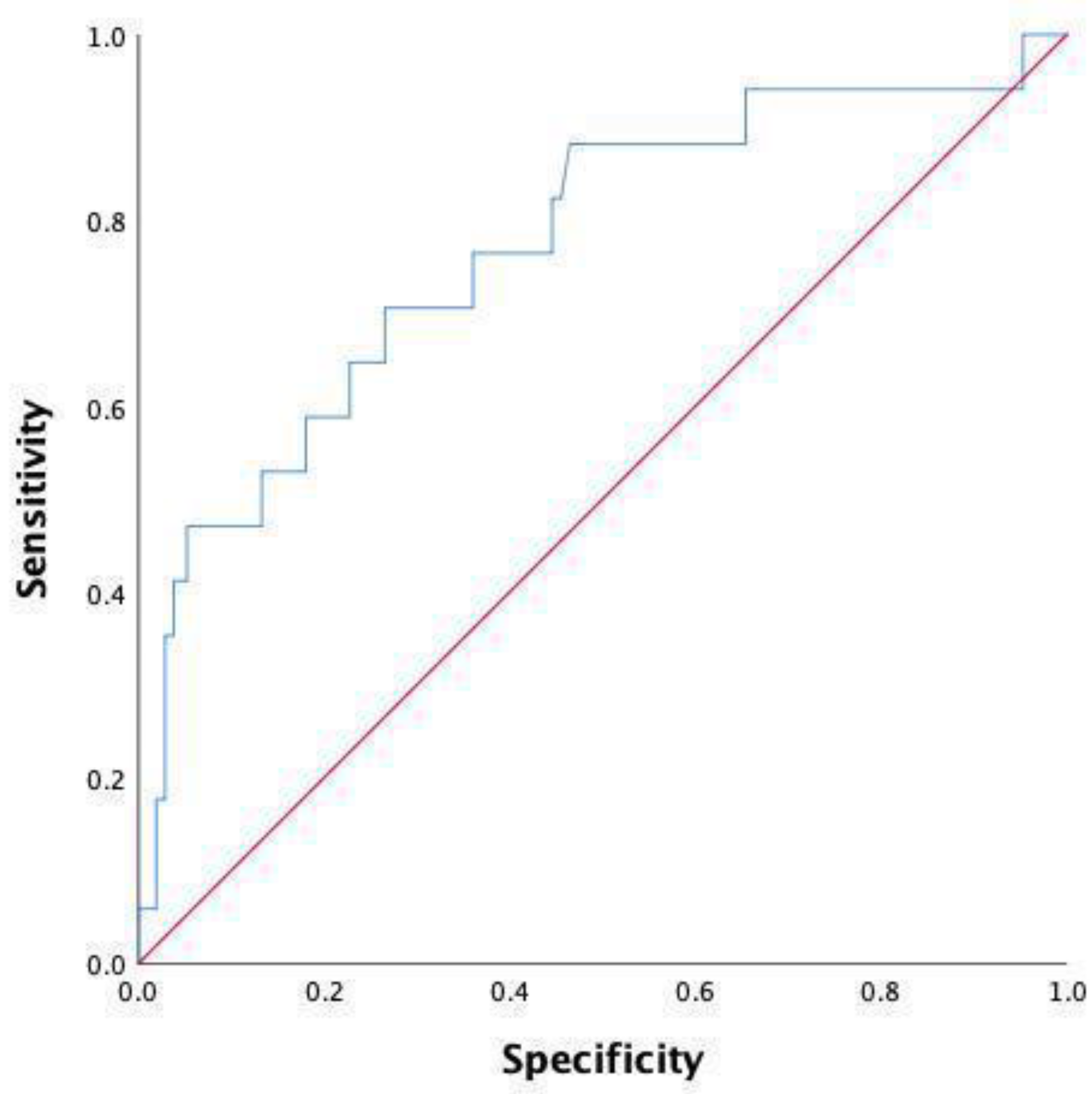
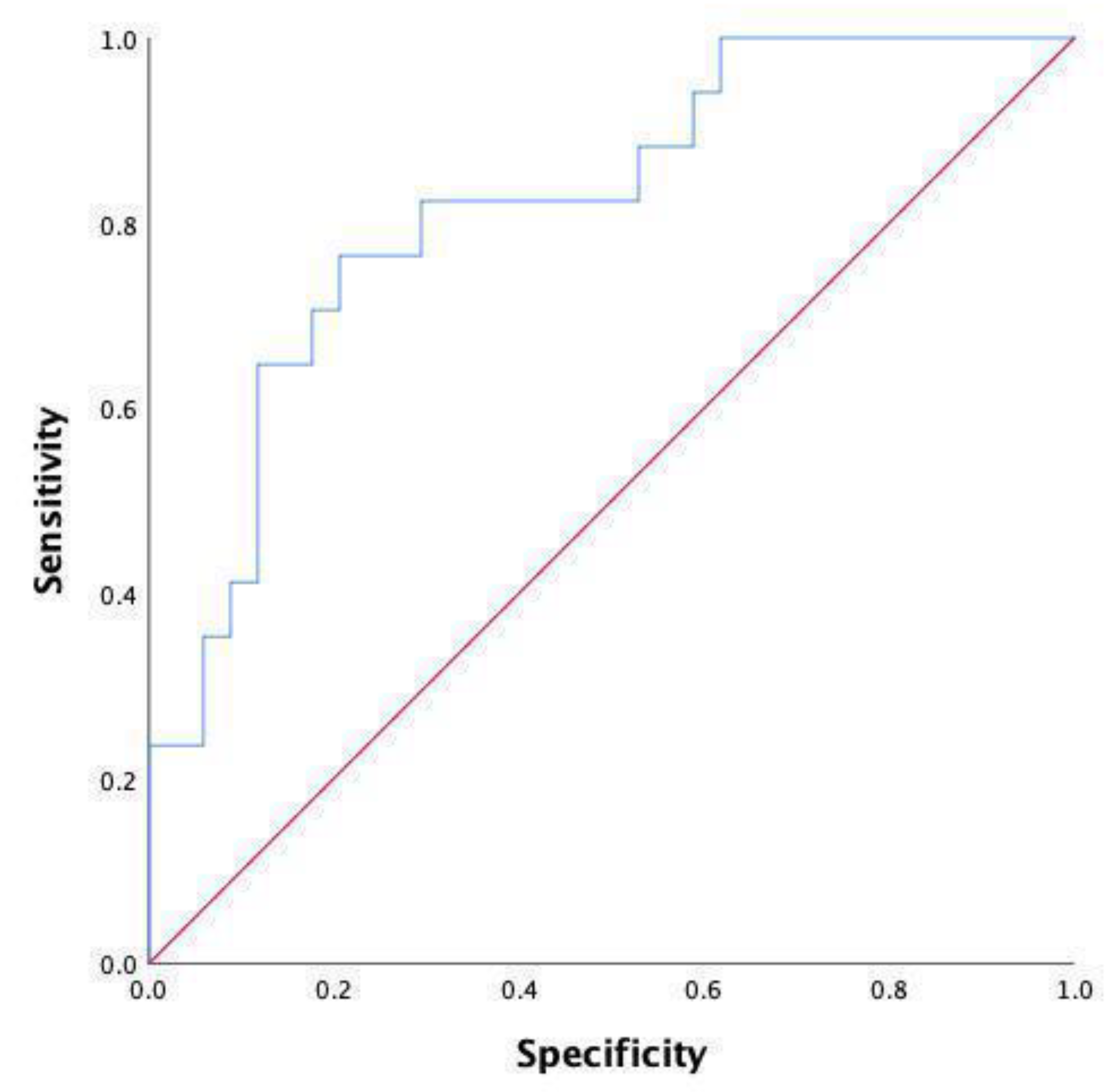
| Variable | AUC | 95% CI | p Value | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| WBC | 0.736 | 0.606–0.865 | 0.001 * | 10.11 | 52.9 | 90 |
| N | 0.770 | 0.642–0.898 | <0.001 * | 6.19 | 70.6 | 73.3 |
| NLR | 0.802 | 0.692–0.912 | <0.001 * | 6.97 | 76.5 | 80.5 |
| NLR and N | 0.771 | 0.643–0.899 | <0.001 * | 0.07 | 70.6 | 73.5 |
| Variable | All Patients | Anastomotic Leakage (N = 16) | Non-Anastomotic Leakage (N = 45) | p Value |
|---|---|---|---|---|
| Gender (n) | 0.156 | |||
| Male | 43 | 14 (87.5) | 29 (64.4) | |
| Female | 18 | 2 (12.5) | 16 (35.6) | |
| Age (median with 95% CI), years | 49 (45.46–59.41) | 51 (51.19–57.88) | 0.393 | |
| Distance from the anal verge (median with 95% CI), cm | 7.5 (5.99–8.14) | 6 (6.21–7.48) | 0.524 | |
| Body mass index (median with 95% CI) kg/m2 | 22.88 (21.91–25.95) | 24.75 (23.76–25.91) | 0.395 | |
| Neoadjuvant therapy (n) | 3 (18.8) | 14 (31.1) | 0.534 | |
| American Society of Anesthesiologists score category (n) | 0.500 | |||
| I | 7 | 3 (18.8) | 4 (8.9) | |
| II | 48 | 11 (68.7) | 37 (82.2) | |
| III | 6 | 2 (12.5) | 4 (8.9) | |
| Smoking (n) | 6 (37.5) | 15 (33.3) | 0.763 | |
| Alcohol consumption (n) | 5 (31.3) | 6 (13.3) | 0.222 | |
| Previous history of abdominal surgery (n) | 2 (12.5) | 8 (17.8) | 0.923 | |
| Preoperative intestinal obstruction (n) | 0 (0) | 1 (2.2) | 1.000 | |
| Hypertension (n) | 2 (12.5) | 9 (20) | 0.771 | |
| Diabetes (n) | 0 (0) | 3 (6.7) | 0.560 | |
| Pulmonary insufficiency (n) | 5 (31.3) | 13 (28.9) | 1.000 | |
| Preoperative hemoglobin (median with 95% CI), g/L | 143 (126.36–151.01) | 132 (123.10–134.90) | 0.064 | |
| Preoperative white blood cell (median with 95% CI), 109/L | 6.58 (5.74–7.28) | 5.73 (3.92–11.27) | 0.121 | |
| Preoperative serum albumin (median with 95% CI), g/L | 46.60 (40.11–47.72) | 45.40 (40.72–45.15) | 0.446 | |
| Preoperative CEA (median with 95% CI), ng/ml | 2.94 (−4.86–26.34) | 3.42 (0.35–25.55) | 0.909 | |
| Duration of operation (median with 95% CI) (min) | 200 (195.90–243.60) | 200 (190.90–240.35) | 0.251 | |
| Intraoperative blood loss (median with 95% CI) (mL) | 50 (44.25–103.25) | 50 (47.39–97.94) | 0.389 | |
| Defunctioning stoma (n) | 6 (32.5) | 29 (64.4) | 0.061 | |
| Pathology type of tumor (n%) | 0.459 | |||
| Adenocarcinoma | 59 | 15 (93.8) | 44 (97.8) | |
| Mucinous carcinoma | 1 | 0 (0) | 1 (2.2) | |
| Signet-ring cell | 1 | 1 (6.2) | 0 (0) | |
| Size of tumor (median with 95% CI), cm | 3.50 (3.22–5.66) | 4.0 (3.22–3.94) | 0.275 | |
| Tumor differentiation (n) | 0.632 | |||
| Well | 3 | 1 (6.2) | 2 (4.4) | |
| Moderate | 45 | 11 (68.7) | 34 (75.6) | |
| Poor | 2 | 1 (6.2) | 1 (2.2) | |
| Well-moderate | 2 | 1 (6.2) | 1 (2.2) | |
| Moderate-poor | 9 | 2 (12.7) | 7 (15.6) | |
| Tumor type (n%) | 0.539 | |||
| Ulcer | 39 | 9 (56.3) | 30 (66.7) | |
| Uplift | 16 | 6 (37.5) | 10 (22.2) | |
| Infiltrating | 6 | 1 (6.2) | 5 (11.1) | |
| Pathological tumor (T) category (n) | 0.480 | |||
| T0 | 2 | 0 (0) | 2 (4.4) | |
| T1 | 2 | 1 (6.2) | 1 (2.2) | |
| T2 | 20 | 5 (31.3) | 15 (33.3) | |
| T3 | 28 | 6 (37.5) | 22 (49) | |
| T4 | 9 | 4 (25) | 5 (11.1) | |
| Pathological node (N) category (n) | 0.413 | |||
| N0 | 34 | 10 (62.5) | 24 (53.4) | |
| N1 | 16 | 5 (31.3) | 11 (24.4) | |
| N2 | 11 | 1 (6.2) | 10 (22.2) | |
| Pathological metastasis (M) category (n) | 0.841 | |||
| M0 | 56 | 14 (87.3) | 42 (93.3) | |
| M1 | 5 | 2 (12.7) | 3 (6.7) |
| Variable | Patients | Postoperative 1 Day | Postoperative 3 Day | Postoperative 5 Day |
|---|---|---|---|---|
| WBC (median with 95% CI) 109/L | Non-AL | 8.71 (8.02–9.65) | 6.81 (6.54–8.59) | 6.27 (6.00–8.00) |
| AL | 10.06 (9.15–10.02) | 8.66 (7.58–11.07) | 10.12 (7.85–12.23) | |
| p value | 0.187 | 0.146 | 0.003 * | |
| Neutrophils (median with 95% CI) 109/L | Non-AL | 7.38 (6.78–8.33) | 5.18 (4.74–6.80) | 4.52 (4.16–5.95) |
| AL | 8.46 (6.80–10.53) | 6.71 (5.70–9.52) | 7.25 (6.16–10.41) | |
| p value | 0.202 | 0.061 | 0.001 * | |
| Lymphocytes (median with 95% CI) 109/L | Non-AL | 0.79 (0.71–0.92) | 1.09 (0.92–1.25) | 1.28 (1.02–1.42) |
| AL | 1.01 (0.72–1.28) | 0.88 (0.71–1.43) | 0.84 (0.71–1.06) | |
| p value | 0.081 | 0.545 | 0.015 * | |
| Monocytes (median with 95% CI) 109/L | Non-AL | 0.44 (0.38–0.53) | 0.48 (0.43–0.63) | 0.52 (0.44–0.64) |
| AL | 0.58 (0.49–0.69) | 0.50 (0.42–0.69) | 0.53 (0.47–0.75) | |
| p value | 0.008 * | 0.816 | 0.372 | |
| Platelets (median with 95% CI) 109/L | Non-AL | 167 (145.63–175.49) | 171 (151.51–183.77) | 189 (176.05–211.79) |
| AL | 173 (156.04–200.20) | 170 (148.77–212.28) | 178 (145.19–208.70) | |
| p value | 0.559 | 0.828 | 0.394 | |
| NLR (median with 95% CI) 109/L | Non-AL | 8.82 (8.41–11.64) | 5.17 (4.62–7.93) | 4.24 (3.58–6.44) |
| AL | 10.62 (7.76–13.67) | 8.25 (6.44–16.49) | 9.98 (7.62–14.36) | |
| p value | 0.679 | 0.219 | <0.001 * | |
| LMR (median with 95% CI) 109/L | Non-AL | 1.79 (1.45–2.83) | 2.06 (1.81–2.78) | 2.31 (2.05–3.22) |
| AL | 1.77 (1.22–3.00) | 1.60 (1.46–2.58) | 1.77 (1.29–2.04) | |
| p value | 0.717 | 0.532 | 0.016 * | |
| PLR (median with 95% CI) 109/L | Non-AL | 200 (180.92–257.88) | 150.44 (144.47–202.58) | 152.87 (151.64–213.23) |
| AL | 187.60 (141.07–343.70) | 193.18 (162.02–311.79) | 163.70 (163.54–330.59) | |
| p value | 0.936 | 0.663 | 0.244 |
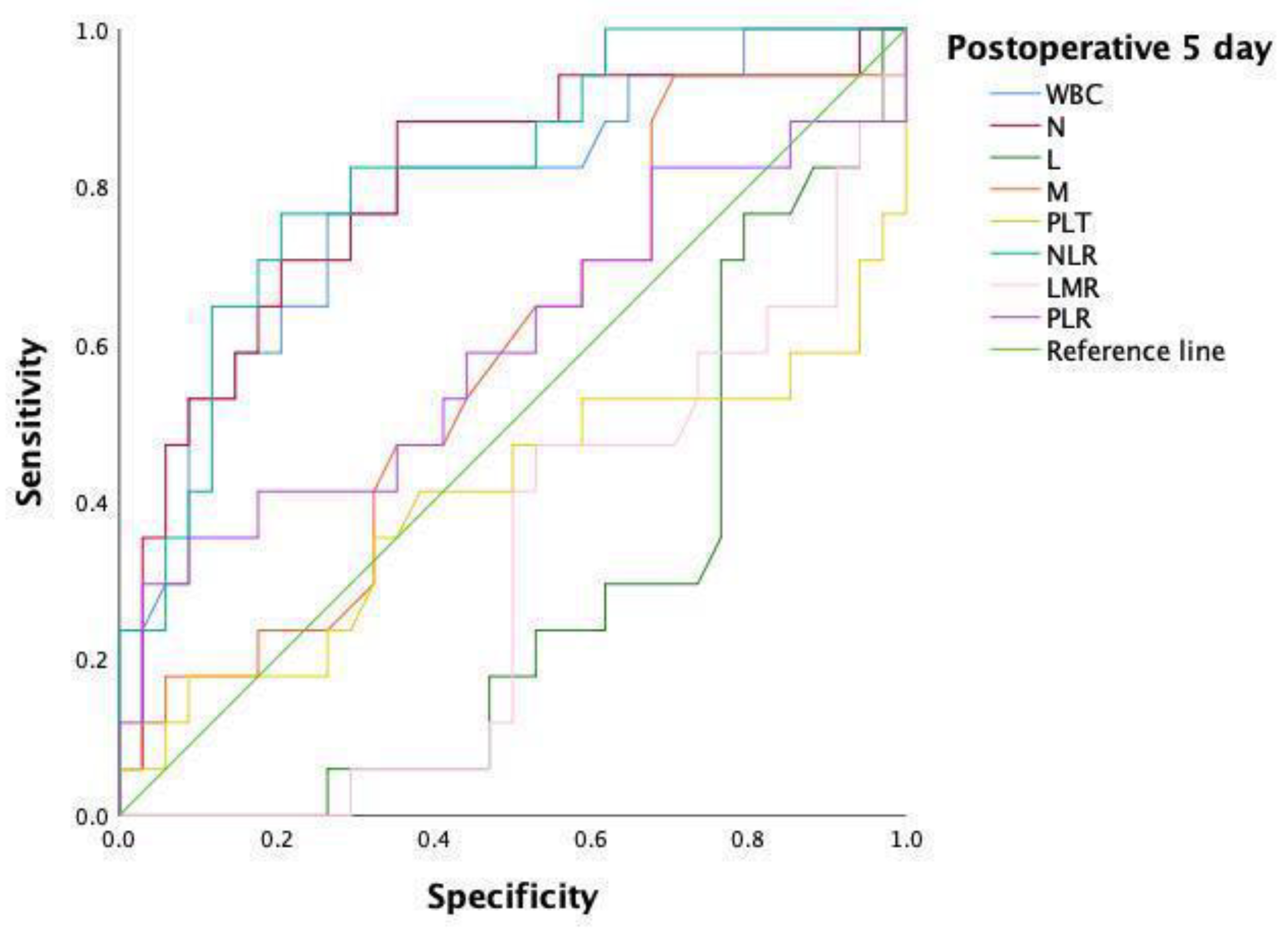
| Variable | AUC | 95% CI | p Value | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| WBC | 0.779 | 0.641–0.916 | 0.001 * | 7.40 | 76.5 | 73.5 |
| N | 0.801 | 0.666–0.936 | 0.001 * | 4.84 | 88.2 | 64.7 |
| NLR | 0.818 | 0.697–0.940 | <0.001 * | 6.54 | 76.5 | 79.4 |
| NLR and N | 0.818 | 0.697–0.940 | <0.001 * | 0.29 | 76.5 | 79.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Xu, K.; Qi, X.; Gao, P.; Liu, M.; Yao, Z.; Zhang, N.; Yang, H.; Zhang, C.; Xing, J.; et al. Neutrophil-to-Lymphocyte Ratio as an Early Predictor of Symptomatic Anastomotic Leakage in Patients after Rectal Cancer Surgery: A Propensity Score-Matched Analysis. J. Pers. Med. 2023, 13, 93. https://doi.org/10.3390/jpm13010093
Tan F, Xu K, Qi X, Gao P, Liu M, Yao Z, Zhang N, Yang H, Zhang C, Xing J, et al. Neutrophil-to-Lymphocyte Ratio as an Early Predictor of Symptomatic Anastomotic Leakage in Patients after Rectal Cancer Surgery: A Propensity Score-Matched Analysis. Journal of Personalized Medicine. 2023; 13(1):93. https://doi.org/10.3390/jpm13010093
Chicago/Turabian StyleTan, Fei, Kai Xu, Xinyu Qi, Pin Gao, Maoxing Liu, Zhendan Yao, Nan Zhang, Hong Yang, Chenghai Zhang, Jiadi Xing, and et al. 2023. "Neutrophil-to-Lymphocyte Ratio as an Early Predictor of Symptomatic Anastomotic Leakage in Patients after Rectal Cancer Surgery: A Propensity Score-Matched Analysis" Journal of Personalized Medicine 13, no. 1: 93. https://doi.org/10.3390/jpm13010093
APA StyleTan, F., Xu, K., Qi, X., Gao, P., Liu, M., Yao, Z., Zhang, N., Yang, H., Zhang, C., Xing, J., Cui, M., & Su, X. (2023). Neutrophil-to-Lymphocyte Ratio as an Early Predictor of Symptomatic Anastomotic Leakage in Patients after Rectal Cancer Surgery: A Propensity Score-Matched Analysis. Journal of Personalized Medicine, 13(1), 93. https://doi.org/10.3390/jpm13010093







