Evaluation of a Novel Technique for Closure of Small Palatal Fistula
Abstract
1. Introduction
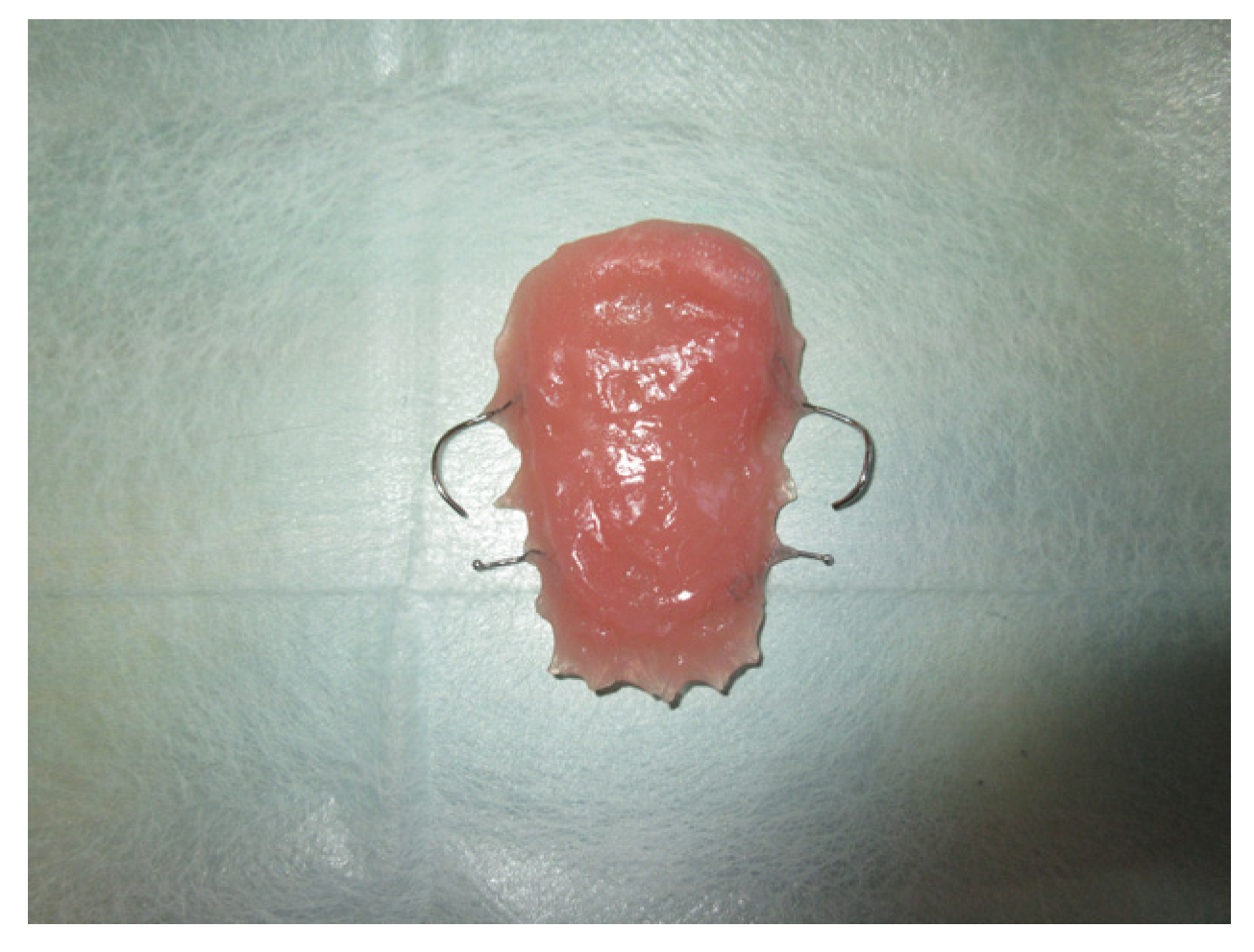
2. Materials and Methods
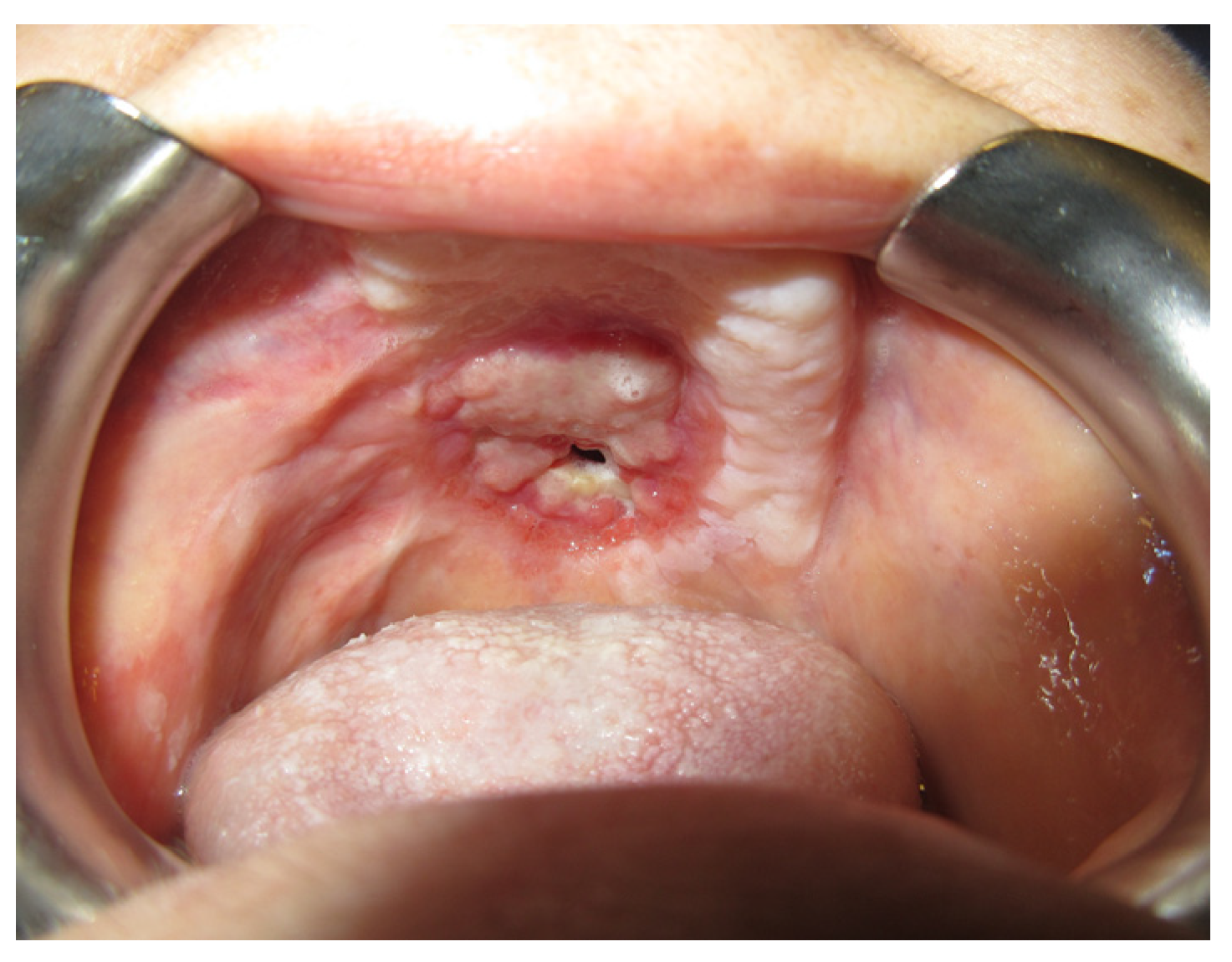
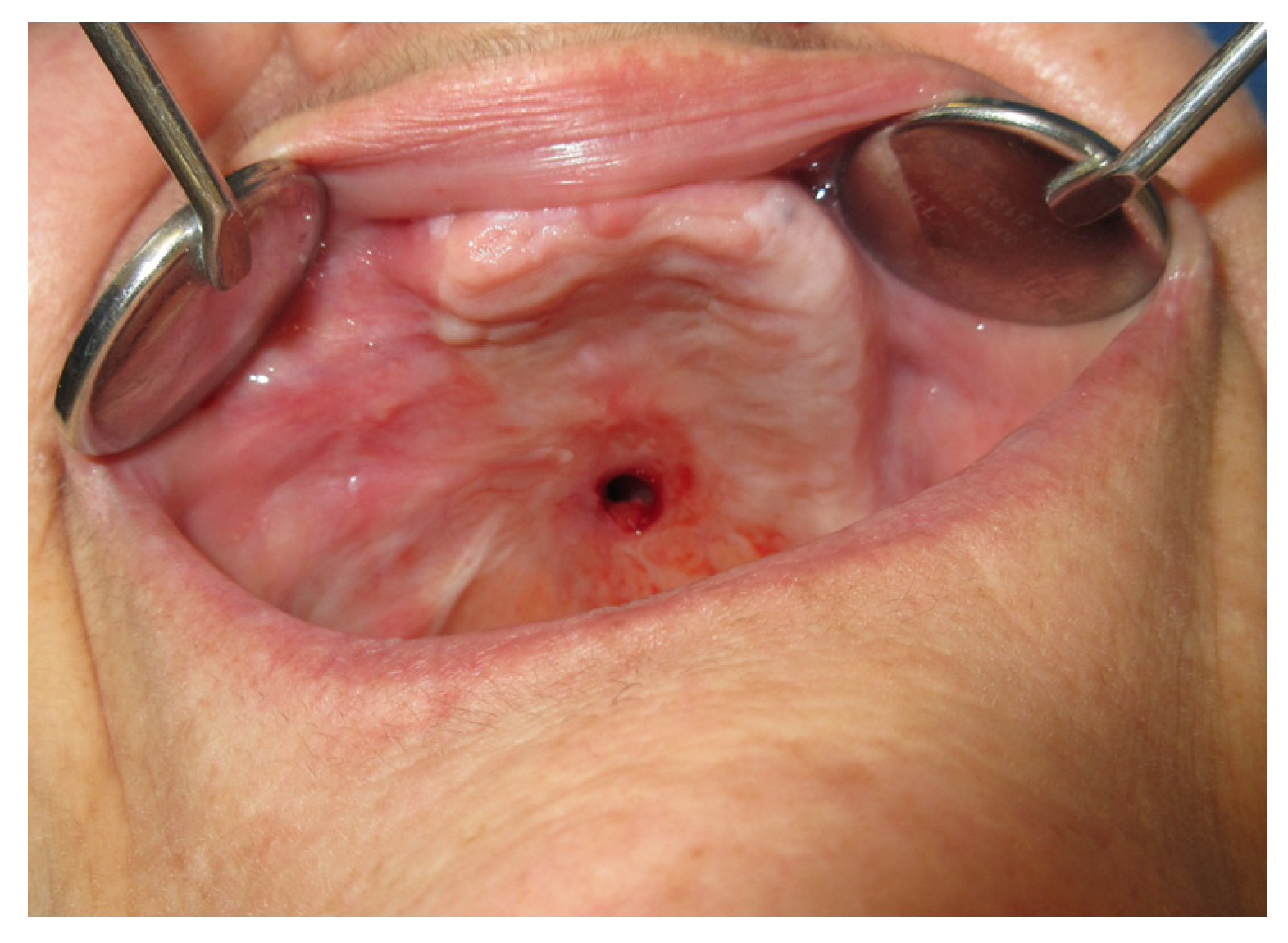
3. Results
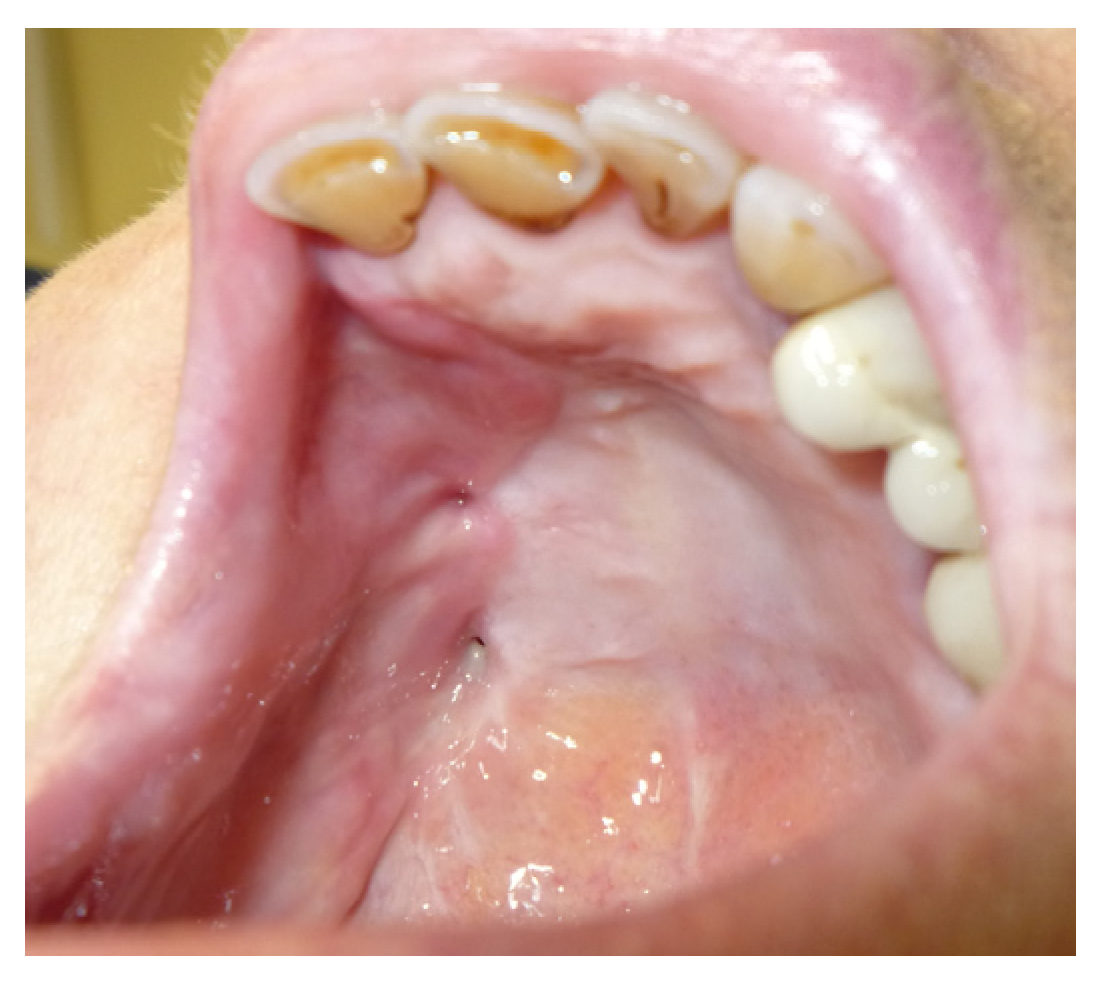
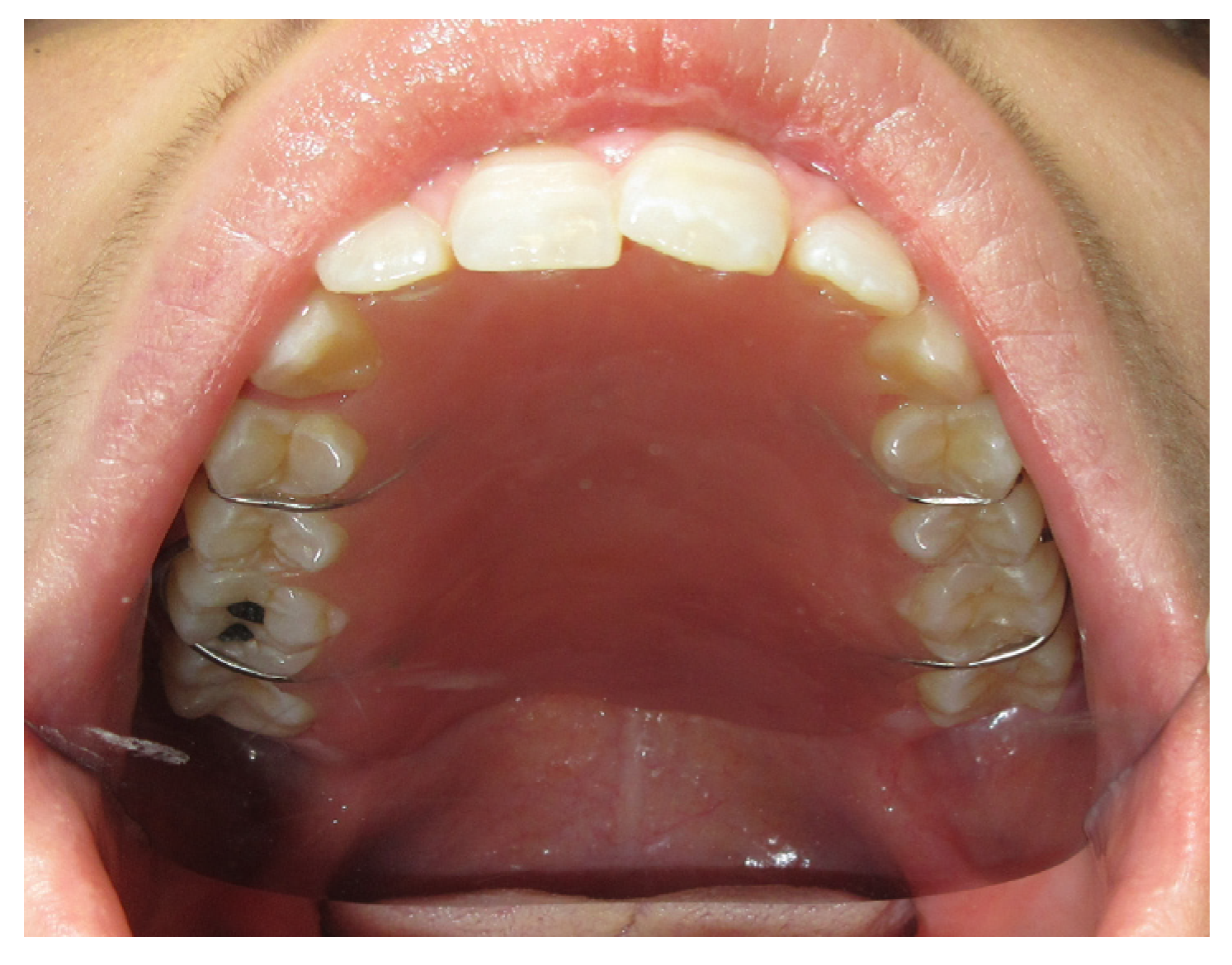
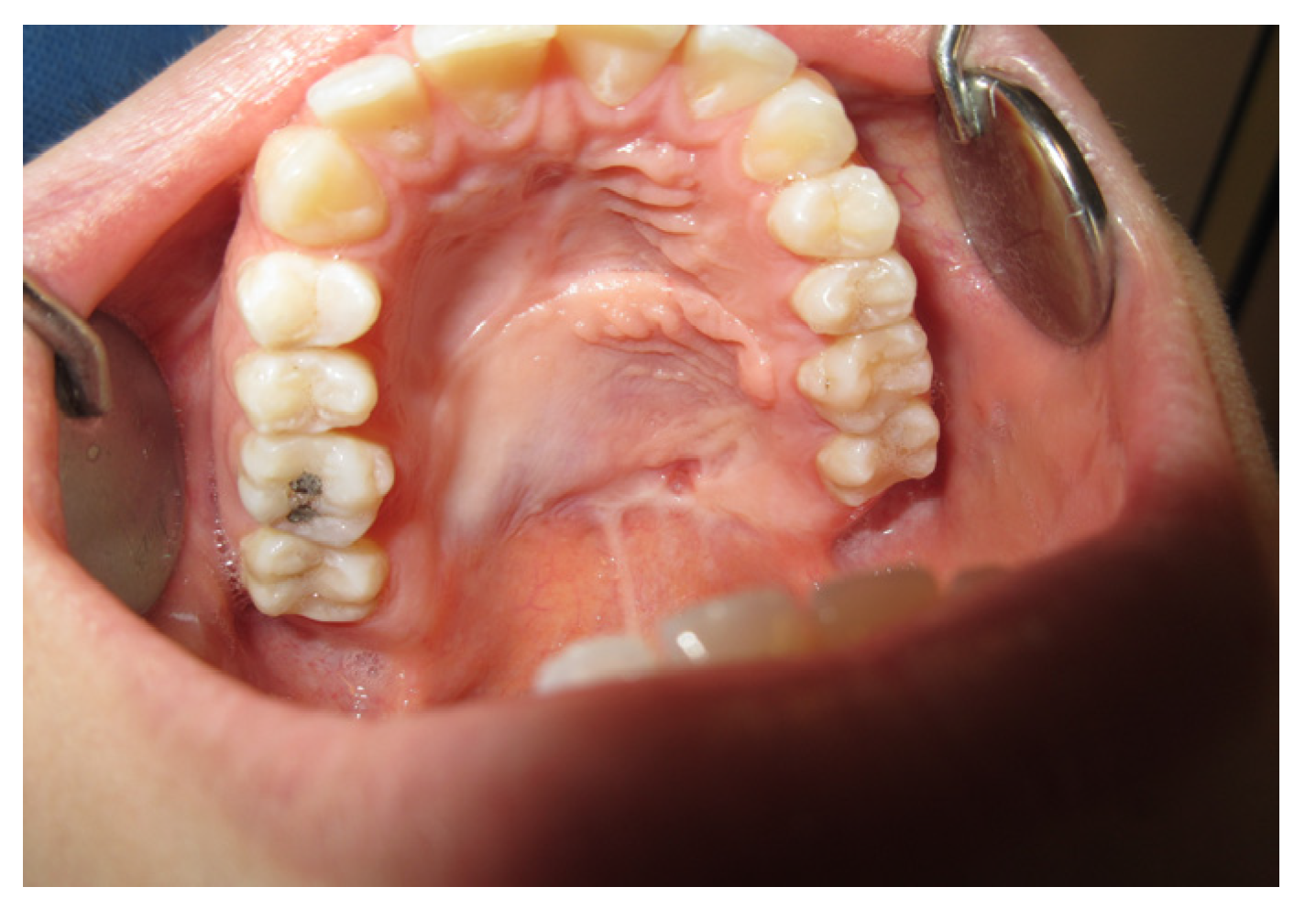
3.1. Case Reports
3.1.1. Case 1: A.R.
3.1.2. Case 2: P.S.
3.1.3. Case 3: R.M.
3.1.4. Case 4: P.M.A.
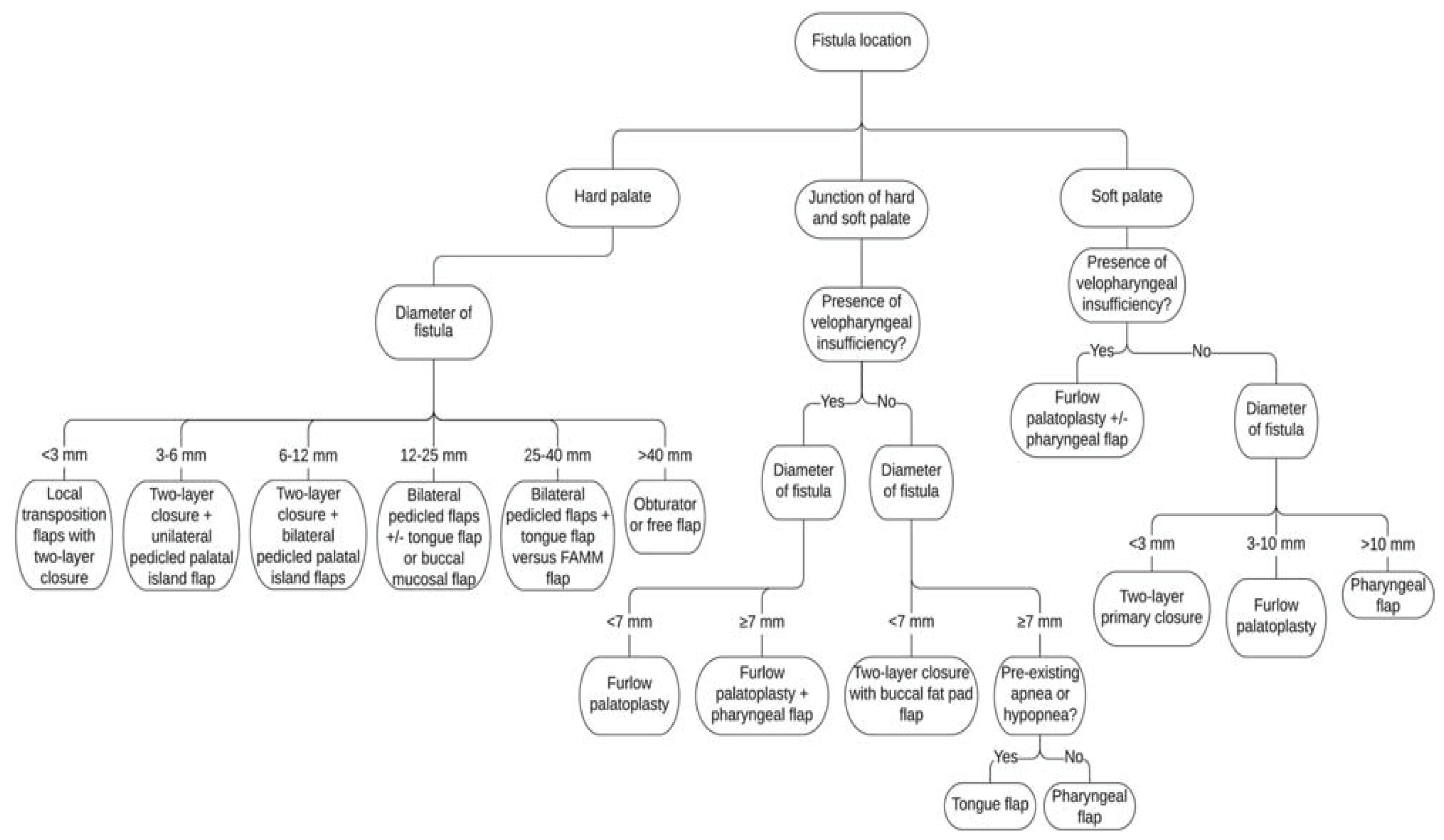
4. Discussion
5. Conclusions
- Age: Regenerative capacity at a young age is likely to be greater.
- The presence of dental elements: A greater reduction in size and a higher rate of closure was observed in partially edentulous patients than in totally edentulous patients. Dental stabilization of the palatal plate probably reduces any micro-movements that prevent the apposition of tissue.
- Presence of basal maxillary bone: The presence of basal bone seems to improve the quantity and quality of the maturation tissue, probably acting as a support for the newly formed tissue.
- The presence of keratinized mucosa: The resistance to trauma of the keratinized epithelium of the mucosa improves the stability over time of the result obtained from the reduction in the diameter of the fistula. The palatal fibromucosa is extremely resistant to trauma compared to the alveolar mucosa [19].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, D.M.; Vecchione, L.; Jiang, S.; Ford, M.; Deleyiannis, F.W.; Haralam, M.A.; Naran, S.; Worrall, C.I.; Dudas, J.R.; Afifi, A.M.; et al. The Pittsburgh Fistula Classification System: A standardized scheme for the description of palatal fistulae. Cleft. Palate. Craniofac. J. 2007, 44, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.M.; Wolfaardt, J.F.; Jha, N.; Seikaly, H. Maxillary Obturators: The Relationship Between Patient Satisfaction and Speech Outcome. Head. Neck. 2003, 25, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Brauner, E.; Cassoni, A.; Battisti, A.; Bartoli, D.; Valentini, V. Prosthetic rehabilitation in post-oncological patients: Report of two cases. Ann. Stomatol. 2010, 1, 19–25. [Google Scholar]
- Kornblith, A.B.; Zlotolow, I.M.; Gooen, J.; Huryn, J.M.; Lerner, T.; Strong, E.W.; Shah, J.P.; Spiro, R.H.; Holland, J.C. Quality of life of maxillectomy patients using an obturator prosthesis. Head Neck 1996, 18, 323–334. [Google Scholar] [CrossRef]
- Brauner, E.; Quarato, A.; De Angelis, F.; Pompa, G.; Jamshir, S.; Valentini, V.; Di Carlo, S. Prosthetic rehabilitation involving the use of implants following a fibula free flap reconstruction in the treatment of Osteosarcoma of the maxilla: A case report. Clin. Ter. 2017, 168, e392–e396. [Google Scholar] [CrossRef] [PubMed]
- Sitzman, T.J.; Allori, A.C.; Matic, D.B.; Beals, S.P.; Fisher, D.M.; Samson, T.D.; Marcus, J.R.; Tse, R.W. Reliability of Oronasal Fistula Classification. Cleft. Palate. Craniofac. J. 2018, 55, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Terenzi, V.; Cassoni, A.; Bosco, S.; Brauner, E.; Shahinas, J.; Pompa, G. Giant Cell Lesion or Langerhans’ Cell Histiocytosis of the Mandible? A Case Report. Eur. J. Inflamm. 2012, 18, 159–164. [Google Scholar] [CrossRef]
- Morton, R.P.; Izzard, M.E. Quality-of-life outcomes in head and neck cancer patients. World. J. Surg. 2003, 27, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Brauner, E.; Di Carlo, S.; Musio, D.; Tombolini, M.; De Angelis, F.; Valentini, V.; Tombolini, V.; Polimeni, A.; Pompa, G. Crestal bone loss around dental implants placed in head and neck cancer patients treated with different radiotherapy techniques: A prospective cohort study. Int. J. Oral. Maxillofac. Surg. 2019, 48, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Berkman, M.D. Early non-surgical closure of postoperative palatal fistulae. Plast. Reconstr. Surg. 1978, 62, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.W.; Wei, W.I. Elimination of palatal fistula after the maxillary swing procedure. Head Neck 2005, 27, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Wible, L.E.; Howard, J.C. Traumatic palatal fistula. Arch. Otolaryngol. 1946, 44, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.Z.; Kilic, A.; Arslan, H. Palatal fistula: A complication of primary tuberculous dacryoadenitis. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Rothermel, A.T.; Lundberg, J.N.; Samson, T.D.; Tse, R.W.; Allori, A.C.; Bezuhly, M.; Beals, S.P.; Sitzman, T.J. A Toolbox of Surgical Techniques for Palatal Fistula Repair. Cleft. Palate. Craniofac. J. 2021, 58, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Agni, N.A. Palatal fistulae: A comprehensive classification and difficulty index. J. Maxillofac. Oral. Surg. 2014, 13, 305–309. [Google Scholar] [CrossRef] [PubMed]
- De Agostino Biella Passos, V.; de Carvalho Carrara, C.F.; da Silva Dalben, G.; Costa, B.; Gomide, M.R. Prevalence, cause, and location of palatal fistula in operated complete unilateral cleft lip and palate: Retrospective study. Cleft. Palate. Craniofac. J. 2014, 51, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Levinson, J.; Rousso, J.J. Revision Surgery of the Cleft Palate. Semin. Plast. Surg. 2020, 34, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, C.; Vuola, P.; Leikola, J.; Heliövaara, A. Pierre Robin Sequence: Incidence of Speech-Correcting Surgeries and Fistula Formation. Cleft. Palate. Craniofac. J. 2020, 57, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Brauner, E.; Valentini, V.; De Angelis, F.; Jamshir, S.; Visca, A.; Romeo, U.; Tenore, G.; Pompa, G.; Di Carlo, S. Gingival hyperplasia around dental implants in jaws reconstructed with free vascularized flaps: A case report series. J. Int. Dent. Med. Res. 2018, 11, 1–7. [Google Scholar]







| Name | Gender | Diagnosis | Therapy | Prosthetic Rehabilitation |
|---|---|---|---|---|
| A.R | F | K superior jaw | Surgery | Superior |
| C.G. | F | Adenocarcinoma | Surgery | Superior |
| C.A. | F | Pleomorphic adenoma left hemimaxilla | Surgery | Superior + inferior |
| D.M. | M | K Adenocystic salivary glands of the palate | Surgery + Rt | Superior |
| D.A. | F | Chondrosarcoma nasal bones | Surgery | Superior |
| D.V. | M | K Squamous cell soft palate | Surgery | Superior |
| G.P. | F | Palate adenocarcinoma | Surgery | Superior |
| I.C. | F | K of the palate | Surgery | Superior |
| L.G. | F | K Squamous cell maxilla | Surgery | Superior + inferior |
| M.R. | M | K Mucoepidermoid maxilla | Surgery | Superior |
| M.R.2 | F | K of the maxilla | Surgery | Superior |
| P.S. | F | K of the palate | Surgery | Superior |
| S.M. | M | K Right cheek and right retromolar spacer | Surgery + Rt adjuvant | Superior + inferior |
| S.L. | F | Chondroblastic osteosarcoma maxilla | Surgery | Superior |
| T.E. | M | Osteosarcoma maxilla | CHT neoadjuvant + surgery + CHT adjuvant | Superior |
| T.G. | F | K Adenocystic maxilla | Surgery | Superior |
| V.F. | M | K Right retromolar space | Surgery | Superior |
| R.D. | M | K Jaw | Surgery | Superior |
| R.M. | M | K Squamous cell maxilla | Surgery | Superior |
| L.M | F | K Squamous cell soft palate | Surgery | Superior |
| Subgroup A | Subgroup B | Total | |
|---|---|---|---|
| Group 1 | 7 | 6 | 13 |
| Group 2 | 4 | 3 | 7 |
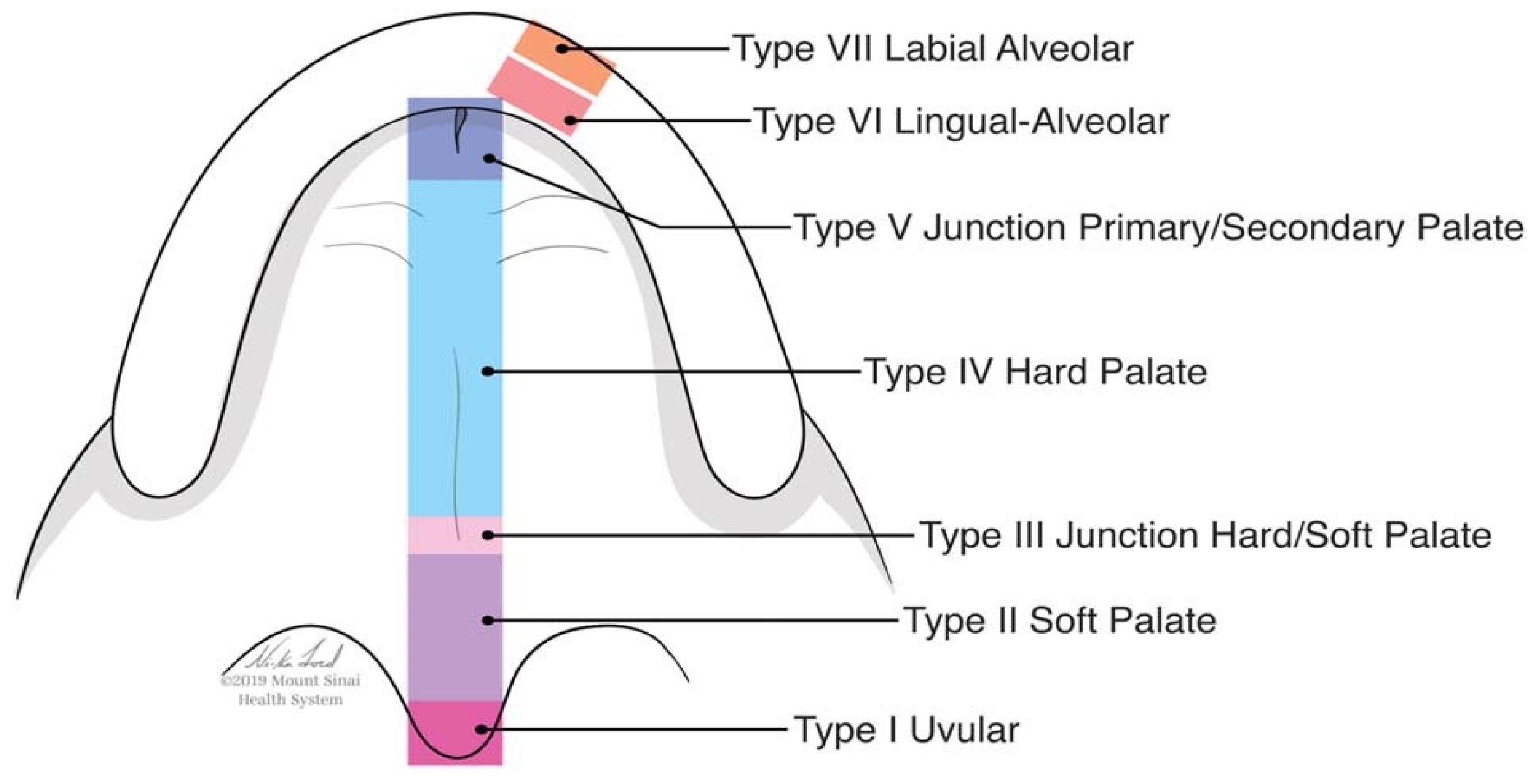
| Pittsburg Classification | N° Patients |
|---|---|
| Type I | 0 |
| Type II | 3 |
| Type III | 6 |
| Type IV | 10 |
| Type V | 0 |
| Type VI | 1 |
| Type VII | 0 |
| N° | Group 1 | Group 2 | Subgroup A | Subgroup B | Initial Size (mm) | After NPSF (mm) |
|---|---|---|---|---|---|---|
| A.R | X | * | 5 | 1 | ||
| C.G. | X | * | 6 | 0 | ||
| C.A. | X | * | 4 | 0 | ||
| D.M. | X | * | 5 | 0 | ||
| D.A. | X | * | 7 | 0 | ||
| D.V. | X | * | 7 | 2 | ||
| G.P. | X | * | 8 | 0 | ||
| I.C. | X | * | 7 | 0 | ||
| L.G. | X | * | 8 | 0 | ||
| M.R. | X | * | 3 | 0 | ||
| M.R.2 | X | * | 4 | 3 | ||
| P.S. | X | * | 7 | 0 | ||
| S.M. | X | * | 4 | 0 | ||
| S.L. | X | * | 6 | 2 | ||
| T.E. | X | * | 2 | 0 | ||
| T.G. | X | * | 5 | 1 | ||
| V.F. | X | * | 3 | 0 | ||
| R.D. | X | * | 6 | 0 | ||
| R.M. | X | * | 9 | 0 | ||
| P.M.A | X | * | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauner, E.; Piccoli, L.; Sallemi, K.; Romeo, U.; Laudoni, F.; Cantore, M.; Tenore, G.; Pranno, N.; De Angelis, F.; Di Cosola, M.; et al. Evaluation of a Novel Technique for Closure of Small Palatal Fistula. J. Pers. Med. 2023, 13, 65. https://doi.org/10.3390/jpm13010065
Brauner E, Piccoli L, Sallemi K, Romeo U, Laudoni F, Cantore M, Tenore G, Pranno N, De Angelis F, Di Cosola M, et al. Evaluation of a Novel Technique for Closure of Small Palatal Fistula. Journal of Personalized Medicine. 2023; 13(1):65. https://doi.org/10.3390/jpm13010065
Chicago/Turabian StyleBrauner, Edoardo, Luca Piccoli, Karim Sallemi, Umberto Romeo, Federico Laudoni, Marco Cantore, Gianluca Tenore, Nicola Pranno, Francesca De Angelis, Michele Di Cosola, and et al. 2023. "Evaluation of a Novel Technique for Closure of Small Palatal Fistula" Journal of Personalized Medicine 13, no. 1: 65. https://doi.org/10.3390/jpm13010065
APA StyleBrauner, E., Piccoli, L., Sallemi, K., Romeo, U., Laudoni, F., Cantore, M., Tenore, G., Pranno, N., De Angelis, F., Di Cosola, M., Valentini, V., & Di Carlo, S. (2023). Evaluation of a Novel Technique for Closure of Small Palatal Fistula. Journal of Personalized Medicine, 13(1), 65. https://doi.org/10.3390/jpm13010065











