Partial Two-Stage Exchange for Infected Total Hip Arthroplasty: A Treatment to Take into Account
Abstract
1. Introduction
2. Patients and Methods
- Well-fixed femoral component.
- Two-year minimum follow-up.
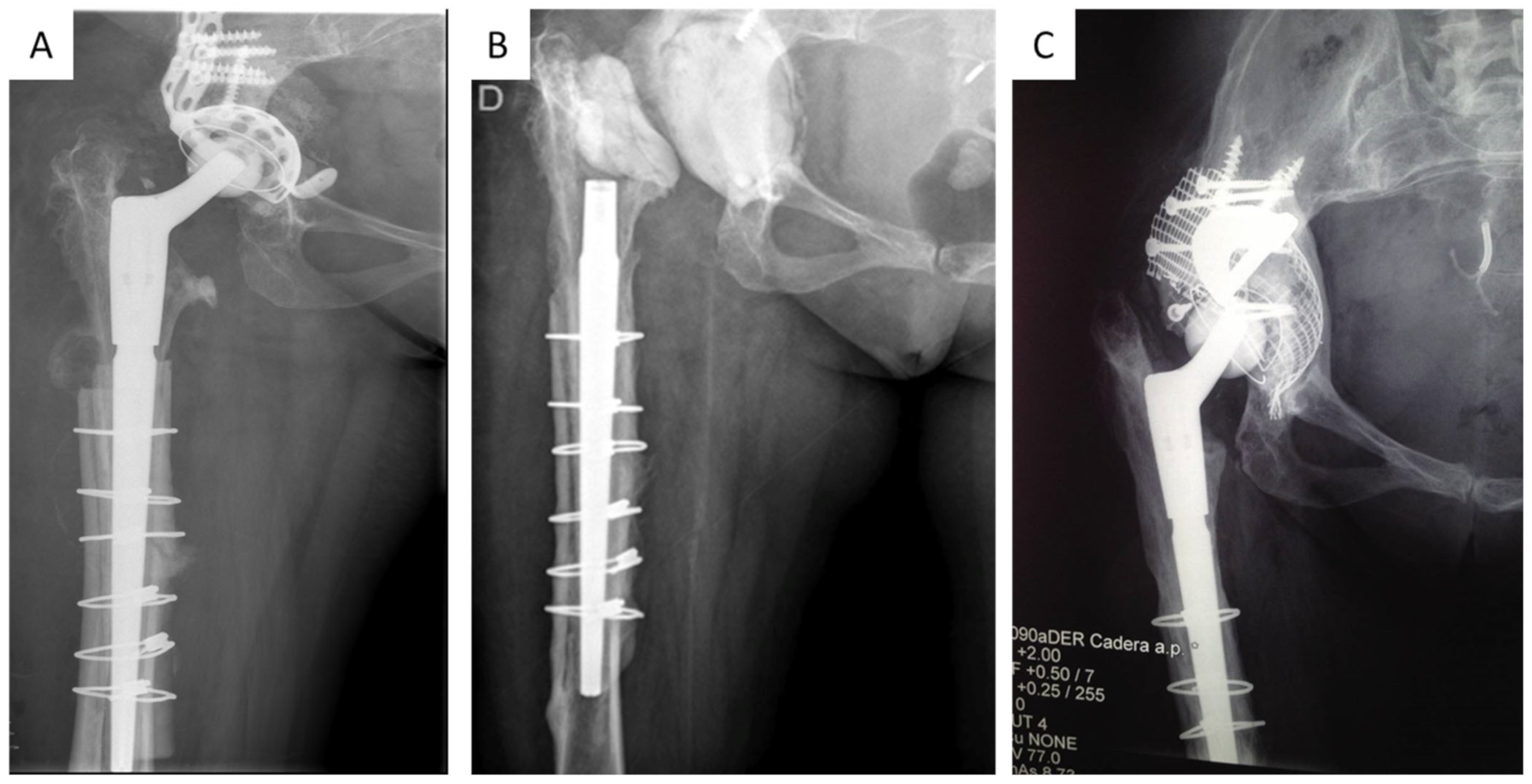
Patient Management and Surgical Technique
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindeque, B.; Hartman, Z.; Noshchenko, A.; Cruse, P. Infection after primary total hip arthroplasty. Orthopedics 2014, 37, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Tsai, S.-W.; Wu, P.-K.; Chen, C.-F.; Wang, H.-Y.; Chen, W.-M. Partial component-retained two-stage reconstruction for chronic infection after uncemented total hip arthroplasty: Results of sixteen cases after five years of follow-up. Int. Orthop. 2017, 41, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Parisi, T.J.; Konopka, J.F.; Bedair, H.S. What is the Long-term Economic Societal Effect of Periprosthetic Infections After THA? A Markov Analysis. Clin. Orthop. Relat. Res. 2017, 475, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.J.; Della Valle, C.J. The two-stage standard in revision total hip replacement. Bone Joint J. 2013, 95, 84–87. [Google Scholar] [CrossRef]
- Triantafyllopoulos, G.K.; Memtsoudis, S.G.; Zhang, W.; Ma, Y.; Sculco, T.P.; Poultsides, L.A. Periprosthetic Infection Recurrence After 2-Stage Exchange Arthroplasty: Failure or Fate? J. Arthroplast. 2017, 32, 526–531. [Google Scholar] [CrossRef]
- Negus, J.J.; Gifford, P.B.; Haddad, F.S. Single-Stage Revision Arthroplasty for Infection-An Underutilized Treatment Strategy. J. Arthroplast. 2017, 32, 2051–2055. [Google Scholar] [CrossRef]
- Chappell, J.D.; Lachiewicz, P.F. Fracture of the femur in revision hip arthroplasty with a fully porous-coated component. J. Arthroplast. 2005, 20, 234–238. [Google Scholar] [CrossRef]
- McAlister, I.P.; Perry, K.I.; Mara, K.C.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Two-Stage Revision of Total Hip Arthroplasty for Infection Is Associated with a High Rate of Dislocation. J. Bone Joint Surg. Am. 2019, 101, 322–329. [Google Scholar] [CrossRef]
- McPherson, E.J.; Woodson, C.; Holtom, P.; Roidis, N.; Shufelt, C.; Patzakis, M. Periprosthetic total hip infection: Outcomes using a staging system. Clin. Orthop. Relat. Res. 2002, 403, 8–15. [Google Scholar] [CrossRef]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef]
- Hargunani, R.; Madani, H.; Khoo, M.; Fotiadou, A.; Pressney, I.; Calleja, M.; O’Donnell, P. Imaging of the Painful Hip Arthroplasty. Can Assoc. Radiol. J. 2016, 67, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Copsey, B.; Thompson, J.Y.; Vadher, K.; Ali, U.; Dutton, S.J.; Fitzpatrick, R.; Lamb, S.E.; Cook, J.A. Problems persist in reporting of methods and results for the WOMAC measure in hip and knee osteoarthritis trials. Qual. Life Res. 2019, 28, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.S.; McAuley, J.P.; Young, A.M.; A Engh, C. Radiographic signs of osseointegration in porous-coated acetabular components. Clin. Orthop. Relat. Res. 2006, 444, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.J.; Sledge, J.B.; Muller, M.E. Total hip arthroplasty with use of an acetabular reinforcement ring in patients who have congenital dysplasia of the hip. Results at five to fifteen years. J. Bone Joint Surg. Am. 1998, 80, 969–979. [Google Scholar] [CrossRef]
- De La Torre-Escuredo, B.; Gómez-García, E.; Álvarez-Villar, S.; Bujan, J.; Ortega, M.A. Bone impaction grafting with trabecular metal augments in large defects in young patients: Unravelling a new perspective in surgical technique. BMC Musculoskelet Disord. 2020, 21, 581. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.J.; Holtzman, J.; Gafni, A.; Saleh, L.; Jaroszynski, G.; Wong, P.; Woodgate, I.; Davis, A.; Gross, A.E. Development, test reliability and validation of a classification for revision hip arthroplasty. J. Orthop. Res. 2001, 19, 50–56. [Google Scholar] [CrossRef]
- Laffosse, J.M. Removal of well-fixed fixed femoral stems. Orthop. Traumatol. Surg. Res. 2016, 102, S177–S187. [Google Scholar] [CrossRef]
- Ekpo, T.E.; Berend, K.R.; Morris, M.J.; Adams, J.B.; Lombardi, A.V. Partial two-stage exchange for infected total hip arthroplasty: A preliminary report. Clin. Orthop. Relat. Res. 2014, 472, 437–448. [Google Scholar] [CrossRef]
- Lombardi, A.V., Jr.; Berend, K.R.; Adams, J.B. Partial two-stage exchange of the infected total hip replacement using disposable spacer moulds. Bone Joint J. 2014, 96, 66–69. [Google Scholar] [CrossRef]
- Crawford, D.A.; Adams, J.B.; Morris, M.J.; Berend, K.R.; Lombardi, A.V. Partial 2-Stage Exchange for Infected Total Hip Arthroplasty: An Updated Report. J. Arthroplast. 2019, 34, 3048–3053. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Jung, J.; Kelm, J.; Schmitt, E. Two-stage treatment protocol for isolated septic acetabular cup loosening. Hip. Int. 2010, 20, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Meyer, C. Partial two-stage exchange at the site of periprosthetic hip joint infections. Arch. Orthop. Trauma. Surg. 2019, 139, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Castagnini, F.; Tella, G.; Montalti, M.; Biondi, F.; Bordini, B.; Busanelli, L.; Toni, A. Mid-term outcomes of a partial 2-stage approach in late chronic periprosthetic hip infections. Hip. Int. 2020, 30, 327–332. [Google Scholar] [CrossRef]
- Faroug, R.; Shah, Y.; McCarthy, M.J.H.; Halawa, M. Two stage one component revision in infected total hip replacements—Two case reports and literature review. Hip. Int. 2009, 19, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Kaneuji, A.; Ueda, S.; Matsumoto, T. Should well-fixed uncemented femoral components be revised in infected hip arthroplasty? Report of five trial cases. J. Orthop. 2016, 13, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Lee, K.H.; Nho, J.-H.; Ha, Y.-C.; Koo, K.-H. Retaining well-fixed cementless stem in the treatment of infected hip arthroplasty. Acta Orthop. 2013, 84, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Yishake, M.; Tang, L.; Chen, X.; Wang, Y.; He, R. Partial two-stage exchange: An alternative method for infected total hip arthroplasty. BMC Musculoskelet Disord. 2021, 22, 686. [Google Scholar] [CrossRef]
- Neut, D.; Van Der Mei, H.C.; Bulstra, S.K.; Busscher, H.J. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 2007, 78, 299–308. [Google Scholar] [CrossRef]
- Leijtens, B.; Sadeghi, N.; Schreurs, B.W.; Rijnen, W.H. Cement-within-cement revision of infected total hip replacement; disappointing results in 10 retrospective cases. Hip. Int. 2016, 26, 67–72. [Google Scholar] [CrossRef]
- Josse, J.; Valour, F.; Maali, Y.; Diot, A.; Batailler, C.; Ferry, T.; Laurent, F. Interaction Between Staphylococcal Biofilm and Bone: How Does the Presence of Biofilm Promote Prosthesis Loosening? Front. Microbiol. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Liebs, T.R. CORR Insights®: The Role of Highly Selective Implant Retention in the Infected Hip Arthroplasty. Clin. Orthop. Relat. Res. 2016, 474, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Romano, C.L.; Mattina, R.; Signori, V.; De Vecchi, E. Does dithiothreitol improve bacterial detection from infected prostheses? A pilot study. Clin. Orthop. Relat. Res. 2012, 470, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
- Ghirardelli, S.T.G.; Antonini, G.; Violante, B.; Fidanza, A.; Indelli, P.F. Debridement, Antibiotic, Pearls, Irrigation and Retention of the Implant and Other Local Strategies on Hip Periprosthetic Joint Infections. Minerva. Chir. 2022, 73, 409–415. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Zhou, Z.; Shen, B.; Kang, P.; Pei, F. Partial implant retention in two-stage exchange for chronic infected total hip arthroplasty. Int. Orthop. 2020, 44, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Anil, U.; Singh, V.; Schwarzkopf, R. Diagnosis and Detection of Subtle Aseptic Loosening in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Urish, K.L.; DeMuth, P.W.; Kwan, B.W.; Craft, D.W.; Ma, D.; Haider, H.; Tuan, R.S.; Wood, T.K.; Davis, C.M., III. Antibiotic-tolerant Staphylococcus aureus Biofilm Persists on Arthroplasty Materials. Clin. Orthop. Relat. Res. 2016, 474, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Xu, B.; Guo, W.; Rehei, A.; Mu, W.; Yang, D.; Cao, L. Retention of the well-fixed implant in the single-stage exchange for chronic infected total hip arthroplasty: An average of five years of follow-up. Int. Orthop. 2017, 41, 901–909. [Google Scholar] [CrossRef]
- El-Husseiny, M.; Haddad, F.S. The Role of Highly Selective Implant Retention in the Infected Hip Arthroplasty. Clin. Orthop. Relat. Res. 2016, 474, 2157–2163. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Board, T.; Kay, P.; Wroblewski, B.M.; Zeller, V.; Chen, S.-Y.; Hsieh, P.-H.; The Global Infection Orthopaedic Management Collaboration; et al. One- and two-stage surgical revision of peri-prosthetic joint infection of the hip: A pooled individual participant data analysis of 44 cohort studies. Eur. J. Epidemiol. 2018, 33, 933–946. [Google Scholar] [CrossRef]
| Gender/Age at Surgery | Primary or Revision/Stem | Morbidities | PJI [9] | |
|---|---|---|---|---|
| Case 1 | F/44 years | Revision/Restoration Modular (Stryker) | III-A-2 | |
| Case 2 | F/74 | Revision/Arcos Modular (Zimmer-Biomet) | Hypertension | III-A-1 |
| Case 3 | M/64 | Revision/Modular Revision (Lima) | Hypertension Diabetes Obesity | III-C-2 |
| Case 4 | F/57 | Primary/Poropalcar (I.Q.L. Spain) | HIV IV drug user HCV | III-C-3 |
| Case 5 | F/72 | Primary/Furlong HA (MBA) | III-A-2 | |
| Case 6 | F/48 | Revision/Arcos Modular (Zimmer-Biomet) | Smoker Obesity | III-B-2 |
| Case 7 | F/57 | Revision/Arcos Modular (Zimmer-Biomet) | Smoker Obesity Kidney disease | III-C-2 |
| Case 8 | M/70 | Revision/Arcos Modular (Zimmer-Biomet) | Hypertension Obesity | III-B-2 |
| Fistula | CRP ESR | WBC | Arthrocentesis | AP | Preoperative Culture | Intraoperative Culture | |
|---|---|---|---|---|---|---|---|
| Case 1 | − | +/+ | + | + | + | S. epidermidis (MR) | S. epidermidis (MR) |
| Case 2 | − | +/+ | + | + | + | Streptococcus mutans | Streptococcus. mutans |
| Case 3 | + | +/+ | + | − | + | − | S. epidermidis (MR) |
| Case 4 | − | +/+ | + | + | + | S. aureus (MS) | S. aureus (MS) |
| Case 5 | + | +/+ | + | + | + | P. aeruginosa | P. aeruginosa |
| Case 6 | + | +/+ | + | + | + | Morganella morgagnii | Morganella morgagnii |
| Case 7 | − | +/+ | + | + | + | S. epidermidis (MR) | S. epidermidis |
| Case 8 | + | +/+ | + | + | + | S. agalactiae | S. agalactiae |
| Case | Preop X-ray Acetabular Loosening | Acetabular Defects [14] | Spacer | Acetabular Reconstruction | Antibiotics Therapy (Weeks) | Time to 2nd Stage (Weeks) | Postop Cultures | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | + | IV | NA | TMT + B.I.G. | 8 | 20 | − | 132 |
| 2 | + | V | NA | PLATE + CUP–CAGE | 8 | 10 | − | 84 |
| 3 | − | II | A | REVISION SHELL | 8 | 16 | − | 96 |
| 4 | + | V | A | PLATE + CUP–CAGE | 8 | 64 | − | 84 |
| 5 | − | II | A | REVISION SHELL | 8 | 12 | − | 48 |
| 6 | + | IV | NA | PLATE + CUP–CAGE | 8 | 44 | − | 84 |
| 7 | − | IV | NA | REVISION SHELL + TMT | 8 | 12 | − | 36 |
| 8 | − | II | NA | REVISION SHELL | 8 | 10 | − | 24 |
| Preoperative Score Median | Postoperative Score Median | Difference | |
|---|---|---|---|
| Pain | 9 | 2 | 7 |
| Stiffness | 4 | 2 | 2 |
| Function | 29 | 18.5 | 10.5 |
| 1st | 2nd | F.U. | 1st | 2nd | F.U. | |
|---|---|---|---|---|---|---|
| Case 1 | 103.2 | 46.7 | 4.3 | 34.3 | 25.3 | 19.4 |
| Case 2 | 76.7 | 34.3 | 4.8 | 56.2 | 28.2 | 18.7 |
| Case 3 | 282.4 | 67.8 | 3.1 | 102.2 | 22.4 | 15.4 |
| Case 4 | 62.9 | 28.2 | 2.9 | 45.3 | 30.1 | 8.3 |
| Case 5 | 93.9 | 54.2 | 5.3 | 48.9 | 22.3 | 15.6 |
| Case 6 | 89.2 | 47.3 | 4.2 | 39.7 | 24.5 | 20.1 |
| Case 7 | 101.3 | 31.4 | 3.1 | 42.4 | 27.4 | 18.4 |
| Case 8 | 253.3 | 89.4 | 2.7 | 45.6 | 26.4 | 15.7 |
| Author | N | Follow-Up (Months) | Success Rate |
|---|---|---|---|
| Faroug et al. | 2 | 39 (36–42) | 100% |
| Anagnostakos et al. | 13 | 55 (12–83) | 91.60% |
| Lee et al. | 19 | 48 (24–96) | 88.20% |
| Ekpo et al. | 19 | 48 (24–132) | 89.50% |
| Lombardi et al. | 26 | 19 (4–36) | 85.70% |
| Fukui et al. | 5 | 50 (42–60) | 100% |
| Baochao et al. | 31 | 60 (24–180) | 87.10% |
| El-Husseiny et al. | 18 | 84 (60–120) | 83.34% |
| Chen et al. | 16 | 70 (38–103) | 81.30% |
| Crawford et al. | 41 | 66 (18–222) | 80.50% |
| Shi et al. | 14 | 67,4 (DS 27,9) | 100% |
| Castagnini et al. | 28 | 60 (24–144) | 78.60% |
| Yishake et al. | 28 | 48 (24–132) | 85.7% |
| Current series | 8 | 38.2 (24–132) | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Romero, M.; Ordas-Bayon, A.; Gomez-Rice, A.; Ortega, M.A.; De La Torre Escuredo, B.J. Partial Two-Stage Exchange for Infected Total Hip Arthroplasty: A Treatment to Take into Account. J. Pers. Med. 2023, 13, 137. https://doi.org/10.3390/jpm13010137
Moreno-Romero M, Ordas-Bayon A, Gomez-Rice A, Ortega MA, De La Torre Escuredo BJ. Partial Two-Stage Exchange for Infected Total Hip Arthroplasty: A Treatment to Take into Account. Journal of Personalized Medicine. 2023; 13(1):137. https://doi.org/10.3390/jpm13010137
Chicago/Turabian StyleMoreno-Romero, Miguel, Alejandro Ordas-Bayon, Alejandro Gomez-Rice, Miguel A. Ortega, and Basilio J. De La Torre Escuredo. 2023. "Partial Two-Stage Exchange for Infected Total Hip Arthroplasty: A Treatment to Take into Account" Journal of Personalized Medicine 13, no. 1: 137. https://doi.org/10.3390/jpm13010137
APA StyleMoreno-Romero, M., Ordas-Bayon, A., Gomez-Rice, A., Ortega, M. A., & De La Torre Escuredo, B. J. (2023). Partial Two-Stage Exchange for Infected Total Hip Arthroplasty: A Treatment to Take into Account. Journal of Personalized Medicine, 13(1), 137. https://doi.org/10.3390/jpm13010137







