Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation
Abstract
1. Introduction
2. Review Methodology
3. The Pathogenesis of AD: A Brief Overview
4. Current Pharmacotherapeutic Trends in the Management of AD
4.1. Non-Pharmacological Interventions
4.2. Pharmacological Interventions
4.2.1. Pros and Cons of AChEIs
4.2.2. Drug Repurposing in AD
4.2.3. Candidate Vaccines in AD
4.2.4. Other Interventional Approaches in AD: One Step Forward
5. Natural Phytochemicals as New Potential Therapeutic Strategies in AD
5.1. Naturally-Occurring Bioactives in AD
5.2. Role of Phytochemicals in AD: Underlying Molecular Mechanisms of Action
5.2.1. Antioxidant Mechanisms
- (1)
- inhibit the formation of free radicals (indirect antioxidants),
- (2)
- directly eliminate chemically generated free radicals (direct antioxidants)
- (3)
- strengthen the cellular capacity to cope with high ROS loads, enzymatically detoxify accumulated ROS or promote the damage repair caused by oxidation (metabolic antioxidants) [116].
5.2.2. Anti-Neuroinflammatory Mechanisms
- (1)
- an inhibitory role in the release of cytokines, such as IL-1β and TNF-α, from activated glia;
- (2)
- an inhibitory action against inducible nitric oxide synthase (iNOS) induction and subsequent NO production in response to glial activation;
- (3)
- an ability to inhibit the activation of NADPH oxidase and subsequent generation of ROS in activated glia;
- (4)
- an inhibitory activity regulation of pro-inflammatory transcription factors such as nuclear factor (NF)-κB through the modulation of various glial and neuronal signaling pathways [158,159]. Ferulic acid, CA, tyrosol, hydroxytyrosol, and vanillic acid have been evaluated as therapeutic agents in neuroinflammation diseases [160].
5.2.3. Anti-Aggregation of β Amyloid Peptides
5.2.4. Anticholinesterase Mechanisms
6. Phytochemicals in AD: From Bioactive Effects to Bioavailability Issues
7. Overall Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- No authors listed. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020; Online ahead of print. [CrossRef]
- Amor, S.; Peferoen, L.A.; Vogel, D.Y.; Breur, M.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 21. [Google Scholar] [CrossRef]
- Siokas, V.; Aloizou, A.M.; Tsouris, Z.; Liampas, I.; Liakos, P.; Calina, D.; Docea, A.O.; Tsatsakis, A.; Bogdanos, D.P.; Hadjigeorgiou, G.M.; et al. ADORA2A rs5760423 and CYP1A2 rs762551 Polymorphisms as Risk Factors for Parkinson’s Disease. J. Clin. Med. 2021, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef] [PubMed]
- Epperly, T.; Dunay, M.A.; Boice, J.L. Alzheimer Disease: Pharmacologic and Nonpharmacologic Therapies for Cognitive and Functional Symptoms. Am. Fam. Physician 2017, 95, 771–778. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Bouyahya, A.; El Menyiy, N.; El Omari, N.; Shahinozzaman, M.; Ara Haque Ovey, M.; Koirala, N.; Panthi, M.; Ertani, A.; et al. Ethnobotany, Phytochemistry, Biological Activities, and Health-Promoting Effects of the Genus Bulbophyllum. Evid. -Based Complementary Altern. Med. 2022, 2022, 6727609. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Lushchak, O. Neuroinflammation in pathogenesis of Alzheimer’s disease: Phytochemicals as potential therapeutics. Mech. Ageing Dev. 2020, 189, 111259. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Saikat, A.S.M.; Jain, D.; Habib, A.; Janmeda, P.; Islam, M.T.; Radha, U.; Daştan, S.D.; Kumar, M.; et al. Biosynthesis of Secondary Metabolites Based on the Regulation of MicroRNAs. Biomed. Res. Int. 2022, 2022, 9349897. [Google Scholar] [CrossRef]
- Hossain, R.; Sarkar, C.; Hassan, S.M.H.; Khan, R.A.; Arman, M.; Ray, P.; Islam, M.T.; Daştan, S.D.; Sharifi-Rad, J.; Almarhoon, Z.M.; et al. In Silico Screening of Natural Products as Potential Inhibitors of SARS-CoV-2 Using Molecular Docking Simulation. Chin. J. Integr. Med. 2021, 28, 249–256. [Google Scholar] [CrossRef]
- Welcome, M.O. Blood brain barrier inflammation and potential therapeutic role of phytochemicals. PharmaNutrition 2020, 11, 100177. [Google Scholar] [CrossRef]
- WFO. WFO The World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 23 February 2022).
- Chemspider. Available online: http://www.chemspider.com/ (accessed on 23 February 2022).
- Arnsten, A.F.T.; Datta, D.; Preuss, T.M. Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer’s-like neuropathology: An evolutionary perspective. Am. J. Primatol. 2021, 83, e23254. [Google Scholar] [CrossRef]
- Ladner, C.J.; Lee, J.M. Pharmacological drug treatment of Alzheimer disease: The cholinergic hypothesis revisited. J. Neuropathol. Exp. Neurol. 1998, 57, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef]
- Zagórska, A.; Jaromin, A. Perspectives for New and More Efficient Multifunctional Ligands for Alzheimer′s Disease Therapy. Molecules 2020, 25, 3337. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Munoz, J.P.; Barbeito, L. The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch. Med. Res. 2001, 32, 367–381. [Google Scholar] [CrossRef]
- Agis-Torres, A.; Solhuber, M.; Fernandez, M.; Sanchez-Montero, J.M. Multi-Target-Directed Ligands and other Therapeutic Strategies in the Search of a Real Solution for Alzheimer’s Disease. Curr. Neuropharmacol. 2014, 12, 2–36. [Google Scholar] [CrossRef]
- Tuppo, E.E.; Arias, H.R. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005, 37, 289–305. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat. Med. 2006, 12, 1005–1015. [Google Scholar] [CrossRef]
- Bacci, A.; Runfola, M.; Sestito, S.; Rapposelli, S. Beyond Antioxidant Effects: Nature-Based Templates Unveil New Strategies for Neurodegenerative Diseases. Antioxidants 2021, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Martorell, M.; Sharopov, F.; Tumer, T.B.; Kurt, B.; Lankatillake, C.; Docea, A.O.; Moreira, A.C.; et al. A Pharmacological Perspective on Plant-derived Bioactive Molecules for Epilepsy. Neurochem. Res. 2021, 46, 2205–2225. [Google Scholar] [CrossRef]
- Nesi, G.; Sestito, S.; Digiacomo, M.; Rapposelli, S. Oxidative Stress, Mitochondrial Abnormalities and Proteins Deposition: Multitarget Approaches in Alzheimer’s Disease. Curr. Top. Med. Chem. 2017, 17, 3062–3079. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Grundman, M.; Petersen, R.C.; Ferris, S.H.; Thomas, R.G.; Aisen, P.S.; Bennett, D.A.; Foster, N.L.; Jack, C.R., Jr.; Galasko, D.R.; Doody, R.; et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch. Neurol. 2004, 61, 59–66. [Google Scholar] [CrossRef]
- Nussbaum, L.; Hogea, L.M.; Calina, D.; Andreescu, N.; Gradinaru, R.; Stefanescu, R.; Puiu, M. Modern treatment approaches in psychoses. pharmacogenetic, neuroimagistic and clinical implications. Farmacia 2017, 65, 75–81. [Google Scholar]
- Zucchella, C.; Sinforiani, E.; Tamburin, S.; Federico, A.; Mantovani, E.; Bernini, S.; Casale, R.; Bartolo, M. The Multidisciplinary Approach to Alzheimer’s Disease and Dementia. A Narrative Review of Non-Pharmacological Treatment. Front. Neurol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Calina, D.; Buga, A.M.; Mitroi, M.; Buha, A.; Caruntu, C.; Scheau, C.; Bouyahya, A.; El Omari, N.; El Menyiy, N.; Docea, A.O. The Treatment of Cognitive, Behavioural and Motor Impairments from Brain Injury and Neurodegenerative Diseases through Cannabinoid System Modulation-Evidence from In Vivo Studies. J. Clin. Med. 2020, 9, 2395. [Google Scholar] [CrossRef]
- Howard, R.; Liu, K.Y. Questions EMERGE as Biogen claims aducanumab turnaround. Nat. Rev. Neurol. 2020, 16, 63–64. [Google Scholar] [CrossRef]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed]
- Finn, L.A. Current Medications for the Treatment of Alzheimer’s Disease. In Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 49–58. [Google Scholar] [CrossRef]
- Tricco, A.C.; Ashoor, H.M.; Soobiah, C.; Rios, P.; Veroniki, A.A.; Hamid, J.S.; Ivory, J.D.; Khan, P.A.; Yazdi, F.; Ghassemi, M.; et al. Comparative Effectiveness and Safety of Cognitive Enhancers for Treating Alzheimer’s Disease: Systematic Review and Network Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 170–178. [Google Scholar] [CrossRef]
- Korabecny, J.; Nepovimova, E.; Cikankova, T.; Spilovska, K.; Vaskova, L.; Mezeiova, E.; Kuca, K.; Hroudova, J. Newly Developed Drugs for Alzheimer’s Disease in Relation to Energy Metabolism, Cholinergic and Monoaminergic Neurotransmission. Neuroscience 2018, 370, 191–206. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Herrera-Bravo, J.; Beltrán, J.F.; Islam, M.T.; Shaheen, S.; Cruz-Martins, N.; Martorell, M.; Kumar, M.; Sharifi-Rad, J.; et al. Neurobiological Promises of the Bitter Diterpene Lactone Andrographolide. Oxid. Med. Cell. Longev. 2022, 2022, 3079577. [Google Scholar] [CrossRef]
- Mallikarjun, V.; Swift, J. Therapeutic Manipulation of Ageing: Repurposing Old Dogs and Discovering New Tricks. EBioMedicine 2016, 14, 24–31. [Google Scholar] [CrossRef][Green Version]
- Bauzon, J.; Lee, G.; Cummings, J. Repurposed agents in the Alzheimer’s disease drug development pipeline. Alzheimers Res. 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Calina, D.; Sarkar, C.; Arsene, A.L.; Salehi, B.; Docea, A.O.; Mondal, M.; Islam, M.T.; Zali, A.; Sharifi-Rad, J. Recent advances, approaches and challenges in targeting pathways for potential COVID-19 vaccines development. Immunol. Res. 2020, 68, 315–324. [Google Scholar] [CrossRef]
- Islam, M.T.; Nasiruddin, M.; Khan, I.N.; Mishra, S.K.; Kudrat-E-Zahan, M.; Riaz, T.A.; Ali, E.S.; Rahman, M.S.; Mubarak, M.S.; Martorell, M.; et al. A Perspective on Emerging Therapeutic Interventions for COVID-19. Front. Public Health 2020, 8, 281. [Google Scholar] [CrossRef]
- Calina, D.; Hartung, T.; Docea, A.O.; Spandidos, D.A.; Egorov, A.M.; Shtilman, M.I.; Carvalho, F.; Tsatsakis, A. COVID-19 vaccines: Ethical framework concerning human challenge studies. Daru 2020, 28, 807–812. [Google Scholar] [CrossRef]
- Kostoff, R.N.; Kanduc, D.; Porter, A.L.; Shoenfeld, Y.; Calina, D.; Briggs, M.B.; Spandidos, D.A.; Tsatsakis, A. Vaccine- and natural infection-induced mechanisms that could modulate vaccine safety. Toxicol. Rep. 2020, 7, 1448–1458. [Google Scholar] [CrossRef]
- Neagu, M.; Calina, D.; Docea, A.O.; Constantin, C.; Filippini, T.; Vinceti, M.; Drakoulis, N.; Poulas, K.; Nikolouzakis, T.K.; Spandidos, D.A.; et al. Back to basics in COVID-19: Antigens and antibodies-Completing the puzzle. J. Cell. Mol. Med. 2021, 25, 4523–4533. [Google Scholar] [CrossRef]
- Calina, D.; Hernández, A.F.; Hartung, T.; Egorov, A.M.; Izotov, B.N.; Nikolouzakis, T.K.; Tsatsakis, A.; Vlachoyiannopoulos, P.G.; Docea, A.O. Challenges and Scientific Prospects of the Newest Generation of mRNA-Based Vaccines against SARS-CoV-2. Life 2021, 11, 907. [Google Scholar] [CrossRef]
- Cacabelos, R. How plausible is an Alzheimer’s disease vaccine? Expert Opin. Drug Discov. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Rafii, M.S.; Sol, O.; Mobley, W.C.; Delpretti, S.; Skotko, B.G.; Burke, A.D.; Sabbagh, M.N.; Yuan, S.H.; Rissman, R.A.; Pulsifer, M.; et al. Safety, Tolerability, and Immunogenicity of the ACI-24 Vaccine in Adults With Down Syndrome: A Phase 1b Randomized Clinical Trial. JAMA Neurol. 2022, 79, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Lacosta, A.M.; Pascual-Lucas, M.; Pesini, P.; Casabona, D.; Pérez-Grijalba, V.; Marcos-Campos, I.; Sarasa, L.; Canudas, J.; Badi, H.; Monleón, I.; et al. Safety, tolerability and immunogenicity of an active anti-Aβ(40) vaccine (ABvac40) in patients with Alzheimer’s disease: A randomised, double-blind, placebo-controlled, phase I trial. Alzheimers Res. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Evaluate the Safety, Tolerability, Immunogenicity and Efficacy of UB-311 in Mild Alzheimer’s Disease (AD) Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT02551809 (accessed on 7 September 2022).
- Wang, C.Y.; Wang, P.N.; Chiu, M.J.; Finstad, C.L.; Lin, F.; Lynn, S.; Tai, Y.H.; De Fang, X.; Zhao, K.; Hung, C.H.; et al. UB-311, a novel UBITh(®) amyloid β peptide vaccine for mild Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2017, 3, 262–272. [Google Scholar] [CrossRef]
- Kwan, P.; Konno, H.; Chan, K.Y.; Baum, L. Rationale for the development of an Alzheimer’s disease vaccine. Hum. Vaccin. Immunother. 2020, 16, 645–653. [Google Scholar] [CrossRef]
- Novak, P.; Schmidt, R.; Kontsekova, E.; Kovacech, B.; Smolek, T.; Katina, S.; Fialova, L.; Prcina, M.; Parrak, V.; Dal-Bianco, P.; et al. FUNDAMANT: An interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res. 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Kwon, H.S.; Choi, S.H.; Jeong, J.H.; Na, H.R.; Lee, C.N.; Yang, Y.; Lee, A.Y.; Lee, J.H.; Park, K.W.; et al. Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: A phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimers Res. 2021, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T.; Greenblatt, C.L.; Chan, E.D.; Dow, J.F. Evaluation of BCG Vaccination and Plasma Amyloid: A Prospective, Pilot Study with Implications for Alzheimer’s Disease. Microorganisms 2022, 10, 424. [Google Scholar] [CrossRef]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. [Google Scholar] [CrossRef]
- Islam, M.T.; Quispe, C.; Martorell, M.; Docea, A.O.; Salehi, B.; Calina, D.; Reiner, Ž.; Sharifi-Rad, J. Dietary supplements, vitamins and minerals as potential interventions against viruses: Perspectives for COVID-19. Int. J. Vitam. Nutr. Res. 2022, 92, 49–66. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Zlatian, O.; Mitroi, M.; Renieri, E.; Tsatsakis, A.; Izotov, B.N.; Burada, F.; Sosoi, S.; Burada, E.; Buga, A.M.; et al. A Novel Nutraceutical Formulation Can Improve Motor Activity and Decrease the Stress Level in a Murine Model of Middle-Age Animals. J. Clin. Med. 2021, 10, 624. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Tumer, T.B.; Moreira, A.C.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharm. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharm. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Kim, M.O.; Ali, T. Editorial: Natural Products-Based Drugs: Potential Therapeutics Against Alzheimer’s Disease and Other Neurological Disorders. Front Pharm. 2019, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Otterdijk, R.; McYbeck, A. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Kamiloglu, S.; Petroni, K.; Mishra, A.P.; Monserrat-Mesquida, M.; Sureda, A.; Martorell, M.; Aidarbekovna, D.S.; Yessimsiitova, Z.; et al. Recent advances in the therapeutic potential of emodin for human health. Biomed. Pharmacother. 2022, 154, 113555. [Google Scholar] [CrossRef] [PubMed]
- Taroncher, M.; Vila-Donat, P.; Tolosa, J.; Ruiz, M.J.; Rodríguez-Carrasco, Y. Biological activity and toxicity of plant nutraceuticals: An overview. Curr. Opin. Food Sci. 2021, 42, 113–118. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer Potential and Other Pharmacological Properties of Prunus armeniaca L.: An Updated Overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luis, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling-from basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Fermin, L.M.; Aparicio-Trejo, O.E.; Avila-Rojas, S.H.; Gomez-Sierra, T.; Martinez-Klimova, E.; Pedraza-Chaverri, J. Natural antioxidants’ effects on endoplasmic reticulum stress-related diseases. Food Chem. Toxicol. 2020, 138, 111229. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. Biomed. Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Buga, A.M.; Docea, A.O.; Sarandi, E.; Mitrut, R.; Renieri, E.; Spandidos, D.A.; Rogoveanu, I.; Cercelaru, L.; Niculescu, M.; et al. Reversal of brain aging by targeting telomerase: A nutraceutical approach. Int. J. Mol. Med. 2021, 48, 199. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.O.; Alegakis, A.K.; Sarandi, E.; Thanasoula, M.; Spandidos, D.A.; Tsatsakis, A.; Razgonova, M.P.; Calina, D. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 2019, 20, 3701–3708. [Google Scholar] [CrossRef] [PubMed]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; D’Errico, S.; Ciccone, R.; De Feo, V.; Secondo, A.; Pannaccione, A. Exploring the Therapeutic Potential of Phytochemicals in Alzheimer’s Disease: Focus on Polyphenols and Monoterpenes. Front Pharm. 2022, 13, 876614. [Google Scholar] [CrossRef]
- Libro, R.; Giacoppo, S.; Soundara Rajan, T.; Bramanti, P.; Mazzon, E. Natural Phytochemicals in the Treatment and Prevention of Dementia: An Overview. Molecules 2016, 21, 518. [Google Scholar] [CrossRef]
- Farooqui, A.A. Therapeutic Potentials of Curcumin for Alzheimer Disease; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ali, S.K.; Hamed, A.R.; Soltan, M.M.; Hegazy, U.M.; Elgorashi, E.E.; El-Garf, I.A.; Hussein, A.A. In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of Alzheimer disease. BMC Complement. Altern. Med. 2013, 13, 121. [Google Scholar] [CrossRef]
- Chen, D. Neuroprotective Effect of Amorphophallus Campanulatus in Stz Induced Alzheimer Rat Model. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 47–54. [Google Scholar] [CrossRef]
- Szilagyi, G.; Nagy, Z.; Balkay, L.; Boros, I.; Emri, M.; Lehel, S.; Marian, T.; Molnar, T.; Szakall, S.; Tron, L.; et al. Effects of vinpocetine on the redistribution of cerebral blood flow and glucose metabolism in chronic ischemic stroke patients: A PET study. J. Neurol. Sci. 2005, 229–230, 275–284. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Williamson, J.D.; Fitzpatrick, A.L.; Kronmal, R.A.; Ives, D.G.; Saxton, J.A.; Lopez, O.L.; Burke, G.; Carlson, M.C.; Fried, L.P.; et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 2008, 300, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Sim, J.Y.; Heo, J.H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Hancianu, M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid beta(1-42) rat model of Alzheimer’s disease. Physiol. Behav. 2013, 120, 193–202. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Yilmaz, S.V.; Yildirim, K.; Pinar, N.M.; Demirci, B.; Brestic, M.; Sytar, O. Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules 2019, 24, 722. [Google Scholar] [CrossRef]
- Arruda, M.; Viana, H.; Rainha, N.; Neng, N.R.; Rosa, J.S.; Nogueira, J.M.; Barreto Mdo, C. Anti-acetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules 2012, 17, 3082–3092. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Khan, M.A.; Ahmad, W.; Shah, M.R.; Imran, M.; Ahmad, S. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: A preliminary anti- Alzheimer’s study. Lipids Health Dis. 2015, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Hancianu, M.; Cioanca, O.; Mihasan, M.; Hritcu, L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine 2013, 20, 446–452. [Google Scholar] [CrossRef]
- Kamal, Z.; Ullah, F.; Ayaz, M.; Sadiq, A.; Ahmad, S.; Zeb, A.; Hussain, A.; Imran, M. Anticholinesterase and antioxidant investigations of crude extracts, subsequent fractions, saponins and flavonoids of atriplex laciniata L.: Potential effectiveness in Alzheimer’s and other neurological disorders. Biol. Res. 2015, 48, 21. [Google Scholar] [CrossRef]
- Geiser, R.J.; Chastain, S.E.; Moss, M.A. Regulation of Bace1 Mrna Expression in Alzheimer’S Disease by Green Tea Catechins and Black Tea Theaflavins. Biophys. J. 2017, 112, 362a. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Liu, A.; Gao, X.; Chen, X.; Ye, M.; Ye, J.; Liu, P.; Xu, S.; Liu, J.; et al. Osthole, a natural coumarin improves cognitive impairments and BBB dysfunction after transient global brain ischemia in C57 BL/6J mice: Involvement of Nrf2 pathway. Neurochem. Res. 2015, 40, 186–194. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Moreno, L.; Puerta, E.; Suarez-Santiago, J.E.; Santos-Magalhaes, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Kaur, N.; Dhiman, M.; Perez-Polo, J.R.; Mantha, A.K. Ginkgolide B revamps neuroprotective role of apurinic/apyrimidinic endonuclease 1 and mitochondrial oxidative phosphorylation against Abeta25-35 -induced neurotoxicity in human neuroblastoma cells. J. Neurosci. Res. 2015, 93, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.H.; Chen, X.; Hua, H.P.; Liang, L.; Liu, L.J. The Oral Pretreatment of Glycyrrhizin Prevents Surgery-Induced Cognitive Impairment in Aged Mice by Reducing Neuroinflammation and Alzheimer’s-Related Pathology via HMGB1 Inhibition. J. Mol. Neurosci. 2017, 63, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Videira, R.; Castanheira, P.; Graos, M.; Resende, R.; Salgueiro, L.; Faro, C.; Cavaleiro, C. Dose-dependent inhibition of BACE-1 by the monoterpenoid 2,3,4,4-tetramethyl-5-methylenecyclopent-2-enone in cellular and mouse models of Alzheimer’s disease. J. Nat. Prod. 2014, 77, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Perez-Severiano, F.; Salvatierra-Sanchez, R.; Rodriguez-Perez, M.; Cuevas-Martinez, E.Y.; Guevara, J.; Limon, D.; Maldonado, P.D.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Santamaria, A. S-Allylcysteine prevents amyloid-beta peptide-induced oxidative stress in rat hippocampus and ameliorates learning deficits. Eur. J. Pharm. 2004, 489, 197–202. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Kamiloglu, S.; Yeskaliyeva, B.; Beyatli, A.; Alfred, M.A.; Salehi, B.; Calina, D.; Docea, A.O.; Imran, M.; Kumar, N.V.A.; et al. Pharmacological Activities of Psoralidin: A Comprehensive Review of the Molecular Mechanisms of Action. Front. Pharmacol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Ali, E.S.; Akter, S.; Ramproshad, S.; Mondal, B.; Riaz, T.A.; Islam, M.T.; Khan, I.N.; Docea, A.O.; Calina, D.; Sharifi-Rad, J.; et al. Targeting Ras-ERK cascade by bioactive natural products for potential treatment of cancer: An updated overview. Cancer Cell Int. 2022, 22, 246. [Google Scholar] [CrossRef]
- Youssif, K.A.; Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Seleem, A.; Salem, M.A.; Hussein, A.S.; Krischke, M.; Mueller, M.J.; et al. Anti-Alzheimer potential, metabolomic profiling and molecular docking of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea aqueous extracts. PLoS ONE 2019, 14, e0223781. [Google Scholar] [CrossRef]
- Suganthy, N.; Sri Ramkumar, V.; Pugazhendhi, A.; Benelli, G.; Archunan, G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: Assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ. Sci. Pollut. Res. Int. 2018, 25, 10418–10433. [Google Scholar] [CrossRef]
- El-Hawwary, S.S.; Abd Almaksoud, H.M.; Saber, F.R.; Elimam, H.; Sayed, A.M.; El Raey, M.A.; Abdelmohsen, U.R. Green-synthesized zinc oxide nanoparticles, anti-Alzheimer potential and the metabolic profiling of Sabal blackburniana grown in Egypt supported by molecular modelling. RSC Adv. 2021, 11, 18009–18025. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Berger, J.; Dorninger, F.; Forss-Petter, S.; Kunze, M. Peroxisomes in brain development and function. Biochim. Biophys. Acta 2016, 1863, 934–955. [Google Scholar] [CrossRef]
- Cheng, Y.; Bai, F. The Association of Tau With Mitochondrial Dysfunction in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 163. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Tutuncu, S.; Aydar, E.F.; Topkaya, C.; Mertdinc, Z.; Ozcelik, B.; Aital, M.; et al. A Review of Recent Studies on the Antioxidant and Anti-Infectious Properties of Senna Plants. Oxid. Med. Cell. Longev. 2022, 2022, 6025900. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharm. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 2005, 26 (Suppl. 1), 128–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, D.; Song, Z.L.; Liu, T.; Hou, Y.; Fang, J. Small molecules regulating reactive oxygen species homeostasis for cancer therapy. Med. Res. Rev. 2021, 41, 342–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Wu, C.T.; Yang, T.H.; Chang, Y.A.; Sheu, M.L.; Liu, S.H. Green Tea Catechin Prevents Hypoxia/Reperfusion-Evoked Oxidative Stress-Regulated Autophagy-Activated Apoptosis and Cell Death in Microglial Cells. J. Agric. Food. Chem. 2016, 64, 4078–4085. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Imran, M.; Moussa, A.Y.; Mostafa, N.M.; El-Shazly, M.; et al. Resveratrol’ biotechnological applications: Enlightening its antimicrobial and antioxidant properties. J. Herb. Med. 2022, 32, 100550. [Google Scholar] [CrossRef]
- Behl, C.; Moosmann, B. Oxidative nerve cell death in Alzheimer’s disease and stroke: Antioxidants as neuroprotective compounds. Biol. Chem. 2002, 383, 521–536. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Escobar, S.J.M.; Fong, G.M.; Winnischofer, S.M.B.; Simone, M.; Munoz, L.; Dennis, J.M.; Rocha, M.E.M.; Witting, P.K. Anti-proliferative and cytotoxic activities of the flavonoid isoliquiritigenin in the human neuroblastoma cell line SH-SY5Y. Chem. Biol. Interact. 2019, 299, 77–87. [Google Scholar] [CrossRef]
- Amin, R.; Quispe, C.; Docea, A.O.; Ydyrys, A.; Kulbayeva, M.; Durna Daştan, S.; Calina, D.; Sharifi-Rad, J. The role of Tumour Necrosis Factor in neuroinflammation associated with Parkinson’s disease and targeted therapies. Neurochem. Int. 2022, 158, 105376. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Prakash Mishra, A.; Sener, B.; et al. Urtica dioica-Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evid. Based Complement. Altern. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Lovell, M.A. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch. Neurol. 2007, 64, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/ RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Prosdocimi, T.; De Gioia, L.; Zampella, G.; Bertini, L. On the generation of OH(.) radical species from H2O2 by Cu(I) amyloid beta peptide model complexes: A DFT investigation. J. Biol. Inorg. Chem. 2016, 21, 197–212. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Kou, J.; Kovacs, G.G.; Hoftberger, R.; Kulik, W.; Brodde, A.; Forss-Petter, S.; Honigschnabl, S.; Gleiss, A.; Brugger, B.; Wanders, R.; et al. Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol. 2011, 122, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Scheper, W.; Hoozemans, J.J. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol. 2015, 130, 315–331. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Stadelmann, C. Problems of cell death in neurodegeneration and Alzheimer’s Disease. J. Alzheimers Dis. 2001, 3, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Fu, S.; Zhang, Y.; Li, Y.; He, D.; Ran, X.; Yan, X.; Du, J.; Meng, T.; et al. alpha-Cyperone Attenuates H2O2-Induced Oxidative Stress and Apoptosis in SH-SY5Y Cells via Activation of Nrf2. Front. Pharm. 2020, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.; Yabe, T. Use of hydrogen peroxide and peroxyl radicals to induce oxidative stress in neuronal cells. Robot. Auton. Syst. 2015, 3, 40–45. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Liu, H.; Zhang, X.; Li, X.; Geng, L.; Zhang, H.; Zhang, Q. Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H(2)O(2)-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway. Mar. Drugs 2017, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, J.; Wang, G.; Jiang, Y.; Shang, L.; Zhao, Y.; Huang, J.; Yang, S.; Wang, J.; Yu, Y. Design, synthesis and biological evaluation of coumarin derivatives as novel acetylcholinesterase inhibitors that attenuate H2O2-induced apoptosis in SH-SY5Y cells. Bioorg. Chem. 2016, 68, 112–123. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, H.; Park, H.; Jeon, J.W.; Cho, W.K.; Ma, J.Y. Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complement. Altern. Med. 2015, 15, 171. [Google Scholar] [CrossRef]
- Izuta, H.; Shimazawa, M.; Tazawa, S.; Araki, Y.; Mishima, S.; Hara, H. Protective effects of Chinese propolis and its component, chrysin, against neuronal cell death via inhibition of mitochondrial apoptosis pathway in SH-SY5Y cells. J. Agric. Food Chem. 2008, 56, 8944–8953. [Google Scholar] [CrossRef]
- Juan-García, A.; Caprioli, G.; Sagratini, G.; Mañes, J.; Juan, C. Coffee Silverskin and Spent Coffee Suitable as Neuroprotectors against Cell Death by Beauvericin and α-Zearalenol: Evaluating Strategies of Treatment. Toxins 2021, 13, 132. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Nunez-Sanchez, M.A.; Tomas-Barberan, F.A.; Espin, J.C. Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef]
- Miyamae, Y.; Han, J.; Sasaki, K.; Terakawa, M.; Isoda, H.; Shigemori, H. 3,4,5-tri-O-caffeoylquinic acid inhibits amyloid beta-mediated cellular toxicity on SH-SY5Y cells through the upregulation of PGAM1 and G3PDH. Cytotechnology 2011, 63, 191–200. [Google Scholar] [CrossRef][Green Version]
- Uberti, D.; Piccioni, L.; Colzi, A.; Bravi, D.; Canonico, P.L.; Memo, M. Pergolide protects SH-SY5Y cells against neurodegeneration induced by H(2)O(2). Eur. J. Pharm. 2002, 434, 17–20. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 108, 53193. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- Angeloni, S.; Freschi, M.; Marrazzo, P.; Hrelia, S.; Beghelli, D.; Juan-Garcia, A.; Juan, C.; Caprioli, G.; Sagratini, G.; Angeloni, C. Antioxidant and Anti-Inflammatory Profiles of Spent Coffee Ground Extracts for the Treatment of Neurodegeneration. Oxid. Med. Cell. Longev. 2021, 2021, 6620913. [Google Scholar] [CrossRef]
- Amato, A.; Terzo, S.; Mule, F. Natural Compounds as Beneficial Antioxidant Agents in Neurodegenerative Disorders: A Focus on Alzheimer’s Disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef]
- Li, Y.; Shi, W.; Li, Y.; Zhou, Y.; Hu, X.; Song, C.; Ma, H.; Wang, C.; Li, Y. Neuroprotective effects of chlorogenic acid against apoptosis of PC12 cells induced by methylmercury. Environ. Toxicol. Pharm. 2008, 26, 13–21. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharm. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Kakio, S.; Nakazawa, Y.; Kobata, K.; Funakoshi-Tago, M.; Suzuki, T.; Tamura, H. Roasted Coffee Reduces beta-Amyloid Production by Increasing Proteasomal beta-Secretase Degradation in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2018, 62, e1800238. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharm. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Corona, G.; Spencer, J.P. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Arch. Biochem. Biophys. 2010, 501, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Abeta25-35-Induced Autophagy and Cognitive Impairment via the mTOR/TFEB Signaling Pathway. Drug Desigh Dev. 2020, 14, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Park, R.Y.; Jeon, H.J.; Kwon, Y.S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, A.G.; Zgavc, T.; Kooijman, R.; Hachimi-Idrissi, S.; Sarre, S.; Michotte, Y. The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. J. Neuroinflammation 2010, 7, 74. [Google Scholar] [CrossRef]
- Taylor, D.L.; Jones, F.; Kubota, E.S.; Pocock, J.M. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. 2005, 25, 2952–2964. [Google Scholar] [CrossRef]
- MacEwan, D.J. TNF receptor subtype signalling: Differences and cellular consequences. Cell Signal. 2002, 14, 477–492. [Google Scholar] [CrossRef]
- Salehi, B.; Sestito, S.; Rapposelli, S.; Peron, G.; Calina, D.; Sharifi-Rad, M.; Sharopov, F.; Martins, N.; Sharifi-Rad, J. Epibatidine: A Promising Natural Alkaloid in Health. Biomolecules 2019, 9, 6. [Google Scholar] [CrossRef]
- Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Tunon, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104 (Suppl. 3), S15–S27. [Google Scholar] [CrossRef]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Pennisi, M.; Crupi, R.; Di Paola, R.; Ontario, M.L.; Bella, R.; Calabrese, E.J.; Crea, R.; Cuzzocrea, S.; Calabrese, V. Inflammasomes, hormesis, and antioxidants in neuroinflammation: Role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 2017, 95, 1360–1372. [Google Scholar] [CrossRef]
- Cheng, K.C.; Chiang, H.C. XBP1 and PERK Have Distinct Roles in Abeta-Induced Pathology. Mol. Neurobiol. 2018, 55, 7523–7532. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, A.U.; Abuine, R.; Palanisamy, S.; Lee, J.K.; Byun, H.-G. Characterization and purification of β−secretase inhibitory peptides fraction from sea cucumber (Holothuria spinifera) enzymatic hydrolysates. Process Biochem. 2021, 111, 86–96. [Google Scholar] [CrossRef]
- Ma, E.-H.; Rathnayake, A.U.; Lee, J.K.; Lee, S.-M.; Byun, H.-G. Characterization of β-secretase inhibitory extracts from sea cucumber (Stichopus japonicus) hydrolysis with their cellular level mechanism in SH-SY5Y cells. Eur. Food Res. Technol. 2021, 247, 2039–2052. [Google Scholar] [CrossRef]
- Chougle, S.; Kumar, D.; Khan, A.; Zehra, S.; Alİ, A. Treatment of Alzheimer’s disease by natural products. J. Exp. Clin. Med. 2021, 38, 634–644. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.L.; Liu, X.Z.; Shen, P.; Zheng, Y.G.; Lan, X.R.; Lu, C.B.; Wang, J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Chan, Y.C.; Liao, J.W.; Wang, M.F.; Yen, G.C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605. [Google Scholar] [CrossRef]
- Yamakawa, M.Y.; Uchino, K.; Watanabe, Y.; Adachi, T.; Nakanishi, M.; Ichino, H.; Hongo, K.; Mizobata, T.; Kobayashi, S.; Nakashima, K.; et al. Anthocyanin suppresses the toxicity of Abeta deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer’s disease. Nutr. Neurosci. 2016, 19, 32–42. [Google Scholar] [CrossRef]
- Wu, P.Q.; Li, B.; Yu, Y.F.; Su, P.J.; Liu, X.; Zhang, Z.P.; Zhi, D.J.; Qi, F.M.; Fei, D.Q.; Zhang, Z.X. Isolation, Characterization, and Possible Anti-Alzheimer’s Disease Activities of Bisabolane-Type Sesquiterpenoid Derivatives and Phenolics from the Rhizomes of Curcuma longa. Chem. Biodivers. 2020, 17, e2000067. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Curcumin effect on non-amyloidogenic pathway for preventing Alzheime’ s disease. Biointerface Res. Appl. Chem. 2019, 9, 4085–4089. [Google Scholar]
- Den Haan, J.; Morrema, T.H.J.; Rozemuller, A.J.; Bouwman, F.H.; Hoozemans, J.J.M. Different curcumin forms selectively bind fibrillar amyloid beta in post mortem Alzheimer’s disease brains: Implications for in-vivo diagnostics. Acta Neuropathol. Commun. 2018, 6, 75. [Google Scholar] [CrossRef]
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by beta-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Seo, E.J.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-kappaB for treatment of Alzheimer’s disease. Pharm. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Goncalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef]
- Yuan, N.N.; Cai, C.Z.; Wu, M.Y.; Su, H.X.; Li, M.; Lu, J.H. Neuroprotective effects of berberine in animal models of Alzheimer’s disease: A systematic review of pre-clinical studies. BMC Complement. Altern. Med. 2019, 19, 109. [Google Scholar] [CrossRef]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029113. [Google Scholar] [CrossRef]
- Santos, G.S.; Sinoti, S.B.P.; de Almeida, F.T.C.; Silveira, D.; Simeoni, L.A.; Gomes-Copeland, K.K.P. Use of galantamine in the treatment of Alzheimer’s disease and strategies to optimize its biosynthesis using the in vitro culture technique. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 143, 13–29. [Google Scholar] [CrossRef]
- Saito, T.; Hisahara, S.; Iwahara, N.; Emoto, M.C.; Yokokawa, K.; Suzuki, H.; Manabe, T.; Matsumura, A.; Suzuki, S.; Matsushita, T.; et al. Early administration of galantamine from preplaque phase suppresses oxidative stress and improves cognitive behavior in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Free Radic. Biol. Med. 2019, 145, 20–32. [Google Scholar] [CrossRef]
- Castillo, W.O.; Palomino, N.V.; Takahashi, C.S.; Giuliatti, S. Genistein and Galantamine Combinations Decrease beta-Amyloid Peptide (1-42)-Induced Genotoxicity and Cell Death in SH-SY5Y Cell Line: An In Vitro and In Silico Approach for Mimic of Alzheimer’s Disease. Neurotox. Res. 2020, 38, 691–706. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Makboul, R.M.; El-Mokhtar, M.A.; Abdel-Rahman, E.A.; Ahmed, I.A.; Nicola, M.A. Terpenoid-rich Elettaria cardamomum extract prevents Alzheimer-like alterations induced in diabetic rats via inhibition of GSK3beta activity, oxidative stress and pro-inflammatory cytokines. Cytokine 2019, 113, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.; Upadhyay, A.K.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Terpenoids as promising therapeutic molecules against Alzheimer’s disease: Amyloid beta- and acetylcholinesterase-directed pharmacokinetic and molecular docking analyses. Mol. Simul. 2017, 44, 1–11. [Google Scholar] [CrossRef]
- Koirala, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Comparative molecular docking studies of lupeol and lupenone isolated from Pueraria lobata that inhibits BACE1: Probable remedies for Alzheimer’s disease. Asian Pac. J. Trop. Med. 2017, 10, 1117–1122. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, H.; Turdu, G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: A review. Bioorg. Chem. 2017, 75, 50–61. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- Suganthy, N.; Pandima Devi, K. Protective effect of catechin rich extract of Rhizophora mucronata against β-amyloid-induced toxicity in PC12 cells. J. Appl. Biomed. 2016, 14, 137–146. [Google Scholar] [CrossRef]
- Okello, E.J.; Mather, J. Comparative Kinetics of Acetyl- and Butyryl-Cholinesterase Inhibition by Green Tea Catechins|Relevance to the Symptomatic Treatment of Alzheimer’s Disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, T.; Cortez-Torres, E.; Perez-Severiano, F.; Espinosa, B.; Guevara, J.; Perez-Benitez, A.; Melendez, F.J.; Diaz, A.; Ramirez, R.E. Antioxidative stress effect of epicatechin and catechin induced by Abeta25-35 in rats and use of the electrostatic potential and the Fukui function as a tool to elucidate specific sites of interaction. Neuropeptides 2016, 59, 89–95. [Google Scholar] [CrossRef]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef]
- Anggreani, E.; Cy, L. Neuroprotective Effect of Chlorogenic Acids against Alzheime’s Disease. Int. J. Food Sci. 2017, 649, 330–337. [Google Scholar]
- Agunloye, O.M.; Oboh, G. Caffeic acid and chlorogenic acid: Evaluation of antioxidant effect and inhibition of key enzymes linked with hypertension. J. Food Biochem. 2018, 42, e12541. [Google Scholar] [CrossRef]
- Walker, D.; Lue, L.F. Anti-inflammatory and immune therapy for Alzheimer’s disease: Current status and future directions. Curr. Neuropharmacol. 2007, 5, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Anekonda, T.S.; Reddy, P.H. Can herbs provide a new generation of drugs for treating Alzheimer’s disease? Brain Res. Brain Res. Rev. 2005, 50, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An Updated Pharmacological Review and Therapeutic Perspectives. Oxidative Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Turgumbayeva, A.; Mertdinç, Z.; Tütüncü, S.; Aydar, E.F.; Özçelik, B.; Anna, S.-W.; Mariola, S.; Koziróg, A.; et al. Santalum Genus: Phytochemical constituents, biological activities and health promoting-effects. Z. Für Nat. C, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A.; et al. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxidative Med. Cell. Longev. 2022, 2022, 5628601. [Google Scholar] [CrossRef]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Raza, Q.; Sadia, H.; Raza, S.; Bhinder, M.; Calina, D.; et al. Myricetin: Targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 2022, 22, 239. [Google Scholar] [CrossRef]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef]
- Ahmad, I.; Aqil, F.; Owais, M. Modern Phytomedicine: Turning Medicinal Plants into Drugs; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Bhattaram, V.A.; Graefe, U.; Kohlert, C.; Veit, M.; Derendorf, H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002, 9 (Suppl. 3), 1–33. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, A.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 20. [Google Scholar] [CrossRef]
- Leyva-Gomez, G.; Cortes, H.; Magana, J.J.; Leyva-Garcia, N.; Quintanar-Guerrero, D.; Floran, B. Nanoparticle technology for treatment of Parkinson’s disease: The role of surface phenomena in reaching the brain. Drug Discov. Today 2015, 20, 824–837. [Google Scholar] [CrossRef]
- Quispe, C.; Herrera-Bravo, J.; Khan, K.; Javed, Z.; Semwal, P.; Painuli, S.; Kamiloglu, S.; Martorell, M.; Calina, D.; Sharifi-Rad, J. Therapeutic applications of curcumin nanomedicine formulations in cystic fibrosis. Prog. Biomater. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Cirone Silva, N.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef]
- Docea, A.O.; Calina, D.; Buga, A.M.; Zlatian, O.; Paoliello, M.M.B.; Mogosanu, G.D.; Streba, C.T.; Popescu, E.L.; Stoica, A.E.; Birca, A.C.; et al. The Effect of Silver Nanoparticles on Antioxidant/Pro-Oxidant Balance in a Murine Model. Int. J. Mol. Sci. 2020, 21, 17. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; do Carmo Pereira, M. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2020, 10, 380–402. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Floran, I.H.; Meza-Toledo, J.A.; Mendoza-Munoz, N.; Gonzalez-Torres, M.; Floran, B.; Cortes, H.; Leyva-Gomez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Quispe, C.; Herrera-Bravo, J.; Javed, Z.; Khan, K.; Raza, S.; Gulsunoglu-Konuskan, Z.; Daştan, S.D.; Sytar, O.; Martorell, M.; Sharifi-Rad, J.; et al. Therapeutic Applications of Curcumin in Diabetes: A Review and Perspective. Biomed. Res. Int. 2022, 2022, 1375892. [Google Scholar] [CrossRef]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Viljoen, A.M. A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils. J. Am. Oil Chem. Soc. 2009, 87, 1–7. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem Toxicol 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Mohar, D.S.; Malik, S. The Sirtuin System: The Holy Grail of Resveratrol? J. Clin. Exp. Cardiol. 2012, 3, 216. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Bahukhandi, A.; Dhyani, P.; Sati, P.; Capanoglu, E.; Docea, A.O.; Al-Harrasi, A.; Dey, A.; Calina, D. Therapeutic Potential of Neoechinulins and Their Derivatives: An Overview of the Molecular Mechanisms Behind Pharmacological Activities. Front. Nutr. 2021, 8, 664197. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
| Target of the Candidate Vaccine in AD | Example/Manufacturer | Results | Trial Status | Ref |
|---|---|---|---|---|
| β-amyloid Vaccines | UB-311 Vaxxinity | improve cognitive function | Phase 2 | [51,52] |
| ABvac 40 Araclon Biotech’s | [50] | |||
| ACI-24 AC Immune | increase clearance of β-amyloid plaques | [49] | ||
| Tau Vaccines | ACI-35.030 AC Immune | no efficacy on tau increase the immune response | Phase 2 | [53] |
| AADvac1 Axon Neuroscience | improve cognitive function | [54] | ||
| Immunomodulatory Vaccines | GV1001 GemVax KAEL Bio | increase clearance of β-amyloid plaques and tau tangles anti-neuroinflammatory | Phase 2 | [55] |
| Bacillus Calmette-Guerin Mindful Diagnostics and Therapeutics | nasal administration impact on β-amyloid plaques immunostimulatory | [56] | ||
| Protollin Brigham and Women’s Hospital | increase immune response | Phase 1 | [48] |
| Tested Phytochemical | Plant Origin | Experimental Model | Effects | Ref. |
|---|---|---|---|---|
Anthocyanin | Phaseolus vulgaris L. Korean Black Beans Morus alba L. (Mulberry) Vaccinium myrtillus L. (Blueberry) | In vivo APP/PS1 mice In vitro murine model SAMP8 SAMR1 Mouse hippocampal cells HT22 Murine model Neuroblastoma cells | Antioxidant Anti-amyloidogenic | [169,170,171] |
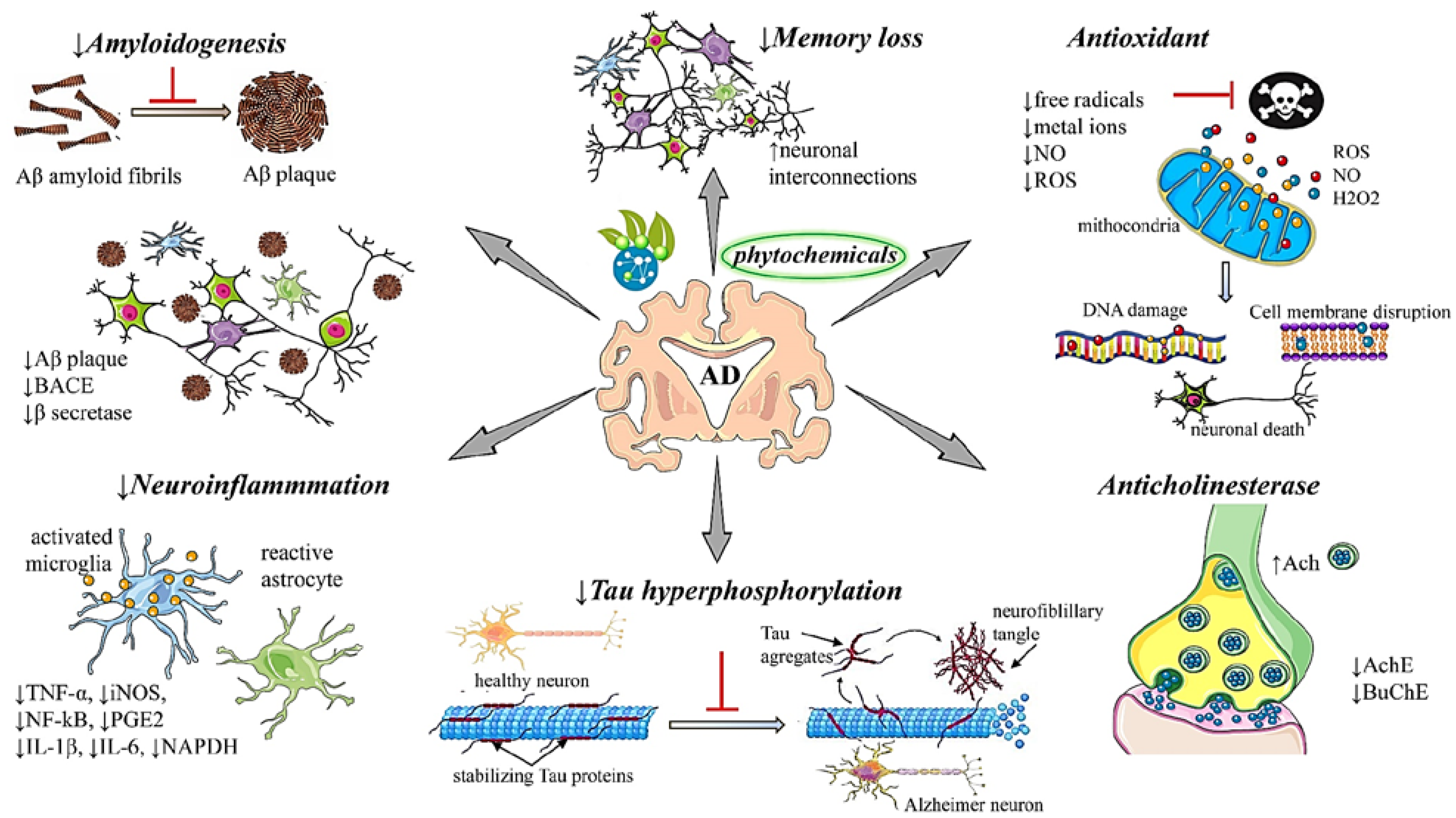
Curcumin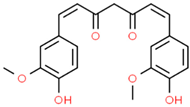 | Curcuma longa L. (Turmeric) | In vivo Alzheimer-Pathological Model of Caenorhabditis elegans In vitro Post-Mortem Brain Tissue Murine model | Antioxidant Anti-amyloidogenic | [172,173,174,175] |
Resveratrol | Vitis vinífera L. (seeds) Veratrum grandiflorum (Maxim. ex Miq.) O.Loes. Vaccinium myrtillus L. Fragaria sp. Rubus ideaeus L. | In vitro Murine macrophages RAW 264.7 Microglia Munira BV-2 Murine models SH-5ySy | Antioxidant Anti-amyloidogenic Anti-hyperphosphorylation Anti neuro-inflammatory | [176,177,178,179] |
Berberin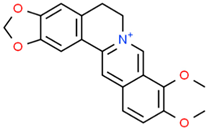 | Berberis sp. | In vitro Neuroblastoma 2a Murine model In vivo Transgenic mice model | Antioxidant Anti-amyloidogenic Anti neuro-inflammatory Tau Anti-hyperphosphorylation | [180,181,182] |
Galantamine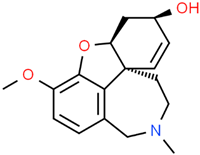 | Galanthus alpinus Sosn. Galanthus woronowii Losinsk. Lycoris radiata (L’Hér.) Herb. | In vitro APPswe/PS1dE9 murine model Brain microvasculating endothelial cells HEK293 (APP-HEK293) Neuroblastoma SH-5ySy cells | Anticholinesterase Anti-amyloidogenic Anti-neuroinflammatory | [64,164,178,183,184,185,186] |
Terpenoid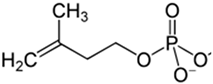 | Ginkgo biloba L. Elettaria cardamomum (L.) Maton Pueraria lobata (Willd.) Ohwi. Ganoderma sp. | In vitro Murine model BACE enzyme acetylcholinesterase SH-5ySy cells Fibroblasts GMK cells | Anticholinesterase Anti-amyloidogenic Antioxidant Anti-neuroinflammatory | [168,187,188,189,190,191] |
Catechin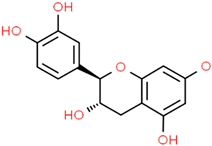 | Rhizophora mucronata Lam. Camellia sinensis (L.) Kuntze | In vitro PC12 cells Acetylcholinesterase enzyme Murine model | Anticholinesterase Anti-amyloidogenic Antioxidant Anti-neuroinflammatory | [192,193,194,195] |
| Hydroxycinnamic acids caffeoylquinic acid 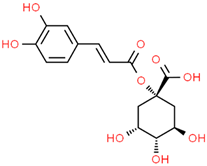 chlorogenic acid 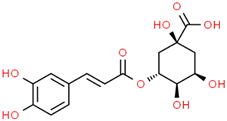 caffeic acid 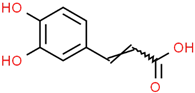 ferulic acid 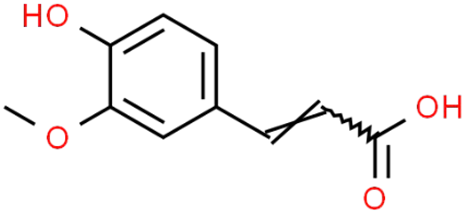 | Coffee grounds (Coffea arabica L.) Green coffee beans (Coffea arabica L.) Roasted coffee beans (Coffea arabica L.) | In vitro SH-5ySy cells Neuro-2A neuroblastoma PC12 cells HepG2 Primary culture cortical Neurons Murine models In vivo Drosophila (Alzheimer’s) models | Anticholinesterase Anti-amyloidogenic Antioxidant Anti -neuroinflammatory Tau Anti-hyperphosphorylation | [144,145,147,148,150,196,197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifi-Rad, J.; Rapposelli, S.; Sestito, S.; Herrera-Bravo, J.; Arancibia-Diaz, A.; Salazar, L.A.; Yeskaliyeva, B.; Beyatli, A.; Leyva-Gómez, G.; González-Contreras, C.; et al. Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. J. Pers. Med. 2022, 12, 1515. https://doi.org/10.3390/jpm12091515
Sharifi-Rad J, Rapposelli S, Sestito S, Herrera-Bravo J, Arancibia-Diaz A, Salazar LA, Yeskaliyeva B, Beyatli A, Leyva-Gómez G, González-Contreras C, et al. Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. Journal of Personalized Medicine. 2022; 12(9):1515. https://doi.org/10.3390/jpm12091515
Chicago/Turabian StyleSharifi-Rad, Javad, Simona Rapposelli, Simona Sestito, Jesús Herrera-Bravo, Alejandra Arancibia-Diaz, Luis A. Salazar, Balakyz Yeskaliyeva, Ahmet Beyatli, Gerardo Leyva-Gómez, Carlos González-Contreras, and et al. 2022. "Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation" Journal of Personalized Medicine 12, no. 9: 1515. https://doi.org/10.3390/jpm12091515
APA StyleSharifi-Rad, J., Rapposelli, S., Sestito, S., Herrera-Bravo, J., Arancibia-Diaz, A., Salazar, L. A., Yeskaliyeva, B., Beyatli, A., Leyva-Gómez, G., González-Contreras, C., Gürer, E. S., Martorell, M., & Calina, D. (2022). Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. Journal of Personalized Medicine, 12(9), 1515. https://doi.org/10.3390/jpm12091515















