Abstract
Objectives: Information on the prediction of improved semen parameters following varicocelectomy is scarce and mostly contradictory. Therefore, we developed and validated a nomogram to predict whether abnormal semen parameters in infertile men could improve following microscopic varicocelectomy (MSV). Methods: From January 2018 to December 2021, 460 consecutive patients who underwent MSV were included. Of them, 336 patients as a development cohort at the Xiang Hua institution. As a validation cohort, Hu Nan Center (124 patients) was used. Clinicopathologic patient information was recorded. The likelihood ratio test using Akaike’s information criteria was employed as the stopping rule, and multivariate logistic regression was utilized to create a prediction model with regression coefficients. The effectiveness of this prediction model was evaluated based on its ability of discrimination, calibration, and clinical utility. Results: The initial total progressively motile sperm count (TPMSC) and vein diameter were predictors of this model. The model demonstrated strong discrimination for the validation cohort, with an area under the receiver operating characteristic (AUROC) of 0.925 (p < 0.001), and strong calibration (unreliability test, p = 0.522). The decision curve study proved the model’s clinical applicability. Conclusion: According to our research, the improvement of semen parameters in infertile men following MSV was significantly predicted by greater vein diameter and higher initial TPMSC. This nomogram aids in individualized decision-making on the varicocele preoperative treatment plan and may help to enhance the therapeutic result.
1. Introduction
Impairment in venous drainage of the testis results in varicocele, which is defined by aberrant dilatation of testicular veins in the pampiniform plexus form [1]. It can eventually have an impact on male fertility and testicular development, as well as cause symptoms of scrotal discomfort and pain, even hypogonadism. Nearly 15% of men with normal sperm quality, 25% of men with impaired sperm quality, and 35 to 40% of men who are infertile suffer from varicocele [2].
The most often used technique is varicocelectomy. Varicocelectomy may enhance semen parameters, spontaneous conception rates, and outcomes of assisted reproductive technologies, according to recent randomized controlled studies and meta-analyses [3,4,5]. Numerous methods have been employed, including open surgery, interventional embolization, microscopic surgery, and laparoscopic surgery [3]. The least invasive procedure should be used in the ideal varicocele treatment plan to achieve high effectiveness and safety.
Due to its benefits of meticulously ligating all veins while sparing the arterial branches, a lower recurrence rate, fewer complications, a higher postoperative semen quality, and a higher postoperative fertility rate, microscopic varicocelectomy (MSV) has gradually come to be the preferred surgical option [6,7,8]. Both the inguinal and subinguinal approaches to MSV are efficient procedures for treating varicocele in infertile men [9]. While varicocelectomy improves semen parameters in only 60–70% of patients and experiences spontaneous pregnancy in only 40–60% of patients, and the fundamental cause of varicocele-related infertility is still unclear [10,11]. The present difficulty is identifying which patients might benefit from surgery the most.
In a retrospective study that included 80 patients who underwent inguinal loupe-assisted varicocelectomy, it was indicated that younger age and higher concentration of preoperative sperm density were prognostic factors for successful varicocelectomy [12]. However, based on a retrospective study that recruited 228 patients who underwent unilateral MSV, Palmisano F et al. [13] found that patients aged older (≥35 years), with higher ultrasound varicocele grading (USVG, grade 3 above), and no concomitant with right subclinical varicocele were more likely to benefit from surgery. Moreover, in a prospective cohort study that included 123 patients treated with subinguinal MSV, Shabana et al. [14] demonstrated that a higher grade of varicocele (II or III), the concentration of sperm density (>8 million/mL), and the ratio of progressive motility (>18%) are significant predictors for excellent outcome after surgery. Data on the prediction of improved semen parameters following varicocelectomy are limited and rather conflicting. As a result, no reliable suggestions can be given. Furthermore, none of studies were validated by external cohorts. By combining and illuminating relevant predictors of critical clinical outcomes and generating a numerical likelihood of the occurrence, a nomogram created from a predictive model is a trustworthy tool for forecasting risk. Therefore, we developed and validated a nomogram to predict whether abnormal semen parameters in infertile men could improve following microscopic varicocelectomy (MSV).
2. Methods
2.1. Study Design
At two centers of Shengjing Hospital of China Medical University, this retrospective study was carried out (Xiang Hua center and Hu Nan center). The current study included 581 consecutive patients who underwent microscopic varicocelectomy between January 2018 and December 2021. There were 460 patients included in the final study after screening. Hu Nan center (124 patients) served as a validation cohort, while the Xiang Hua center (336 patients) served as a development cohort.
The Ethics Committee of Shengjing Hospital Affiliated China Medical University granted its approval (2022PS719K). ChiCTR2200060504 is the UIN for the clinical research registry. The 1975 Declaration of Helsinki’s ethical principles were followed by the study procedure.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria: patients over the age of 18 who underwent MSV (subinguinal or inguinal approach) for infertility with at least one aberrant semen parameter (concentration, total number, motility, or morphology), as well as varicocele that was clinically palpable, are included in the study.
Exclusion criteria: patients with a history of previous varicocelectomy (recurrent) or other inguinal surgeries (such as hernia repair), chromosomal abnormalities (such as AZF microdeletions or karyotype disorders), reproductive system malformations (such as cryptorchidism), lower urinary tract infections, prostatitis, epididymitis, and seminal vesiculitis, hypopituitarism, hyperthyroidism, Cushing’s syndrome; abnormal levels of luteinizing hormone, follicle-stimulating hormone, or serum testosterone were excluded. The details of flowchart were presented in Figure 1.
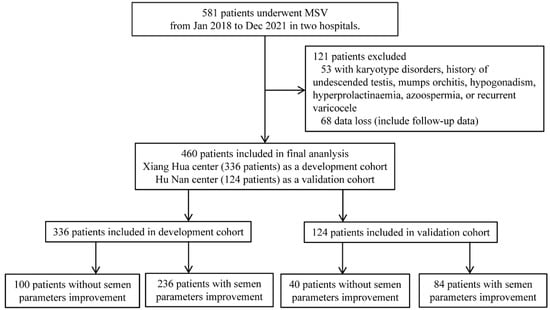
Figure 1.
Flowchart of this study.
The technique of MSV: Testicular artery and lymph vessels sparing and free ligation of gubernacular veins strategy were used.
2.3. Measurement of Characteristics and Follow-Up
Patient characteristics (age, body mass index (BMI)), the length of infertility (months), smoking history (never vs. current or former), varicocele data (surgical side (left vs. bilateral), grade (left, II vs. III), diameter of veins (left, mm), testicular volume (left, mL), semen parameters prior to MSV (baseline total progressively motile sperm count (TPMSC, 106/mL), and intraoperative data (surgical approach (subinguinal vs. Inguinal), number of ligated veins).
The physical examination in an upright position led to the diagnosis of varicocele (VC). The VC was categorized based on the Dubin and Amelar grading system: Grade 1: palpable during Valsalva maneuver. Grade 2: palpable at rest. Grade 3: visible and palpable at rest. By using Doppler ultrasonography to quantify all varicoceles, it was possible to establish their existence and determine their true diameters (The maximum venous diameter). The lack of a desired pregnancy after regular, unprotected sexual activity for at least one year is referred to as infertility. A patient is considered to be a current smoker if he has smoked 100 cigarettes during his life. The formula V = L × W × H × 0.71 was used to calculate the volume of the testicles. The total sperm count and the ratio of the progressively motile was used to determine total progressively motile sperm count (TPMSC = total sperm number (106/ejaculate) × progressive motility (PR, %); PR, progressive (a + b motility)] [15]. In post-operative semen analysis, we defined semen parameter improvement as a greater than 50% increase in total motile sperm count (TMSC) within six months of surgery [15]. Semen analysis was conducted repeatedly and assessed in accordance with WHO guidelines [16]. The follow-up assessment was carried out by phone calls or clinical visits.
2.4. Statistical Analysis
The data were analyzed using STATA 15.0 (Stata Corporation, College Station, TX, USA), R software (version 3.0.1; https://www.r-project.org/, accessed on 7 November 2022), and IBM SPSS Statistics for Windows, version 22.0 (IBM Corporation, Armonk, NY, USA). The R packages “rms” and “glmnet” were used in this work. With statistical significance being defined as a probability (p) value of less than 0.05, all of the statistical significance levels that were reported were two-sided.
The Kolmogorov–Smirnov test was used to establish the normality of continuous variables. The mean and standard deviation were used to describe regularly distributed continuous data, whereas the median (interquartile range) was employed to represent continuous variables that were not normally distributed. The independent-samples t test is used to compare the means of two continuous normally distributed variables. The t-test for students was used. To compare two continuous non-normally distributed variables, the Mann–Whitney U test was applied. Number was given as the categorical variable (percentage). To compare categorical variables, the Fisher’s exact test and the chi-squared test were used. A predictive nomogram with regression coefficients was constructed using multivariate unconditional logistic regression analysis. With Akaike’s information criteria serving as the stopping rule, the likelihood ratio test was used to apply the backward stepwise selection [17,18].
The effectiveness of the model was assessed in a different validation cohort. The logistic regression model created in the development cohort was utilized to compute the likelihood for each patient in the validation cohort. The area under the receiver operating characteristic (AUROC) curve was evaluated to gauge how well the model discriminated. An AUROC of 0.5 meant there was no prejudice, while an AUROC of 1.0 meant there was total discrimination. Calibration plots, the unreliability test, and the Hosmer-Lemeshow (H-L) chi-square statistic were all used to assess the model’s calibration (p > 0.05 indicating good calibration). A slope on the 45-degree line showed that the calibration was perfect. Using decision curve analysis, which evaluated the net benefits at various thresholds, it was possible to assess the clinical relevance of the model.
3. Results
There were 336 patients enrolled in the development cohort and 124 individuals included in the validation cohort following screening using the same inclusion and exclusion criteria. Semen parameter improvement after MSV was shown in 70.2% (236/336) of patients in the development cohort and 67.7% (84/124) of patients in the validation cohort, respectively; see more information in Table 1.

Table 1.
Demographics and clinical data in this cohort according to the development cohort and validation cohort.
Patients with improved semen parameters in the development cohort’s univariate analysis had greater vein diameters and higher baseline TPMSC levels (Table 2). A predictive nomogram with regression coefficients was constructed using multivariate binary logistic regression. The likelihood ratio test using Akaike’s information criteria was employed as the stopping rule. Backward stepwise selection was also used. The final model displays the outcomes (diameter of veins, and baseline TPMSC). Based on these findings, we created a predictive model, from which we constructed a nomogram that predicts an improvement in the semen parameter following MSV (Table 2 and Figure 2).

Table 2.
Demographics and clinical data in this cohort according to the semen parameters improvement type after MSV.
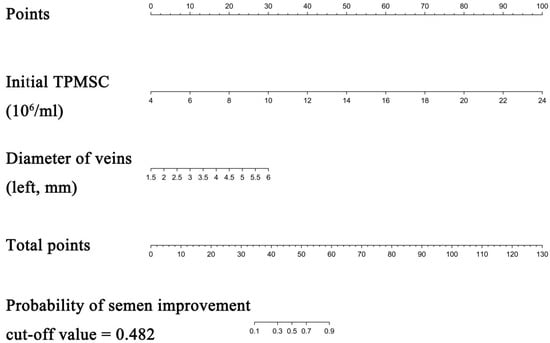
Figure 2.
Nomogram to predict semen parameters improvement after MSV. Each clinicopathologic factor corresponds to a specific point by drawing a line straight upward to the Points axis. After summing the points located on the Total points axis, the sum represents the probability of semen parameters improvement after MSV by drawing a line straight down to the risk axis.
The cutoff value of probability in this model was 48.2%, with a sensitivity of 93.64% and a specificity of 80.00%. The AUROC values for the development and validation cohorts were 0.9423 and 0.9256, respectively (Table 3 and Figure 3). The unreliability test statistic for calibration in the validation cohort was −0.011, with a p-value of 0.522, while the Emax and Eavg values were 0.124 and 0.045, respectively. With a p-value of 0.4231 and an H-L chi-square statistic of 10.2, the calibration was deemed to be adequate. The decision curve showed that utilizing this nomogram to predict semen parameter improvement after MSV was more beneficial than using either the treat-all-patients or treat-none schemes if a patient’s threshold probability was between 10% and 100%. The net advantage fell within this range (Figure 3).

Table 3.
Multivariate binary logistic regression of semen parameter improvement after MSV.
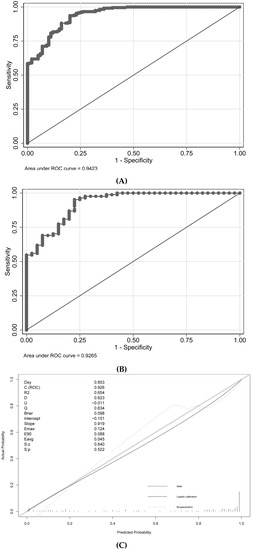
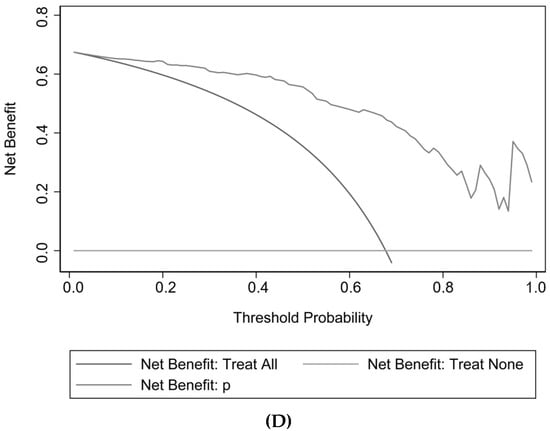
Figure 3.
Discrimination, calibration, and decision curve analysis for the model. (A) ROC in the development cohort; (B) ROC in the validation cohort; (C) Calibration plot; (D) Decision Curve Analysis.
Each clinicopathological characteristic was assigned to a particular point by drawing a line straight upward to the Points axis. The probability of improving the semen parameter was calculated by drawing a straight line from the risk axis straight down to the sum of the points on the Total Points axis. For instance, the baseline TPMSC level is 12 × 106/mL (13 points) and the vein width is 3.5 mm (40 points). The total score for this patient was 53. The estimated chance of semen parameter improvement following MSV was around 72%; the cutoff value was 48.2%. The decision-making process for a treatment plan may benefit from this result. Further information is provided in Supplement Figure S1.
4. Discussion
The precise relationship between varicocele and decreased male fertility is unknown. This has been explained by a number of theories, including hypoxia and hemostasis, elevated scrotal temperature, autoimmune, reflux of adrenal metabolites, and enhanced oxidative stress [2]. At least one-third of infertile men do not report an improvement in semen parameters [19,20], it is yet unknown why this is the case and which individuals would benefit from surgery. Therefore, the goal of this study was to develop and validate a nomogram based on a large cohort of infertile men with abnormal semen parameters for predicting semen parameter improvement following MSV. Our research indicates that the improvement of semen parameters in infertile men following MSV is significantly predicted by a greater vein diameter and a higher initial TPMSC.
Total sperm count multiplies the ratio of progressively motile sperm yielded the combined indication known as TPMSC. This study discovered a favorable correlation between greater initial TPMSC and the improvement of semen parameters following MSV. Those who claimed recovery after MSV had a considerably greater TPMSC than patients who did not recover (15.51 million vs. 9.82 million, respectively; p < 0.001). Wang et al. [21] found that the semen parameters improvement rate was higher in patients who presented a better baseline TMSC after varicocelectomy; it was highest (increased by 49.68 million) in the TMSC > 10 million group, and lowest in the TMSC 2 million group (increased by 10.20 million). This meta-systemic study, which included nine studies, supports these findings. Therefore, while estimating the semen parameter improvement rate of planned varicocelectomy, the combined indication of TPMSC should be employed.
In this study, improvement in semen parameters following varicocelectomy was significantly predicted by the diameter of veins (as determined by ultrasonography). In agreement with this, Palmisano F et al. [13] discovered that patients with greater USVG (grade 3 and above) were more likely to benefit from surgery based on a retrospective research study that enrolled 228 patients who underwent unilateral MSV. A greater varicocele grade (grade 2 or 3) is a major predictor of excellent results following surgery, according to Shabana et al. [14], who conducted another prospective cohort research with 123 patients who had subinguinal MSV. The physical examination is frequently misleading because to its subjective character and dependence on the surgeon’s expertise, hence in our opinion US grading is superior to palpable examination and more connected to the treatment success. Particularly in patients who are obese, have high-located testes, or have had inguinal surgery in the past may find it to be of limited benefit. However, several investigations have revealed no correlation between varicocele grade and improved semen parameter [12]. The variation in inclusion criteria and the standard of metrics assessed may account for this discrepancy.
The study includes several limitations. First, a single-center retrospective design was used. Second, certain potential predictors were absent despite their lack of widespread acceptance in clinical practice, such as DNA fragmentation index and anti-sperm antibodies. The anti-sperm antibody (ASA) can decrease sperm motility and, therefore, cause male infertility. However, current evidence has shown that positivity for ASA is not a predictor of the outcome after varicocelectomy [22]. Third, a recent study demonstrated that laparoscopic varicocelectomy is also a valid therapeutic approach to improve semen parameters for further assisted reproductive techniques; and it is particularly preferred in those who underwent bilateral varicocelectomy [23]. However, because microscopic surgery is the more preferred practice for varicocelectomy, laparoscopic or open procedures were excluded in this study. The short follow-up time was the fourth drawback (at least six months), which may underestimate the improvement rate of semen parameters. However, the typical duration to improve semen parameters following varicocelectomy is up to two spermatogenic cycles (less than six months) [21]. Fifth, we could not explore whether these predictors are associated with spontaneous pregnancy, which should be considered the ultimate goal of varicocelectomy; Therefore, further studies were needed to solve this issue. Finally, this study excluded patients with a history of previous varicocelectomy; thus, this nomogram cannot be applied to patients with recurrent varicocele. Despite the drawbacks, this is the first nomogram for predicting the improvement of the semen parameters in infertile men following MSV with excellent external validation.
According to this study, the improvement of semen parameters in infertile men following MSV was significantly predicted by greater vein diameter and higher initial TPMSC. This nomogram aids in individualized decision making on the varicocele preoperative treatment plan and may help to enhance the therapeutic result.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13010011/s1, Figure S1: Example of nomogram to predict semen parameters improvement after MSV.
Author Contributions
H.S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. H.S.: Protocol/project development; X.L., D.L., C.P. and H.S.: Data collection or management; X.L., D.L., C.P. and H.S.: Data analysis; X.L., D.L., C.P. and H.S.: Manuscript writing/editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by The 345 Talent Project of Shengjing Hospital.
Institutional Review Board Statement
The Ethics Committee Shengjing Hospital Affiliated China Medical University granted its approval (2022PS719K). ChiCTR2200060504 is the UIN for the clinical research registry. The 1975 Declaration of Helsinki’s ethical principles were followed by the study procedure. The clinical research registry UIN is ChiCTR2200060504 (Chinese Clinical Trial Registry: http://www.chictr.org.cn/index.aspx, accessed on 8 November 2022).
Informed Consent Statement
Informed consent from all eligible patients was obtained.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We give special thanks to all the colleagues at Shengjing Hospital for their help and support. The authors would like to thank all of the study participants.
Conflicts of Interest
The authors declare no conflict of interest. These sponsors had no role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
MSV: microscopic varicocelectomy.
References
- Damsgaard, J.; Joensen, U.N.; Carlsen, E.; Erenpreiss, J.; Jensen, M.B.; Matulevicius, V.; Zilaitiene, B.; Olesen, I.A.; Perheentupa, A.; Punab, M.; et al. Varicocele is associated with impaired semen quality and reproductive hormone levels: A study of 7035 healthy young men from six European countries. Eur. Urol. 2016, 70, 1019–1029. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Baazeem, A.; Belzile, E.; Ciampi, A.; Dohle, G.; Jarvi, K.; Salonia, A.; Weidner, W.; Zini, A. Varicocele and male factor infertility treatment: A new meta-analysis and review of the role of varicocele repair. Eur. Urol. 2011, 60, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Dubin, J.M.; Greer, A.B.; Kohn, T.P.; Masterson, T.A.; Ji, L.; Ramasamy, R. Men with severe oligospermia appear to benefit from varicocele repair: A cost-effectiveness analysis of assisted reproductive technology. Urology 2018, 111, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kohn, T.P.; Kohn, J.R.; Pastuszak, A.W. Varicocelectomy before assisted reproductive technology: Are outcomes improved? Fertil. Steril. 2017, 108, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Tian, J.; Du, W.; Zhang, L.; Wang, H.; Wang, Z. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: A meta-analysis of randomized controlled trials. BJU Int. 2012, 110, 1536–1542. [Google Scholar] [CrossRef]
- Wang, H.; Ji, Z.G. Microsurgery versus laparoscopic surgery for varicocele: A meta-analysis and systematic review of randomized controlled trials. J. Investig. Surg. 2020, 33, 40–48. [Google Scholar] [CrossRef]
- Bryniarski, P.; Taborowski, P.; Rajwa, P.; Kaletka, Z.; Życzkowski, M.; Paradysz, A. The comparison of laparoscopic and microsurgical varicocoelectomy in infertile men with varicocoele on paternity rate 12 months after surgery: A prospective randomized controlled trial. Andrology 2017, 5, 445–450. [Google Scholar] [CrossRef]
- Pan, F.; Pan, L.; Zhang, A.; Liu, Y.; Zhang, F.; Dai, Y. Comparison of two approaches in microsurgical varicocelectomy in Chinese infertile males. Urol. Int. 2013, 90, 443–448. [Google Scholar] [CrossRef]
- Almekaty, K.; Zahran, M.H.; Zoeir, A.; Minhas, S.; Salem, K. The role of artery-preserving varicocelectomy in subfertile men with severe oligozoospermia: A randomized controlled study. Andrology 2019, 7, 193–198. [Google Scholar] [CrossRef]
- Bozhedomov, V.A.; Lipatova, N.A.; Alexeev, R.A.; Alexandrova, L.M.; Nikolaeva, M.A.; Sukhikh, G.T. The role of the antisperm antibodies in male infertility assessment after microsurgical varicocelectomy. Andrology 2014, 2, 847–855. [Google Scholar] [CrossRef]
- Huang, H.C.; Huang, S.T.; Chen, Y.; Hsu, Y.C.; Chang, P.C.; Hsieh, M.L. Prognostic factors for successful varicocelectomy to treat varicocele-associated male infertility. Reprod. Fertil. Dev. 2014, 26, 485–490. [Google Scholar] [CrossRef]
- Palmisano, F.; Moreno-Mendoza, D.; Ievoli, R.; Veber-Moisés-Da Silva, G.; Gasanz-Serrano, C.; Villegas-Osorio, J.F.; Peraza-Godoy, M.F.; Vives, Á.; Bassas, L.; Montanari, E.; et al. Clinical factors affecting semen improvement after microsurgical subinguinal varicocelectomy: Which subfertile patients benefit from surgery? Ther. Adv. Urol. 2019, 11, 1756287219887656. [Google Scholar] [CrossRef]
- Shabana, W.; Teleb, M.; Dawod, T.; Elsayed, E.; Desoky, E.; Shahin, A.; Eladl, M.; Sorour, W. Predictors of improvement in semen parameters after varicocelectomy for male subfertility: A prospective study. Can. Urol. Assoc. J. 2015, 9, E579–E582. [Google Scholar] [CrossRef]
- Ok, F.; Erdogan, O.; Durmus, E. Can preoperative gonadotropin and testosterone levels predict the success of varicocelectomy? Andrologia 2020, 52, e13887. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010; pp. 1–286. Available online: https://www.who.int/docs/default-source/reproductive-health/srhr-documents/infertility/examination-and-processing-of-human-semen-5ed-eng.pdf (accessed on 7 November 2022).
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Boulesteix, A.L.; Binder, H. Stability investigations of multivariable regression models derived from low- and high-dimensional data. J. Biopharm. Stat. 2011, 21, 1206–1231. [Google Scholar] [CrossRef]
- Abdel-Meguid, T.A.; Al-Sayyad, A.; Tayib, A.; Farsi, H.M. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur. Urol. 2011, 59, 455–461. [Google Scholar] [CrossRef]
- Samplaski, M.K.; Lo, K.C.; Grober, E.D.; Zini, A.; Jarvi, K.A. Varicocelectomy to “upgrade” semen quality to allow couples to use less invasive forms of assisted reproductive technology. Fertil. Steril. 2017, 108, 609–612. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Liu, Y.; Wang, L. Outcome of varicocelectomy on different degrees of total motile sperm count: A systematic review and meta-analysis. Syst. Biol. Reprod. Med. 2019, 65, 430–436. [Google Scholar] [CrossRef]
- Al-Adl, A.M.; El-Karamany, T.; Issa, H.; Zaazaa, M. The influence of antisperm antibodies, intratesticular haemodynamics and the surgical approach to varicocelectomy on seminal variables. Arab J. Urol. 2014, 12, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Seiler, F.; Kneissl, P.; Hamann, C.; Jünemann, K.P.; Osmonov, D. Laparoscopic varicocelectomy in male infertility: Improvement of seminal parameters and effects on spermatogenesis. Wien. Klin. Wochenschr. 2022, 34, 51–55. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).